Abstract
Riociguat (Adempas): a novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension
INTRODUCTION
Pulmonary hypertension is a condition defined by a mean pulmonary artery pressure greater than or equal to 25 mm Hg at rest measured by right heart catheterization.1 Previously classified into two categories (primary pulmonary hypertension or secondary pulmonary hypertension due to identified causes or risk factors), pulmonary hypertension is now categorized into five groups based on pathological findings, hemodynamic characteristics, and management (Table 1).2 Because of the multiple etiologies of pulmonary hypertension, a thorough diagnostic evaluation is essential in patients who present with symptoms (dyspnea, fatigue, chest pain, syncope, edema) so that appropriate therapy may be initiated.3
Table 1.
Clinical Classification of Pulmonary Hypertension*
| 1. Pulmonary arterial hypertension (PAH; WHO Group 1) |
|
| 2. Pulmonary hypertension due to left heart disease (WHO Group 2) |
|
| 3. Pulmonary hypertension due to lung diseases and/or hypoxia (WHO Group 3) |
|
| 4. Chronic thromboembolic pulmonary hypertension (WHO Group 4) |
| 5. Pulmonary hypertension with unclear multifactorial mechanisms (WHO Group 5) |
|
ALK-1 = activin receptor-like kinase 1; BMPR2 = bone morphogenic protein receptor type II; CAV1 = caveolin-1; ENG = endoglin; HIV = human immunodeficiency virus; WHO = World Health Organization
Updated at the Fifth World Symposium on Pulmonary Hypertension in Nice, France, 2013. Adapted from Simonneau et al.2
Over the last decade, great strides have been made in the understanding and management of pulmonary arterial hypertension (PAH; WHO Group 1). PAH is a chronic, progressive disorder of the pulmonary arterial circulation that leads to pathological increases in peripheral vascular resistance (PVR) and ultimately to right heart failure.3 Hemodynamically, PAH is characterized by a mean pulmonary artery pressure greater than or equal to 25 mm Hg at rest with a pulmonary artery wedge pressure less than or equal to 15 mm Hg and elevated PVR (greater than 3 mm Hg/l*min).1 Though PAH may be idiopathic in nature, it may also be heritable, acquired via drug exposure, or the result of disorders ranging from connective tissue disease and human immunodeficiency virus (HIV) infection to portal hypertension, among others.2 Based on data from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL), the incidence of PAH in U.S. adults is 2.0 per million, with approximately equal numbers of patients with idiopathic or heritable PAH versus PAH due to associated disorders.4 Survival rates progressively decline from the time of diagnosis (85% at one year versus 49% at seven years).5 However, data from various registries indicate that overall survival rates have improved in recent years, likely as a result of improved treatment options.6
The treatment of PAH is difficult, largely because of the multiple pathophysiological processes involved, including excessive vascular proliferation, reduced apoptosis, thrombosis, inflammation, and vasoconstriction.3 General measures recommended for all PAH patients include supervised exercise training, avoidance of pregnancy, routine immunizations (influenza and pneumococcal), psychosocial support, and referral to expert centers.7 Additional supportive therapy includes the use of oral anticoagulants, diuretics, oxygen, and digoxin. Calcium-channel blocker therapy is recommended in those patients exhibiting a positive response to an acute vasoreactivity test.7 In those without such a response, and in patients who cannot sustain a response to calcium-channel blocker therapy, a PAH-approved drug should be selected, taking into account multiple variables—including the patient’s functional class, the medication’s route of administration, side-effect and drug-interaction profiles, patient preference, physician experience, and cost of therapy.7
PAH-specific therapy includes endothelin receptor antagonists (ambrisentan, bosentan, macitentan), phosphodiesterase type-5 (PDE-5) inhibitors (sildenafil, tadalafil), and prostanoids (iloprost, treprostinil, epoprostenol). Such drugs may cautiously be considered in non-PAH pulmonary hypertension as well, although there are few data to guide the decision-making process.3 Typically, treatment for the other more prevalent forms of pulmonary hypertension (WHO Groups 2–5) is directed at the underlying disease state, whether it is cardiac, pulmonary, or thromboembolic in nature.3 Notably, for chronic thromboembolic pulmonary hypertension (CTEPH; WHO Group 4) the treatment of choice is surgical; the first-line therapy, pulmonary endarterectomy, is potentially curative.3
In October 2013, the Food and Drug Administration approved riociguat (Adempas, Bayer Healthcare Pharmaceuticals), the first agent in a novel therapeutic class called soluble guanylate cyclase (sGC) stimulators.8 Riociguat was approved for the treatment of adults with PAH, thereby adding to the treatment armamentarium of this condition. Notably, riociguat was also approved for the treatment of adults whose CTEPH persists or recurs following pulmonary endarterectomy, and in those with inoperable CTEPH.9 Approval for use in patients with CTEPH is unique to riociguat, as no other PAH-approved agent is indicated for other forms of pulmonary hypertension.10–20 The purpose of this article is to review the pharmacological and clinical characteristics of riociguat and discuss their implications for its use.
CHEMICAL STRUCTURE9
Chemically, riociguat is methyl 4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b]pyridin-3-yl]-5-pyrimidinyl(methyl) carbamate. The molecular formula and molecular weight of riociguat are C20H19FN8O2 and 422.42 g/mol, respectively. The chemical structure is shown in Figure 1.
Figure 1.
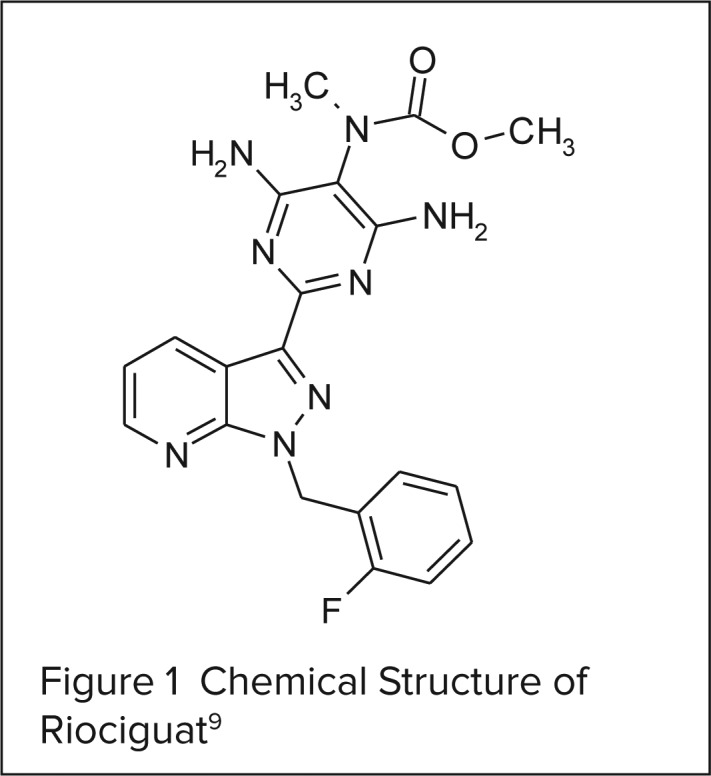
Chemical Structure of Riociguat9
CLINICAL PHARMACOLOGY
In healthy subjects, endothelial cell-derived nitric oxide (NO) induces vasodilation in vascular smooth muscle cells through the activation of sGC and the subsequent increase in production of cyclic guanosine monophosphate (cGMP). The production of endothelial cell-derived NO is reduced in patients with PAH, so targeting the NO/sGC/cGMP pathway is a logical therapeutic approach.21 Riociguat does so via a dual mechanism of action: It sensitizes sGC to endogenous NO, and it directly stimulates sGC receptors independent of NO availability, resulting in vasorelaxation and antiproliferative effects.9,21 This is in contrast to PDE-5 inhibitors, which target a component of the NO signaling pathway further downstream and have restricted efficacy in the presence of reduced NO levels.21
PHARMACODYNAMICS AND PHARMACOKINETICS
In an early hemodynamic study evaluating doses of 1 mg or 2.5 mg of riociguat in patients with pulmonary hypertension, riociguat significantly reduced PVR, mean pulmonary arterial pressure, systolic blood pressure, and systemic vascular resistance in addition to increasing the cardiac index in a dose-dependent manner.22 Comparable hemodynamic effects were subsequently seen in a phase 2 study.23 The effects of riociguat on PVR and N-terminal pro-brain natriuretic peptide (NT-proBNP) were further evaluated in phase 3 clinical studies as prespecified secondary outcomes (whereas the impact on other hemodynamic variables was exploratory in nature).24,25 Compared with placebo, statistically significant reductions in PVR were evident in riociguat-treated patients; least-squares mean differences (LSMD) from baseline were –226 dyn*sec*cm–5 in patients with PAH24 and –246 dyn*sec*cm–5 in patients with CTEPH (P < 0.001).25 Similarly, NT-proBNP levels were significantly lower (P < 0.001) in riociguat-treated patients with PAH (LSMD, –432 pg/mL) and CTEPH (LSMD, –444 pg/mL).24,25
Riociguat is readily absorbed, with an absolute oral bioavailability of approximately 94% that is not affected by food.9 At doses ranging from 0.5 mg to 2.5 mg, riociguat exhibits dose-dependent increases in plasma concentrations with pronounced variability among individuals.22,26 Peak plasma concentrations occur within 1.5 hours after oral intake.9,22 Riociguat is not extensively distributed; its volume of distribution is approximately 30 L in both healthy subjects and patients with pulmonary hypertension.9,27 Riociguat is approximately 95% bound to human plasma proteins, mainly serum albumin and α1- acidic glycoprotein. 9,27 Several cytochrome P450 (CYP) enzymes are involved in the metabolism of riociguat (CYP1A1, CYP3A4/5, CYP2C8, and CYP2J2), though the formation of the major active metabolite, M1, is largely catalyzed by CYP1A1. Clinically, this is noteworthy because CYP1A1 is inducible by exposure to polycyclic aromatic hydrocarbons such as those found in cigarette smoke, thereby warranting dosage adjustments in smokers.9,27 Furthermore, riociguat is a substrate of the efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), making the compound prone to drug interactions. The drug is eliminated in the urine (40%) and feces (53%), largely as metabolites.9,27 The elimination half-life is longer in patients with PAH (approximately 12 hours) than in healthy subjects (approximately seven hours), likely because of impaired elimination in PAH patients.22,26,27 Average systemic clearance is 1.8 L/hr in patients with PAH, approximately half of that seen in healthy subjects (3.4 L/hr).9,22,27
Dose adjustments based on sex, age, weight, or race/ethnicity are not necessary because these factors have not demonstrated a clinically relevant effect on the pharmacokinetics of riociguat and its active metabolite.9,27 Despite significant hepatic metabolism and renal elimination, studies indicate that impairment in hepatic or renal function also do not necessitate dosage adjustments.9,27 Of note, the effect of riociguat in patients with end-stage renal disease (creatinine clearance less than 15 mL/min) or severe hepatic impairment (Child Pugh C) has not been evaluated.9
CLINICAL STUDIES
The efficacy of riociguat was established in two pivotal phase 3 studies involving patients with PAH (WHO Group 1) or CTEPH (WHO Group 4).24,25 Additionally, phase 2 studies evaluating the effect of riociguat in patients with pulmonary hypertension associated with systolic left ventricular dysfunction (PH-sLVD; WHO Group 2) and interstitial lung disease (WHO Group 3) have been published and are briefly summarized here.28,29
Pulmonary Arterial Hypertension
The PATENT-1 trial was an international, multicenter, randomized, double-blind, placebo-controlled, 12-week study evaluating the efficacy and safety of riociguat in patients with symptomatic PAH who were either not receiving treatment for the condition or were being treated with endothelin receptor antagonists or nonintravenous prostanoids.24 A total of 443 patients received placebo (n = 126), riociguat administered orally at individually adjusted doses up to 2.5 mg three times daily (n = 254), or riociguat at doses adjusted up to 1.5 mg three times daily (n = 63). The latter treatment arm was included solely for exploratory purposes and was therefore excluded from the efficacy analysis. Most randomized patients were white (61%) and female (79%) with idiopathic PAH (61%) or PAH associated with connective tissue disease (25%). Almost all patients were in WHO functional class II or III (42% and 53%, respectively) and half were receiving treatment with other PAH therapies, largely endothelin receptor antagonists.24
With respect to the primary endpoint, the change from baseline to the end of week 12 in six-minute walk distance (6MWD), there was a statistically significant difference in the riociguat 2.5-mg-maximum group versus the placebo group (LSMD, 36 m; P < 0.001).24 The effect was consistent across numerous subgroups of patients, including those who were treatment-naïve and those on concomitant endothelin receptor antagonists or prostanoids. Patients with more advanced disease (WHO functional class III or IV) experienced greater improvements in exercise capacity than those in WHO functional class I or II. Statistically significant improvements from baseline were also noted with riociguat therapy in several secondary endpoints, including PVR (P < 0.001), NT-proBNP levels (P < 0.001), WHO functional class (P = 0.003), Borg dyspnea score (P = 0.002), and time to clinical worsening (P = 0.005). Quality of life, as assessed by the EuroQol Group 5-Dimension Self-Report Questionnaire, did not significantly differ between groups.24
Study discontinuation because of adverse events occurred in 3% of the riociguat 2.5-mg-maximum group versus 7% in the placebo group.24 The most frequently occurring serious adverse events in the riociguat 2.5-mg-maximum group and placebo group were syncope (1% versus 4%, respectively), worsening pulmonary hypertension (less than 1% versus 2%, respectively), chest pain (1% in both groups), and right ventricular failure (1% in both groups).24 Additional adverse events data are discussed in the Safety and Tolerability section.
Ninety-eight percent (N = 396) of patients who completed the PATENT-1 study entered PATENT-2, an ongoing, open-label, long-term extension study.24 An exploratory analysis of the first 12 weeks revealed additional improvements in 6MWD in patients receiving riociguat up to 2.5 mg three times daily; there was a mean increase of 53 m over the baseline distance noted in PATENT-1.24 Sustained benefits in 6MWD and functional class were also noted in an interim one-year unpublished analysis of this study.30
Chronic Thromboembolic Pulmonary Hypertension
Ghofrani et al25 evaluated the efficacy and safety of riociguat in patients with CTEPH in an international, multicenter, randomized, double-blind, placebo-controlled study (CHEST-1). Patients (N = 261) with inoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy were randomly assigned to receive placebo (n = 88) or riociguat (n = 173) up to 2.5 mg three times daily for 16 weeks. Clinical outcome measures were similar to those in PATENT-1.
The majority of randomized patients (mean age, 59 years; 66% female) had inoperable CTEPH (72%) and, as in PATENT-1, most were in WHO functional class II (31%) or III (64%).25 Similar to the findings seen in patients with PAH, there was a statistically significant improvement from baseline in the 6MWD (LSMD, 46 m; P < 0.001). This effect was consistent across all subgroups of patients, though less pronounced in patients with persistent/recurrent pulmonary hypertension. Riociguat-treated patients also experienced statistically significant improvements in several secondary endpoints, notably PVR (P < 0.001), NT-proBNP level (P < 0.001), and WHO functional class (P = 0.003); clinical worsening was not significantly delayed. Few patients discontinued the study because of adverse events (3% in the riociguat group versus 2% in the placebo group). Right ventricular failure (3% of patients in each group), syncope (2% in the riociguat group and 3% in the placebo group), and hemoptysis (2% in the riociguat group) were the most frequent serious adverse events.25 The most commonly reported adverse events are summarized in the Safety and Tolerability section. Preliminary data from the long-term extension study (CHEST-2), which have been presented but not yet published, suggest that effects on 6MWD and functional class are sustained in this patient population and that riociguat is generally well tolerated with a good long-term safety profile.31 As with PATENT-2, these data must be viewed cautiously until the full results of the study are available.
Unlabeled Uses
Bonderman et al28 evaluated the effects of riociguat on hemodynamic and clinical outcomes in heart failure patients with PH-sLVD in a double-blind, multicenter, phase 2b study. Eligible patients (N = 201) were randomized in a 2:1:1:2 fashion to one of four treatment arms: placebo, riociguat 0.5 mg, riociguat 1 mg, or riociguat 2 mg, three times daily for a 16-week treatment period. In addition, participants received standard medical treatment (angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, beta blockers, and aldosterone antagonists) or cardiac device therapy. The primary endpoint, placebo-corrected change from baseline to week 16 in mean pulmonary artery pressure, was not significantly different between the riociguat 2-mg group and the placebo group (LSMD, –2.7, P = 0.10). Use of riociguat did, however, result in significant improvements in several hemodynamic variables (cardiac index, stroke volume index, PVR, and systemic vascular resistance) without significantly affecting heart rate and blood pressure. Notably, echocardiographic parameters and exploratory clinical outcomes, such as 6MWD, clinical worsening events, and change in functional class, were also not improved, although an improvement in health-related quality of life was found.28 More studies are needed to better characterize the role of riociguat in this patient population.
In another phase 2, open-label, uncontrolled pilot trial, Hoeper et al29 evaluated the efficacy of riociguat (target dose of 2.5 mg three times daily) in patients with pulmonary hypertension due to interstitial lung disease (N = 22), though the study was primarily designed to assess safety and tolerability. Although cardiac output and PVR improved, this did not translate into substantial improvements in clinically relevant outcomes. There was only a slight increase in 6MWD, while functional class and quality of life measures remained unchanged. During the initial 12 weeks of treatment and at the interim 12-month mark of the long-term extension phase, 104 adverse events were reported, 70% of which were considered drug-related. Dyspnea, peripheral edema, dyspepsia, headache, and “feeling hot” were the most frequently reported adverse events.29 The clinical relevance and applicability of this data is limited owing to the small, uncontrolled, open-label design of this study.
SAFETY AND TOLERABILITY
Adverse Effects
Information on the adverse-event profile of riociguat is restricted to what is known from the fairly short-term phase 3 clinical trials involving patients with PAH and CTEPH. The most commonly reported adverse events in PATENT-1 and CHEST-1 were headache, dizziness, dyspepsia, peripheral edema, nausea, vomiting, diarrhea, and nasopharyngitis (Table 2).24,25 Hypotension was also frequently reported and significantly greater in riociguat-treated patients enrolled in PATENT-1; this would be expected because of the drug’s vasodilatory actions. Notably, this did not translate into an increased incidence of syncope. Syncope was more frequently reported in the placebo-treated patients in both PATENT-1 and CHEST-1 (4% and 3%, respectively) than in patients treated with riociguat (1% and 2%, respectively).24,25
Table 2.
Adverse Events Reported in at Least 5% of Riociguat-Treated Patients And at a Frequency Greater Than Placebo in Phase 3 Clinical Trials24,25
| Adverse Events | Number of Patients (%) | ||||
|---|---|---|---|---|---|
| PATENT-1 | CHEST-1 | ||||
| Riociguat, maximum 2.5 mg t.i.d. (n = 254) | Riociguat, maximum 1.5 mg t.i.d. (n = 63) | Placebo (n = 126) | Riociguat, maximum 2.5 mg t.i.d. (n = 173) | Placebo (n = 88) | |
| Headache | 69 (27) | 20 (32) | 25 (20) | 43 (25) | 12 (14) |
| Dyspepsia | 48 (19) | 8 (13) | 10 (8) | 31 (18) | 7 (8) |
| Peripheral edema | 44 (17) | 14 (22) | 14 (11) | 27 (16) | 18 (20) |
| Dizziness | 40 (16) | 15 (24) | 15 (12) | 39 (23) | 11 (12) |
| Nasopharyngitis | 26 (10) | 6 (10) | 14 (11) | 26 (15) | 8 (9) |
| Nausea | 40 (16) | 10 (16) | 16 (13) | 19 (11) | 7 (8) |
| Diarrhea | 35 (14) | 6 (10) | 13 (10) | 17 (10) | 4 (5) |
| Vomiting | 26 (10) | 7 (11) | 11 (9) | 17 (10) | 3 (3) |
| Pyrexia | 8 (3) | 6 (10) | 4 (3) | — | — |
| Hypotension | 25 (10)* | 2 (3) | 3 (2) | 16 (9) | 3 (3) |
| Anemia | 21 (8) | 1 (2) | 3 (2) | — | — |
| Palpitations | 20 (8) | 5 (8) | 6 (5) | — | — |
| Gastroesophageal reflux disease | 14 (6) | 4 (6) | 4 (3) | — | — |
| Gastritis | 4 (2) | 4 (6) | 0 | — | — |
| Nasal congestion | 11 (4) | 4 (6) | 3 (2) | — | — |
| Upper respiratory tract infection | — | — | — | 10 (6) | 4 (5) |
| Increase in INR | — | — | — | 10 (6) | 4 (5) |
| Constipation | — | — | — | 10 (6) | 1 (1) |
| Prolonged aPTT | — | — | — | 8 (5) | 2 (2) |
aPTT = activated partial-thromboplastin time; INR = international normalized ratio; t.i.d. = three times daily
P = 0.005
It is worth noting that various bleeding events were reported more frequently in riociguat-treated patients than in the placebo groups in both studies.32 Treatment-emergent bleeding events were reported for 11.0% of subjects in the riociguat 2.5-mg group versus 9.5% of subjects in the placebo group in the PATENT-1 study, and 13.3% of subjects in the riociguat group compared with 11.4% of subjects in the placebo group in the CHEST-1 trial. The most frequently reported bleeding events were epistaxis and hemoptysis.32 The occurrence of serious bleeding events during the clinical development of riociguat, albeit low (2.4% versus 0% in placebo), prompted the inclusion of a warning in the prescribing information.9 Ultimately, the long-term extension studies and post-marketing reports will shed more light on the overall safety of this agent.
Contraindications
Because of teratogenicity documented in preclinical studies, riociguat is classified as pregnancy category X and contraindicated for use during pregnancy.9 A restricted distribution program is in place (the Adempas REMS Program) for all females on riociguat therapy, irrespective of reproductive potential. The program requires all prescribers and pharmacies dispensing riociguat to be to certified with the program, and in females of reproductive potential, pregnancy must be ruled out prior to initiation of therapy, monthly during treatment, and one month after drug discontinuation. Acceptable methods of contraception must be utilized during and for one month after treatment.33 Concurrent administration of riociguat with nitrates or NO donors as well as with PDE-5 or nonspecific PDE inhibitors (e.g., dipyridamole, theophylline) is also contraindicated because of additive hemodynamic effects resulting in hypotension.9
Warnings and Precautions
As a result of its blood-pressure-lowering effects, the riociguat label warns of the potential for symptomatic hypotension or ischemia when riociguat is used in patients with hypovolemia, severe left ventricular outflow obstruction, resting hypotension, and autonomic dysfunction, and when it is used concurrently with antihypertensive or strong CYP and P-gp/BCRP inhibitors (see the Drug Interactions section).9 Initial lower doses in those at risk for hypotension and/or dose reductions in those developing hypotension while on riociguat therapy are warranted. As previously noted, the labeling also warns of the increased incidence of bleeding noted with riociguat.9 Furthermore, since the cardiovascular status of patients with pulmonary veno-occlusive disease may be significantly worsened by agents with vasodilatory effects on the pulmonary vasculature, administration of riociguat to such patients is not recommended. The possibility of associated pulmonary vaso-occlusive disease should be considered in the event that pulmonary edema develops while on riociguat therapy.9
DRUG INTERACTIONS
Both riociguat and NO delivered by nitrates act on sGC to generate an increase in cGMP. PDE-5 inhibitors also increase cGMP by inhibiting its breakdown. Because of their mechanisms of action on the NO/sGC/cGMP pathway, an additive hypotensive effect is expected. This has been demonstrated with concurrent administration of riociguat and nitrates or NO donors (such as amyl nitrate) and PDE-5 inhibitors.32 As a result, concurrent use is contraindicated. With respect to pharmacokinetic interactions,in vitro and in vivo studies suggest that riociguat is unlikely to appreciably induce or inhibit CYP enzymes and drug transporters, lowering its interaction potential as a precipitant drug.9,34,35 However, plasma concentrations of riociguat are affected by other precipitant compounds, warranting dosage and administration adjustments (see the Dosage and Administration section). As alluded to previously, plasma concentrations of riociguat are reduced by 50% to 60% in smokers because of induction of the CYP1A1 enzyme by polycyclic aromatic hydrocarbons.9 Strong CYP3A inducers (e.g., rifampin, phenytoin, carbamazepine, phenobarbital, St. John’s wort) may also significantly decrease riociguat exposure, although data to guide dose adjustments are lacking.9 Concurrent administration of ketoconazole, a strong CYP and P-gp/BCRP inhibitor, led to a 150% increase in riociguat’s mean area under the curve (AUC).32 A similar increase in riociguat exposure is expected with HIV protease inhibitors.9 Concurrent administration with antacids such as aluminum hydroxide/magnesium hydroxide resulted in reduced riociguat bioavailability (mean Cmax decrease of 56% and mean AUC decrease of 34%), an effect that was also seen, although to a lesser extent, with proton-pump inhibitor co-administration (decrease in mean Cmax of 35% and mean AUC of 26%).9,32 Importantly, riociguat demonstrates no clinically relevant interactions with warfarin and aspirin, agents that are typically used in patients with PAH.9,35
DOSAGE AND ADMINISTRATION9
Riociguat is available as oral tablets in five different strengths (0.5, 1, 1.5, 2, and 2.5 mg) to allow for dosage flexibility. The recommended starting dose is 1 mg taken three times a day. In patients at risk for hypotension, consideration should be given to initiating treatment at 0.5 mg three times a day. The dose may be increased no sooner than every two weeks to a maximum dose of 2.5 mg three times a day if tolerated, though patients who smoke may require higher doses. Up-titration should occur in increments of 0.5 mg taken three times a day and only if the patient’s systolic blood pressure remains above 95 mm Hg and there are no signs or symptoms of hypotension. Should hypotension occur, a decrease in dose (by 0.5 mg taken three times a day) is recommended. In patients concomitantly using riociguat with strong CYP and P-gp/BCRP inhibitors (e.g., azole antifungals and protease inhibitors), a starting dose of 0.5 mg three times daily should be considered and close monitoring for signs and symptoms of hypotension is necessary. Because of the impact on riociguat absorption, antacids should not be taken within one hour of riociguat. Any treatment interruption for three days or more warrants retitration of the riociguat dose.
COST
The average wholesale price of a 30-day supply (90 tablets) of riociguat, regardless of the strength, is $9,270.36 This is in line with the cost of endothelin receptor antagonists, but significantly greater than the cost of PDE-5 inhibitors. A cost comparison of agents approved for PAH that are administered orally or via inhalation is provided in Table 3. Parenterally administered prostanoids are excluded from the table because use of these agents results in additional indirect costs associated with continuous subcutaneous or intravenous administration.
Table 3.
Cost of Therapy With Orally Administered Or Inhaled PAH-Approved Agents
| Drug | Usual Adult Maintenance Dose9–17 | Costa |
|---|---|---|
| Soluble guanylate cyclase (sGC) stimulator | ||
| Riociguat (Adempas, Bayer) | 2.5 mg by mouth three times daily | $9,270 |
| Endothelin receptor antagonists | ||
| Ambrisentan (Letairis, Gilead) | 5–10 mg by mouth once daily | $8,272 |
| Bosentan (Tracleer, Actelion) | 125 mg by mouth twice daily | $9,126 |
| Macitentan (Opsumit, Actelion) | 10 mg by mouth once daily | $8,208 |
| Phosphodiesterase type-5 (PDE-5) inhibitors | ||
| Sildenafil (Revatio, Pfizer) (generic: multiple manufacturers) | 20 mg by mouth three times daily | $2,751 $1,710b |
| Tadalafil (Adcirca, Eli Lilly) | 40 mg by mouth once daily | $2,486 |
| Prostanoids | ||
| Iloprost (Ventavis, Actelion) | 2.5–5 mcg/inhalation six to nine times per day | $21,049c |
| Treprostinil (Tyvaso, United Therapeutics) | Nine inhalations (54 mcg) four times daily | $15,622d |
| Treprostinil (Orenitram, United Therapeutics) | Oral dosing, individualized according to responsee | $9,828e |
Cost is calculated for a 30-day supply unless otherwise specified. Cost is based on average wholesale price (AWP) at the usual adult maintenance dose and rounded to the nearest dollar.
The lowest AWP noted in Red Book Online is provided.
Cost is based on 180 ampules of either the 10-mcg/mL or 20-mcg/mL ampule.
Cost for a 28-day supply of the refill kit containing seven foil pouches each with four 2.9-mL ampules.
The mean dose in a 12-week study was 3.4 mg twice daily.17 Cost is based on a dose of 3.5 mg twice daily (2.5-mg and 1-mg tablets).
P&T COMMITTEE CONSIDERATIONS
The efficacy of riociguat in PAH is comparable to that seen with other PAH-approved agents; riociguat improved 6MWD by 36 m, whereas this endpoint was improved by 33 m and 35 m with tadalafil and bosentan, respectively.11,14 This effect size has been characterized as modest, as only 21% of patients had improvement in functional class and the improvement in 6MWD just about surpassed the minimal clinically important difference (33 m).37 Still, the current evidence-based treatment algorithm for PAH recommends riociguat as one of several agents that may be used as initial therapy in patients of WHO functional class II or III or as a component of combination therapy (except with PDE-5 inhibitors) in cases of inadequate clinical response.7 Since there are no studies comparing the different PAH-approved compounds, no evidence-based first-line treatment is proposed; instead, treatment selection must be based on patient- and medication-specific factors.7
As an additional point to consider, riociguat is the only medication to be shown in a randomized, controlled clinical study to be effective in improving 6MWD in select patients with CTEPH. Hence, recommendations developed out of the Fifth World Symposium on Pulmonary Hypertension include the use of riociguat in cases where pulmonary endarterectomy is not feasible or CTEPH does not abate following surgery.38 Moreover, clinical trials suggest that riociguat has a relatively tolerable side-effect profile, although adverse effects of concern include the apparent increased risk of bleeding and hypotension. Most of the frequently reported adverse events can be attributed to its mode of action as a smooth muscle dilator.
Potential barriers to the use of this agent include its multiple daily dosing schedule, several drug interactions, the need for a risk evaluation and mitigation strategy, and its cost, which is significantly higher than PDE-5 inhibitors. Despite these limitations, riociguat does appear to have a role for use in patients in whom other therapies may not be suitable and as a component of combination therapy. It certainly has a place in therapy for CTEPH, though it is important to underscore the fact that it should not be used in lieu of surgery.
CONCLUSION
PAH is a chronic disorder in which morbidity and mortality remain high despite the availability of medical therapies. Riociguat is a first-in-class oral medication that adds to the treatment options for PAH. It is also the only agent approved for CTEPH, albeit in select patients. Riociguat appears to be effective and well tolerated. Additional long-term studies are necessary to further elucidate its place in therapy.
REFERENCES
- 1.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 4.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest. 2011;139(1):128–137. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 6.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 suppl):D51–59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D60–72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration FDA approves Adempas to treat pulmonary hypertension. Oct 10, 2013. Available at: www.fda.gov/newsevents/newsroom/pressannouncements/ucm370866.htm. Accessed April 29, 2014.
- 9.Adempas (riociguat) prescribing information. Whippany, New Jersey: Bayer HealthCare Pharmaceuticals Inc.; Oct, 2013. [Google Scholar]
- 10.Letairis (ambrisentan) prescribing information. Foster City, California: Gilead Sciences, Inc.; Jan, 2014. [Google Scholar]
- 11.Tracleer (bosentan) prescribing information. South San Francisco, California: Actelion Pharmaceuticals US, Inc.; Oct, 2012. [Google Scholar]
- 12.Opsumit (macitentan) prescribing information. South San Francisco, California: Actelion Pharmaceuticals US, Inc.; Oct, 2013. [Google Scholar]
- 13.Revatio (sildenafil) prescribing information. New York, New York: Pfizer Inc.; Jan, 2014. [Google Scholar]
- 14.Adcirca (tadalafil) prescribing information. Indianapolis, Indiana: Eli Lilly and Company; Dec, 2013. [Google Scholar]
- 15.Ventavis (iloprost) prescribing information. South San Francisco, California: Actelion Pharmaceuticals US, Inc.; Nov, 2013. [Google Scholar]
- 16.Tyvaso (treprostinil) prescribing information. Research Triangle Park, North Carolina: United Therapeutics Corp.; May, 2013. [Google Scholar]
- 17.Orenitram (treprostinil) prescribing information. Research Triangle Park, North Carolina: United Therapeutics Corp.; Feb, 2014. [Google Scholar]
- 18.Remodulin (treprostinil) prescribing information. Research Triangle Park, North Carolina: United Therapeutics Corp.; Oct, 2013. [Google Scholar]
- 19.Flolan (epoprostenol sodium) prescribing information. Research Triangle Park, North Carolina: GlaxoSmithKline, LLC; Mar, 2011. [Google Scholar]
- 20.Veletri (epoprostenol) prescribing information. South San Francisco, California: Actelion Pharmaceuticals US, Inc.; Jun, 2012. [Google Scholar]
- 21.Ghofrani HA, Grimminger F. Soluble guanylate cyclase stimulation: an emerging option in pulmonary hypertension therapy. Eur Respir Rev. 2009;18(111):35–41. doi: 10.1183/09059180.00011112. [DOI] [PubMed] [Google Scholar]
- 22.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33(4):785–792. doi: 10.1183/09031936.00039808. [DOI] [PubMed] [Google Scholar]
- 23.Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36(4):792–799. doi: 10.1183/09031936.00182909. [DOI] [PubMed] [Google Scholar]
- 24.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 25.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 26.Frey R, Mück W, Unger S, et al. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63–2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol. 2008;48(8):926–934. doi: 10.1177/0091270008319793. [DOI] [PubMed] [Google Scholar]
- 27.Food and Drug Administration, Center for Drug Evaluation and Research Application number: 204819Orig1s000: clinical pharmacology and biopharmaceutics review(s) Jun, 2013. Available at: http://www.accessdata.fda.gov/drug-satfda_docs/nda/2013/204819Orig1s000ClinPharmR.pdf. Accessed April 29, 2014.
- 28.Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502–511. doi: 10.1161/CIRCULATIONAHA.113.001458. [DOI] [PubMed] [Google Scholar]
- 29.Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41(4):853–860. doi: 10.1183/09031936.00213911. [DOI] [PubMed] [Google Scholar]
- 30.Rubin LJ, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): a phase III long-term extension study (PATENT-2). Presentation at American Thoracic Society 2013 International Conference; Philadelphia. May 17–22, 2013; Available at: http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2013.187.1_MeetingAbstracts.A3531. Accessed April 29, 2014. [Google Scholar]
- 31.Bayer HealthCare Press Center Interim results from CHEST-2 study support benefits of Bayer’s riociguat as demonstrated in phase III CHEST-1 study. Mar 4, 2013. Available at: http://press.healthcare.bayer.com/en/press/auth/news-details-page.php/14933/2013-0143. Accessed April 29, 2014.
- 32.Food and Drug Administration Center for Drug Evaluation and Research. Application number: 204819Orig1s000: medical review(s) Apr 26, 2013. Available at: http://www.access-data.fda.gov/drugsatfda_docs/nda/2013/204819Orig1s000MedR.pdf. Accessed April 29, 2014.
- 33.Food and Drug Administration Adempas (riociguat tablets): Risk Evaluation and Mitigation Strategy (REMS) Jun 11, 2014. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationfor-PatientsandProviders/UCM370907.pdf. Accessed October 9, 2014.
- 34.Rickert V, Haefeli WE, Weiss J. Pharmacokinetic interaction profile of riociguat, a new soluble guanylate cyclase stimulator, in vitro. Pulm Pharmacol Ther. 2014;28(2):130–137. doi: 10.1016/j.pupt.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Frey R, Mück W, Kirschbaum N, et al. Riociguat (BAY 63–2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. J Clin Pharmacol. 2011;51(7):1051–1060. doi: 10.1177/0091270010378119. [DOI] [PubMed] [Google Scholar]
- 36.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Accessed October 9, 2014. [Google Scholar]
- 37.Archer SL. Riociguat for pulmonary hypertension—a glass half full. N Engl J Med. 2013;369(4):386–388. doi: 10.1056/NEJMe1306684. [DOI] [PubMed] [Google Scholar]
- 38.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D92–99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]


