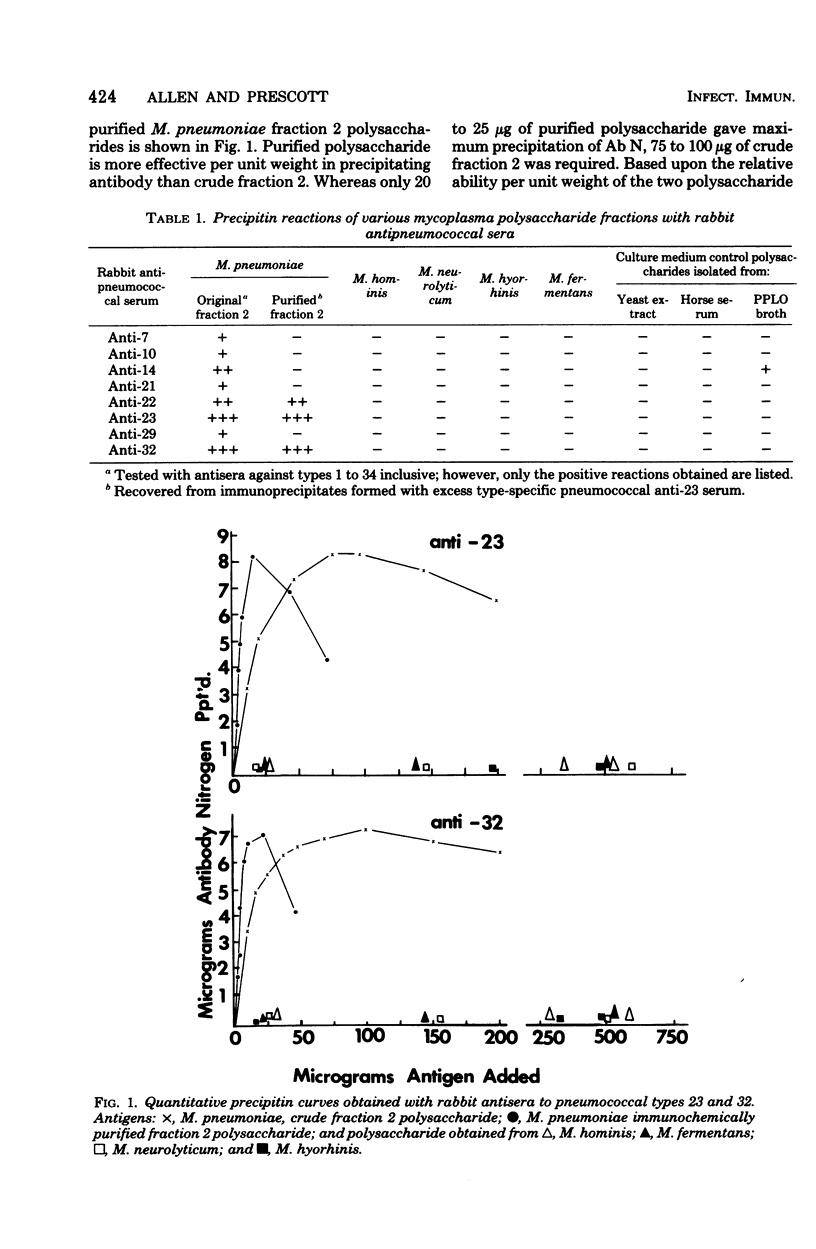
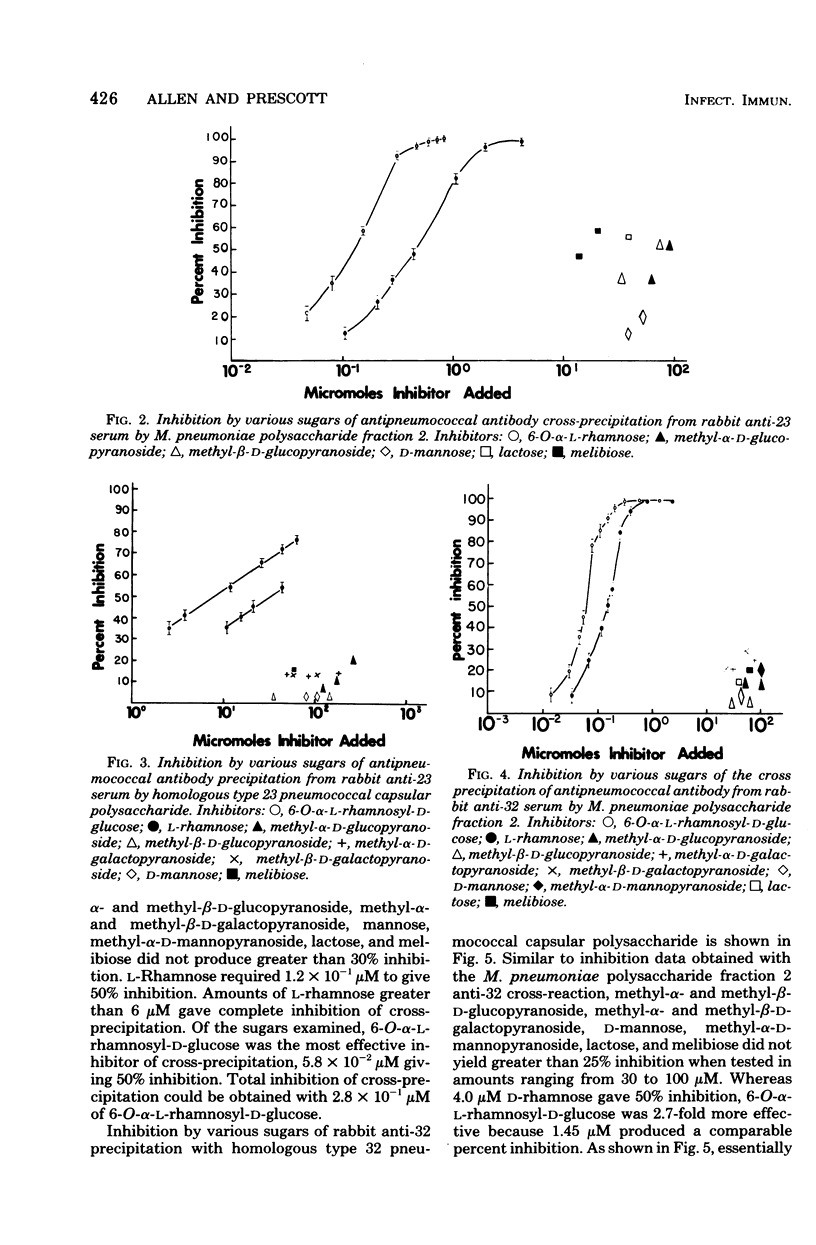
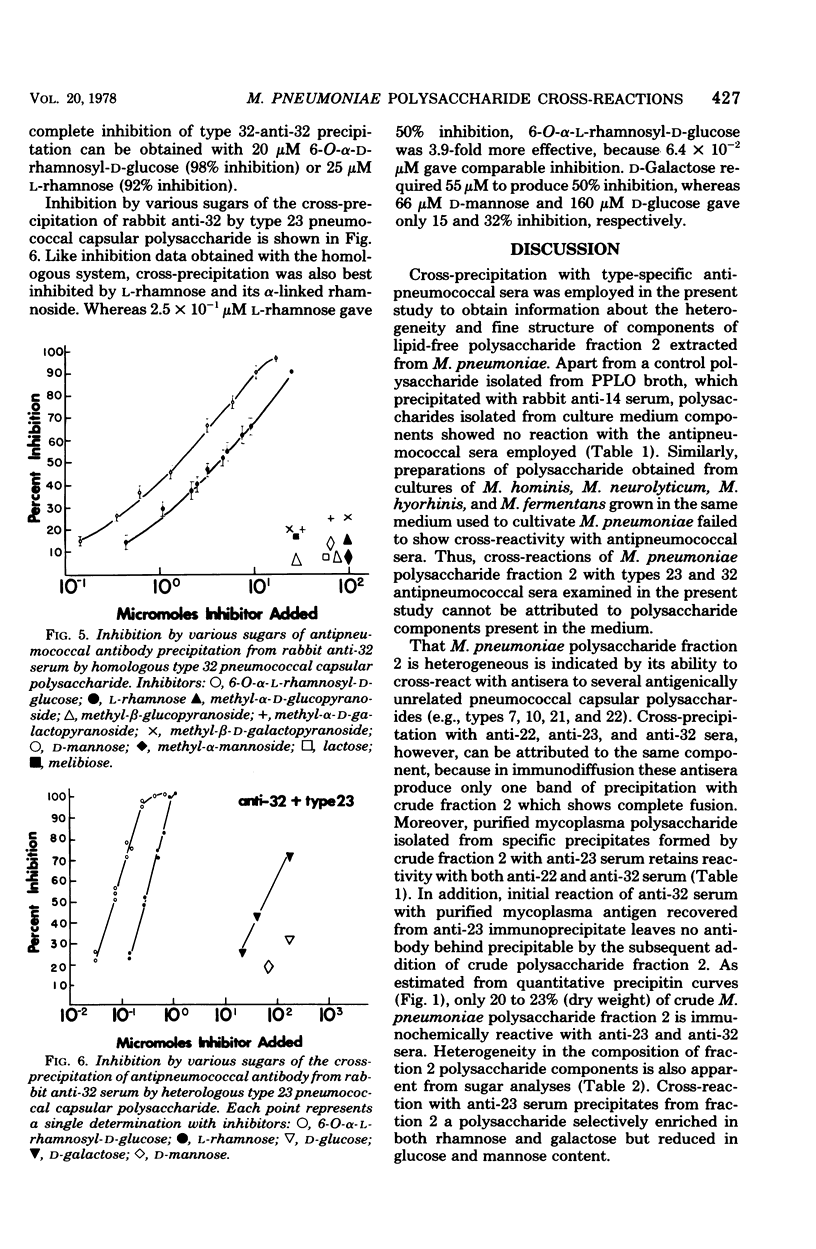
Abstract
Lipid-free polysaccharide fraction 2 extracted from Mycoplasma pneumoniae strain FH by Prescott et al. (J. Bacteriol. 91:2117-2115, 1966) was examined for its ability to cross-precipitate antibody from type-specific rabbit antipneumococcal sera types 1 to 34 inclusive. Cross-precipitation in type-specific pneumococcal anti-type 23 and anti-type 32 sera was examined in detail and could be attributed to a rhamnose-galactose-rich component of crude M. pneumoniae polysaccharide fraction 2 recovered from immunoprecipitates formed with anti-type 23 serum. Immunochemically isolated mycoplasma polysaccharide was found to contain glucose, galactose, rhamnose, and mannose in 1:14:5:4 molar proportions. Comparison of the ability of 6-O-alpha-L-rhamnosyl-D-glucose and free L-rhamnose to inhibit precepitation by homologous pneumococcal and heterologous mycoplasma polysaccharide antigens indicates a combining site specificity for anti-type 23 and anti-type 32 antibodies directed largely against the alpha-linked L-rhamnosyl determinants and the occurrence of alpha-L-rhamnosyl units in type 32 and M. pneumoniae polysaccharides. Hapten inhibition of the cross-precipitation of pneumococcal type 23 capsular polysaccharide in anti-type 32 serum helps to establish that cross-reactivity can be attributed to interaction of recurrent, alpha-L-rhamnosyl units of type 23 with anit-alpha-L-rhamnoside combining sites of anti-type 32 antibodies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldes G., Prescott B. A comparison of the chemical composition of Mycobacterium tuberculosis muris with Mycobacterium tuberculosis bovis (BCG). Biochem Biophys Res Commun. 1971 Aug 20;44(4):852–858. doi: 10.1016/0006-291x(71)90789-3. [DOI] [PubMed] [Google Scholar]
- Felton L. D., Kauffmann G., Stahl H. J. The Precipitation of Bacterial Polysaccharides with Calcium Phosphate : Pneumococcus. J Bacteriol. 1935 Feb;29(2):149–161. doi: 10.1128/jb.29.2.149-161.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger M., Davie J. M., Krause R. M. Cross-reactions of the group-specific polysaccharides of streptococcal groups B and G in anti-pneumococcal sera with especial reference to type 23 and its determinants. J Immunol. 1967 Oct;99(4):794–796. [PubMed] [Google Scholar]
- Heidelberger M., Jann K., Jann B., Orskov F., Orskov I., Westphal O. Relations between structures of three K polysaccharides of Escherichia coli and cross-reactivity in antipneumococcal sera. J Bacteriol. 1968 Jun;95(6):2415–2417. doi: 10.1128/jb.95.6.2415-2417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABAT E. A., SCHIFFMAN G. Immunochemical studies on blood groups. 28. Further studies on the oligosaccharide determinats of blood group B and BP1 specificity. J Immunol. 1962 Jun;88:782–787. [PubMed] [Google Scholar]
- Michel M. F., Krause R. M. Immunochemical studies on the group and type antigens of group F streptococci and the identification of a grouplike carbohydrate in a type II strain with an undesignated group antigen. J Exp Med. 1967 Jun 1;125(6):1075–1089. doi: 10.1084/jem.125.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott B., Sobeslavsky O., Caldes G., Chanock R. M. Isolation and characterization of fractions of Mycoplasma pneumoniae. I. Chemical and chromatographic separation. J Bacteriol. 1966 Jun;91(6):2117–2125. doi: 10.1128/jb.91.6.2117-2125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABAROVA Z. A., BUCHANAN J. G., BADDILEY J. The composition of pneumococcus type-specific substances containing phosphorus. Biochim Biophys Acta. 1962 Feb 12;57:146–148. doi: 10.1016/0006-3002(62)91091-0. [DOI] [PubMed] [Google Scholar]
- Schiefer H. G., Gerhardt U., Brunner H., Krüpe M. Studies with lectins on the surface carbohydrate structures of mycoplasma membranes. J Bacteriol. 1974 Oct;120(1):81–88. doi: 10.1128/jb.120.1.81-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., James W. D., Chanock R. M. Isolation and characterization of fractions of Mycoplasma pneumoniae. II. Antigenicity and immunogenicity. J Bacteriol. 1966 Jun;91(6):2126–2138. doi: 10.1128/jb.91.6.2126-2138.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavský O., Prescott B., James W. D., Chanock R. M. Serological and immunogenic activities of different fractions of Mycoplasma pneumoniae. Ann N Y Acad Sci. 1967 Jul 28;143(1):682–690. doi: 10.1111/j.1749-6632.1967.tb27714.x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WILLERS J. M., OTTENS H., MICHEL M. F. IMMUNOCHEMICAL RELATIONSHIP BETWEEN STREPTOCOCCUS MG, F 3 AND STREPTOCOCCUS SALIVARIUS. J Gen Microbiol. 1964 Dec;37:425–431. doi: 10.1099/00221287-37-3-425. [DOI] [PubMed] [Google Scholar]
- Welch H., Borman E. K., Mickle F. L. Preparation and Analysis of Diagnostic Antipneumococcus Serum. Am J Public Health Nations Health. 1939 Jan;29(1):35–42. doi: 10.2105/ajph.29.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]


