Abstract
IMPORTANCE
Vitamin D deficiency has been associated with hypertension, diabetes mellitus, and incident stroke. Little is known about the association between vitamin D and subclinical cerebrovascular disease.
OBJECTIVE
To examine the relationship of 25-hydroxyvitamin D (25[OH]D) levels with cerebrovascular abnormalities as assessed on brain magnetic resonance imaging (MRI) among participants of the Atherosclerosis Risk in Communities (ARIC) Brain MRI study.
DESIGN, SETTING, AND PARTICIPANTS
Participants were white and black adults aged 55 to 72 years with no history of clinical stroke who underwent a cerebral MRI at ARIC visit 3 (n = 1622) and a second cerebral MRI approximately 10 years later (n = 888).
EXPOSURES
The 25(OH)D level was measured by mass spectrometry at visit 3, with levels adjusted for calendar month and categorized using race-specific quartiles.
MAIN OUTCOMES AND MEASURES
The cross-sectional and prospective associations of 25(OH)D levels with white matter hyperintensities (WMHs) and MRI-defined infarcts were investigated using multivariable regression models.
RESULTS
The mean age of the participants was 62 years, 59.6% were women, and 48.6% were black. Lower 25(OH)D levels were not significantly associated with WMH score of severity, prevalent high-grade WMH score (≥3), or prevalent infarcts in cross-sectional, multivariable-adjusted models (all P > .05). Similarly, no significant prospective associations were found for lower 25(OH)D levels with change in WMH volume, incident high WMH score (≥3), or incident infarcts on the follow-up MRI, which occurred approximately 10 years later.
CONCLUSIONS AND RELEVANCE
A single measure of 25(OH)D was not cross-sectionally associated with WMH grade or prevalent subclinical infarcts and was not prospectively associated with WMH progression or subclinical brain infarcts seen on serial cerebral MRIs obtained approximately 10 years apart. These findings do not support optimizing vitamin D levels for brain health.
White matter hyperintensities (WMHs) and subclinical infarcts are commonly seen on brain magnetic resonance imaging (MRI) scans of older adults.1 Because of their wide variability in prevalence among older adults and their associations with cardiovascular disease (CVD) risk factors and prior stroke, WMHs are believed to be at least partially preventable through identification and treatment of modifiable risk factors.1 White matter hyperintensities, even in the absence of obvious neurologic deficits, are associated with reduced functioning on cognitive testing and subjective mental decline.2 Furthermore, progression of WMHs has a stronger association with persistent cognitive impairment than a single measure of WMHs.3
In the Atherosclerosis Risk in Communities (ARIC) Brain MRI study, Mosley et al4 found that both high-grade WMHs and silent infarcts seen on brain MRI were independently associated with lower scores on cognitive testing. Among a subset of these ARIC Brain MRI study participants who underwent brain MRI imaging a second time 10 years later, worsening status indicated on MRI, including WMH progression and incident subclinical infarction, was significantly associated with neurologic symptoms during follow-up.5 Thus, the identification of novel and modifiable risk factors (eg, potential vitamin D deficiency) associated with silent infarcts, WMHs, and their progression could have important clinical implications.
Cumulative systolic blood pressure is a strong predictor of WMH progression,6 and adequate vitamin D status may play an important role in blood pressure regulation. In mice, activated vitamin D is an inhibitor of the renin-angiotensin system.7 Observational studies8 have linked low serum 25-hydroxyvitamin D (25[OH]D) levels with incident hypertension. Numerous observational studies and meta-analyses have found low 25(OH)D levels to be associated with CVD risk factors9 and increased risk for CVD events.10
Emerging data suggest that vitamin D also may be important for cognitive functioning11 and protective against neurovascular injury.12 Low 25(OH)D status is associated with increased risk for symptomatic ischemic stroke.13,14 However, despite the association of vitamin D with clinical stroke, very little is known about the relationship of 25(OH)D with subclinical cerebrovascular abnormalities. Only one small (N = 318) cross-sectional study15 among elderly adults receiving home care services found that lower vitamin D levels were associated with increased WMH volume and severity and the prevalence of large-vessel infarcts. However, reverse causation may be one plausible explanation for the association found because sicker individuals are less likely to be able to participate in physical activity outdoors and exposure to sunlight.16
Therefore, further exploration of the relationship between presymptomatic cerebrovascular abnormalities and vitamin D levels is needed to determine whether there might be a window for preventing clinically symptomatic disease. We set out to examine both the cross-sectional and prospective associations of vitamin D levels with cerebrovascular brain MRI abnormalities among participants in the ARIC Brain MRI ancillary study. We hypothesized that vitamin D deficiency would be associated with a higher prevalence of WMHs and subclinical infarcts at baseline and with increased progression of WMHs and increased risk of silent brain infarcts over time. We hypothesized that this association of low vitamin D and subclinical cerebrovascular disease would be mediated through vascular risk factors, particularly hypertension.
Methods
Participants
The ARIC study was a prospective, population-based study of CVD, which at baseline recruited 15 792 middle-aged, predominantly black and white participants in 4 US communities in 1987–1989.17 The ARIC Brain MRI ancillary study is a subset of ARIC study cohort participants aged 55 years or older from the Forsyth County, North Carolina, and Jackson, Mississippi, sites who were invited for a cerebral MRI and cognitive testing during ARIC visit 3 (1993–1994). Inclusion and exclusion criteria for the ARIC Brain MRI study have been published.4 A total of 1934 participants underwent cerebral MRI (59.7% women and 49.6% blacks).
All participants without MRI contraindications who completed the ARIC visit 3 MRI were invited for a second brain MRI between 2004 and 2006. Two interpretable brain scans were available for 983 participants.6 Blood samples were collected during each full cohort visit under careful conditions. Serum or plasma was separated at 4°C and promptly stored at −70°C.
For the purposes of these analyses, ARIC visit 3 (the date of the first brain MRI) was considered the baseline time point for the ARIC Brain MRI study. Of the 1934 participants with brain MRI data at ARIC visit 3, we excluded those with a history of stroke (n = 44), including 12 individuals who had an ARIC-adjudicated clinical stroke between visit 1 and visit 3 and 32 who had a self-reported stroke before visit 1. We additionally excluded those with missing stored serum samples, an insufficient amount of serum for 25(OH)D measurement, samples that did not pass internal quality control measures (n = 165), participants of self-reported nonwhite and nonblack race (n = 5), and participants missing covariates included in statistical models (n = 98). This left a total of 1622 participants with measured 25(OH)D levels and interpretable brain MRIs at visit 3 for cross-sectional analyses and 888 with a second brain MRI available for prospective analyses.
The institutional review boards at all ARIC study sites approved study protocols, and all participants provided written informed consent. Participants received financial compensation.
Laboratory Assays
Frozen stored plasma or serum samples of 25(OH)D have been shown18 to be extremely stable, making it a useful bio-marker for epidemiologic studies. With the use of stored serum from ARIC visit 3, the 25(OH)D level was measured using liquid chromatography–tandem mass spectrometry (Shimadzu LC20AD XR-ABISciex 5500) at the Molecular Epidemiology and Biomarker Research Laboratory and Advanced Research and Diagnostic Laboratory, University of Minnesota, in 2012–2013. The interassay coefficients of variation (CVs) for 25(OH)D2 were 6.2% and 5.3% at concentrations of 7.9 and 12.9 ng/mL, respectively (to convert to nano-moles per liter, multiply by 2.496). The interassay CVs for 25(OH)D3 were 4.8% and 4.8% for concentrations of 30.1 and 55.8 ng/mL. The total 25(OH)D concentration was determined by adding 25(OH)D2 and 25(OH)D3.
Serum calcium, phosphorus, and parathyroid hormone (PTH) levels were also measured using the same stored samples from ARIC visit 3. Calcium and phosphate levels were measured (Modular P Chemistry Analyzer; Roche Diagnostics) using a colorimetric method. The laboratory interassay CV was 2.3% for calcium and 2.2% for phosphorus. With serum PTH measurement (Elecsys 2010; Roche Diagnostics), the interassay CV was 7%.
Brain MRI
The ARIC Brain MRI ancillary study cerebral MRI scanning protocol has been described.4 At ARIC visit 3, WMH severity was qualitatively scored from barely detectable WM change (grade 1) to extensive confluent changes (grade 8). The absence of WMHs was scored grade 0, and WMHs with changes worse than grade 8 were scored as grade 9. This rating scale (0–9) was developed and validated in the Cardiovascular Health Study.1 For these analyses, scores of 3 or more were considered high because there were few participants at the very high end of the scale.
At the 2004–2006 second ARIC Brain MRI study visit, in addition to qualitatively scoring WMH by visual inspection into grades 0 to 9, a semiautomated quantitative volumetric analysis was performed.6 Because quantitative WMHs were not available for visit 3 scans, WMH volume scores were imputed for visit 3(using the qualitative scores from both brain scans and the quantitative volume from the second MRI), as previously described.6
At both ARIC Brain MRI study visits, the MRIs were also scored separately for subclinical infarcts by size and location. Because abnormalities smaller than 3 mm could not be reliably detected on MRI, infarcts considered in these analyses were lesions of 3 mm or more.19 Incident infarcts were defined as those seen on the second brain MRI among individuals with no infarcts on their first brain MRI.
Other Clinical Covariates
A physical examination including blood pressure, body mass index (BMI), and waist circumference was performed at visit 3 per standard ARIC protocol.17 Information on age, sex, race, educational level, alcohol use, smoking status, history of stroke, and medication use (including vitamin supplements) was assessed with standard ARIC questionnaires. Prevalence of hypertension (blood pressure ≥140/90 mmHg and/or antihypertensive medication use) and diabetes mellitus (fasting glucose level ≥126 mg/dL [to convert to millimoles per liter, multiply by 0.0555] and/or use of hypoglycemic medication) were determined at visit 3. Physical activity was assessed at visit 3 via the Baecke Physical Activity questionnaire,20 and a composite score (ranging from 1 to 5) was used to qualitatively assess leisure time intentional exercise through sports-related activities; scoring was based on frequency and intensity, with 5 being the highest score. The 10-year coronary heart disease risk and 10-year stroke risk using the respective Framingham equations were calculated for all participants.
Lipid levels were remeasured at ARIC visit 3. Comprehensive metabolic panel (for creatinine to estimate glomerular filtration rate [eGFR]) and high-sensitivity C-reactive protein were not measured at visit 3, so ARIC visit 2 data (1990–1992) were used for these laboratory variables. The eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.21
Statistical Analysis
Levels of 25(OH)D are known to vary by season.9 Therefore, we adjusted 25(OH)D for seasonal changes by computing the residuals from a linear regression model with 25(OH)D as the dependent variable and month of visit as the independent variable. The residuals were added back to the overall mean to determine an estimated annual 25(OH)D value. We performed this adjustment separately for whites and blacks because 25 (OH)D concentrations vary by race. This monthly adjusted vitamin D level was used in all analyses.
Clinical characteristics of the ARIC Brain MRI study population were tabulated across race-specific quartiles of 25 (OH)D. We tested for linear trend across quartiles of vitamin D separately by race using linear (continuous variables reporting means), quantile (continuous variables reporting medians), logistic (binary variables), and ordered logistic (categorical variables) regression.
Using multivariable linear regression, we determined the associations of 25(OH)D with WMH grade at visit 3 and change in WMH volume during follow-up. Logistic regression was used to determine the odds of having a prevalent high WHM score (≥3) at visit 3 and the risk of an incident high WMH score (≥3) on the second MRI for participants with baseline WMH scores lower than 3 by 25(OH)D quartiles. Logistic regression also was performed to test the association of 25(OH)D with the risk of having a prevalent infarct at visit 3 or an incident subclinical infarct at the second brain MRI visit.
The regression models described above compared risk across race-specific 25(OH)D quartiles. We also evaluated associations using 25(OH)D levels as a continuous variable compared per 10-ng/mL decrease in 25(OH)D). In addition, in supplemental analyses we evaluated all associations using clinical categories of 25(OH)D: 30 ng/mL or more as sufficient, 20 to less than 30 ng/mL as insufficient, and less than 20 ng/mL as deficient.
Our primary model was adjusted for demographic factors (age and sex) and behavioral and socioeconomic variables (educational level, income, physical activity, smoking, alcohol use, BMI, waist circumference, and vitamin D supplementation). Although vitamin D supplementation contributes to 25(OH)D levels, it is also a marker of health-seeking behavior and a potential confounder in observational studies and thus was included in the model. We evaluated 2 additional models: (1) adding potential mediators (diabetes, systolic and diastolic blood pressure, use of hypertension medication, total and high-density lipoprotein cholesterol, and eGFR) and (2) adding biomarkers related to vitamin D metabolism (calcium, phosphate, and PTH levels).
Analyses were performed using SAS, version 9.3 (SAS Institute Inc) and Stata/IC, version 12.1 (StataCorp). All P values are 2-sided, and significance was set at P < .05.
Results
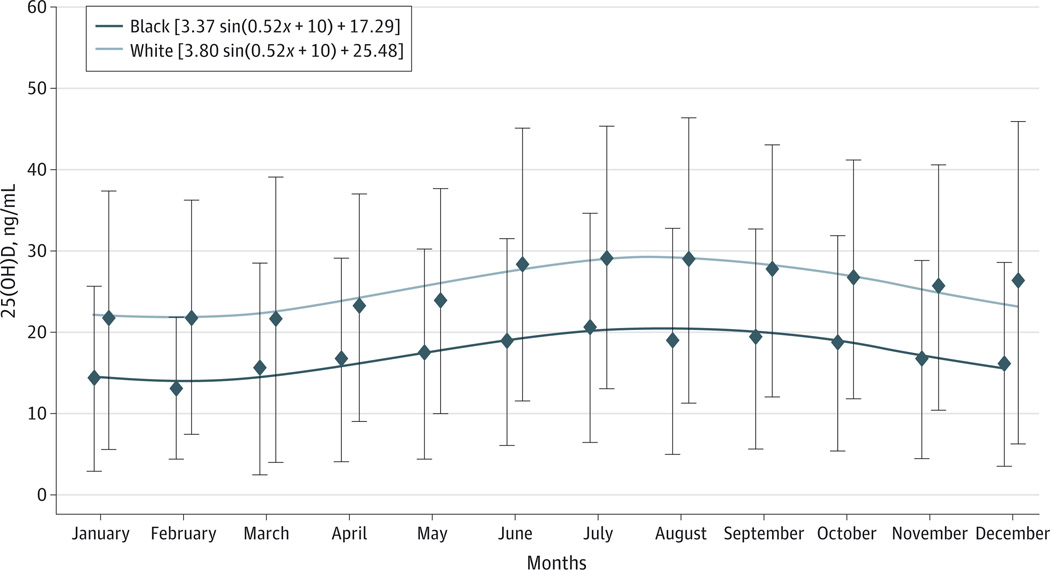
The unadjusted relationship of 25(OH)D levels by calendar month and race is shown in the Figure. As anticipated, blacks had lower levels of 25(OH)D than whites, and a sinusoidal pattern associated with seasonal change was demonstrated. Therefore, all subsequent analyses used monthly adjusted 25 (OH)D levels.
Figure. Mean 25-Hydroxyvitamin D (25[OH]D) Levels by Calendar Month and Race.
Unadjusted means (diamonds) and SDs (limit lines) of 25(OH)D levels by calendar month and race showing the sinusoidal relationship of 25(OH)D with calendar month. To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Clinical characteristics of study participants by race-specific quartiles of 25(OH)D are reported in Table 1. Women had lower 25(OH)D levels than men. Among whites in the highest 25(OH)D quartile compared with the lowest, mean values for BMI and waist circumference were lower and the prevalence of diabetes was lower. In both whites and blacks, PTH was lower and mean intentional physical activity score was higher across increasing quartiles of 25(OH)D. Surprisingly, eGFR was slightly higher among the lower quartiles of 25 (OH)Din unadjusted data in both whites and blacks, but eGFR was measured at ARIC visit 2 (1990–1992) and not concurrently with 25(OH)D measurement. In the ARIC visit 3 time period (1993–1994), there was a low prevalence of reported use of vitamin D supplements, but the percentage of supplement use was higher among blacks with higher vitamin D status. Across race-specific 25(OH)D quartiles, the median WMH score was 1 in all vitamin D categories, and there were no significant differences in the prevalence of high WMH scores (≥3) or subcortical infarcts.
Table 1.
Participant Characteristics at ARIC Visit 3 (1993–1994) by Race-Specific Quartiles of 25(OH)Da
| Characteristic | Whites | Blacks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D Quartileb |
P Value for Trend |
25(OH)D Quartilec |
P Value for Trend |
|||||||
| 1 (n = 209) |
2 (n = 209) |
3 (n = 204) |
4 (n = 211) |
1 (n = 196) | 2 (n = 199) | 3 (n = 199) | 4 (n = 195) | |||
| 25(OH)D, mean (SD), ng/mL | 16.2 (3.0) | 22.6 (1.4) | 27.5 (1.5) | 36.0 (5.5) | NP | 10.1 (2.0) | 14.6 (1.1) | 18.6 (1.4) | 26.0 (4.2) | NP |
| Age, mean (SD), y | 62.6 (4.3) | 63.3 (4.4) | 63.3 (4.4) | 63.0 (4.3) | .45 | 61.0 (4.4) | 61.4 (4.6) | 61.5 (4.6) | 62.4 (4.3) | .002 |
| Female sex, No. (%) | 143 (68.4) | 121 (57.9) | 101 (49.5) | 99 (46.9) | <.001 | 156 (79.6) | 131 (65.8) | 123 (61.8) | 93 (47.7) | <.001 |
| Study site, No. (%) | ||||||||||
| Jackson, Mississippi | 0 | 0 | 0 | 0 | .99 | 167 (85.2) | 170 (85.4) | 171 (85.9) | 180 (92.3) | .03 |
| Forsyth County, North Carolina | 209 (100.0) | 209 (100.0) | 204 (100.0) | 211 (100.0) | 29 (14.8) | 29 (14.6) | 28 (14.1) | 15 (7.7) | ||
| Educational level, No. (%) | ||||||||||
| <High school | 23 (11.0) | 31 (14.8) | 30 (14.7) | 31 (14.7) | .64 | 61 (31.1) | 73 (36.7) | 83 (41.7) | 90 (46.2) | .005 |
| High school or vocational school | 99 (47.4) | 83 (39.7) | 89 (43.6) | 91 (43.1) | 54 (27.6) | 53 (26.6) | 45 (22.6) | 42 (21.6) | ||
| College, graduate or professional school | 87 (41.6) | 95 (45.5) | 85 (41.7) | 89 (42.2) | 81 (41.3) | 73 (36.7) | 71 (35.7) | 63 (32.3) | ||
| BMI, mean (SD) | 26.8 (5.2) | 27.0 (4.8) | 26.5 (4.1) | 25.7 (4.2) | .01 | 29.8 (5.1) | 29.7 (5.7) | 29.6 (5.3) | 28.9 (4.9) | .06 |
| Waist circumference, mean (SD), cm | 97.9 (14.1) | 98.0 (13.0) | 97.6 (11.7) | 94.5 (13.0) | .007 | 102.2 (13.6) | 101.6 (13.8) | 101.3 (13.4) | 100.2 (13.4) | .15 |
| Diabetes mellitus, No. (%) | 31 (14.8) | 25 (12.0) | 18 (8.8) | 16 (7.6) | .01 | 49 (25.0) | 52 (26.1) | 48 (24.4) | 40 (20.5) | .23 |
| Hypertension, No. (%) | 66 (31.6) | 74 (35.6) | 66 (32.5) | 66 (31.3) | .78 | 126 (65.3) | 128 (65.0) | 122 (61.9) | 117 (60.0) | .22 |
| Smoking status, No. (%) | ||||||||||
| Never | 85 (40.7) | 85 (40.7) | 93 (45.6) | 72 (34.1) | .66 | 107 (54.6) | 95 (47.7) | 103 (51.8) | 88 (45.1) | .26 |
| Former | 69 (33.0) | 84 (40.2) | 90 (44.1) | 104 (49.3) | 49 (25.0) | 72 (36.2) | 67 (33.7) | 70 (35.9) | ||
| Current | 55 (26.3) | 40 (19.1) | 21 (10.3) | 35 (16.6) | 40 (20.4) | 32 (16.1) | 29 (14.6) | 37 (19.0) | ||
| Alcohol use, No. (%) | ||||||||||
| Never | 86 (41.2) | 69 (33.0) | 62 (30.4) | 68 (32.2) | .15 | 89 (45.4) | 89 (44.7) | 86 (43.2) | 77 (39.5) | .48 |
| Former | 27 (12.9) | 44 (32.2) | 45 (22.1) | 28 (18.0) | 53 (27.0) | 53 (26.6) | 57 (28.6) | 66 (33.9) | ||
| Current | 96 (45.9) | 96 (45.9) | 97 (47.6) | 105 (49.8) | 54 (27.6) | 57 (28.6) | 56 (28.1) | 52 (26.7) | ||
| Creatinine, mean (SD), mg/dLd | 1.08 (0.18) | 1.11 (0.20) | 1.13 (0.18) | 1.18 (0.19) | <.001 | 1.14 (0.38) | 1.19 (0.40) | 1.16 (0.21) | 1.24 (0.23) | .005 |
| eGFR, mean (SD), mL/mind | 87.6 (12.6) | 86.2 (13.5) | 86.7 (12.4) | 83.4 (13.5) | .002 | 95.8 (17.9) | 94.5 (17.9) | 96.0 (17.0) | 90.5 (16.8) | .005 |
| Total cholesterol level, mean (SD), mg/dL | 209.4 (38.2) | 207.0 (34.7) | 209.6 (32.5) | 210.6 (37.3) | .56 | 207.2 (38.4) | 209.5 (39.2) | 211.6 (42.9) | 206.2 (41.2) | .81 |
| Triglycerides level, mean (SD), mg/dL | 156.8 (86.0) | 152.1 (94.6) | 155.9 (119.5) | 146.5 (100.2) | .36 | 115.9 (63.7) | 122.6 (66.0) | 109.9 (62.9) | 116.1 (64.9) | .61 |
| LDL-C level, mean (SD), mg/dL | 125.7 (33.1) | 123.6 (33.8) | 127.5 (28.9) | 125.1 (33.3) | .91 | 126.9 (35.4) | 131.9 (37.3) | 131.9 (39.2) | 127.0 (39.2) | .87 |
| HDL-C level, mean (SD), mg/dL | 52.0 (19.2) | 53.1 (19.9) | 52.0 (18.5) | 56.0 (21.9) | .06 | 57.1 (23.2) | 53.0 (16.4) | 58.2 (17.1) | 56.1 (20.3) | .76 |
| 10-y Coronary heart disease risk, mean (SD), % | 7.9 (8.2) | 8.3 (8.7) | 8.4 (9.2) | 8.4 (8.1) | .56 | 7.5 (8.7) | 7.8 (8.0) | 7.1 (6.6) | 8.0 (6.4) | .67 |
| 10-y Incident stroke risk, mean (SD), % | 4.1 (7.1) | 3.8 (5.3) | 3.7 (5.0) | 3.5 (3.7) | .27 | 8.1 (12.6) | 7.1 (7.7) | 6.8 (7.9) | 7.1 (7.2) | .31 |
| Physical Activity Index (range 0–5), mean (SD) | 2.3 (0.7) | 2.6 (0.7) | 2.7 (0.8) | 3.0 (0.9) | <.001 | 2.3 (0.7) | 2.3 (0.7) | 2.4 (0.7) | 2.5 (0.8) | .001 |
| PTH, mean (SD), pg/mL | 49.1 (46.5) | 40.4 (12.8) | 40.7 (14.9) | 36.3 (13.1) | <.001 | 57.1 (23.6) | 53.5 (30.6) | 46.1 (17.7) | 42.0 (15.3) | <.001 |
| Calcium, mean (SD), mg/dL | 9.4 (0.4) | 9.4 (0.4) | 9.4 (0.3) | 9.4 (0.4) | .84 | 9.5 (0.5) | 9.6 (0.4) | 9.6 (0.4) | 9.6 (0.4) | .14 |
| Phosphate, mean (SD), mg/dL | 3.5 (0.6) | 3.4 (0.5) | 3.4 (0.5) | 3.3 (0.5) | .009 | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.4) | 3.5 (0.5) | .18 |
| High-sensitivity C-reactive protein, median (IQR), mg/L | 2.4 (1.0–5.3) | 1.9 (1.0–4.9) | 2.4 (1.2–6.2) | 2.1 (0.9–4.7) | .70 | 3.6 (1.5–7.1) | 3.0 (1.4–7.0) | 3.1 (1.2–6.5) | 3.3 (1.2–7.0) | .74 |
| Vitamin D supplement use, No. (%) | 4 (1.9) | 5 (1.9) | 3 (1.5) | 10 (4.7) | .08 | 1 (0.5) | 0 (0.0) | 0 (0.0) | 6 (3.1) | .02 |
| White matter score (range 0–9), median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | .99 | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | .99 |
| White matter score ≥3, No. (%) | 22 (10.5) | 21 (10.1) | 22 (10.8) | 23 (10.9) | .85 | 25 (12.8) | 23 (11.6) | 22 (11.1) | 28 (14.4) | .56 |
| White matter progression, mean (SD), cm3 | 3.8 (8.7) | 3.5 (6.7) | 3.9 (8.8) | 3.5 (7.2) | .90 | 5.9 (9.1) | 7.4 (8.3) | 7.2 (9.4) | 8.2 (11.7) | .12 |
| Prevalent subclinical infarcts ≥3 mm, No. (%) | 17 (8.1) | 18 (8.6) | 21 (10.3) | 16 (7.6) | .94 | 31 (15.8) | 27 (13.6) | 19 (9.6) | 30 (15.4) | .80 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; NP, not performed; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone.
SI conversion factors: To convert C-reactive protein to nanomoles per liter, multiply by 9.524; calcium to millimoles per liter, 0.25; creatinine to micromoles per liter, 88.4; HDL-C to millimoles per liter, 0.0259; LDL-C to millimoles per liter, 0.0259; 25(OH)D to nanomoles per liter, 2.496; PTH to nanograms per liter, 1; total cholesterol to millimoles per liter, 0.0259; and triglycerides to millimoles per liter, 0.0113.
Of the total population (N = 1622), the following variables had missing data: diabetes mellitus (n = 2), hypertension (n = 9), creatinine (n = 19), eGFR (n = 19), total cholesterol (n = 1), triglycerides (n = 1), LDL-C (n = 23), HDL-C (n = 1), 10-year coronary heart disease risk (n = 135), 10-year stroke risk (n = 95), high-sensitivity C-reactive protein (n = 202), and white matter progression (n = 734).
Quartiles of 25(OH)D for whites were as follows: 1, less than 20.0 ng/mL; 2, 20.0 to less than 24.9 ng/mL; 3, 24.9 to less than 30.2 ng/mL; and 4, 30.2 ng/mL or more.
Quartiles of 25(OH)D for blacks were as follows: 1, less than 12.7 ng/mL; 2, 12.7 to less than 16.4 ng/mL; 3, 16.4 to less than 21.1 ng/mL; and 4, 21.1 ng/mL or more.
Measured at ARIC visit 2 (1990–1992).
Characteristics of the participants stratified by race and clinical vitamin D categories (≥30 ng/mL, 20 to <30 ng/mL, and <20 ng/mL) of 25(OH)D are listed in the Supplement (eTable 1).
In adjusted cross-sectional regression analyses using ARIC visit 3 data, we did not find any associations between 25 (OH)D and WMH score (Table 2), prevalence of high WMH score (≥3) (Table 3), or prevalent subclinical infarcts of 3 mm or more (Table 4) for the overall group, whites only, or blacks only (all P > .05). Compared with those in the highest quartile, participants in the lowest quartile had WMH β coefficient scores that were 0.02 (95% CI, −0.17 to 0.21) higher for whites and 0.08 (−0.15 to 0.32) higher for blacks. Per 10-ng/mL decrease in 25 (OH)D levels, the odds ratios of having a prevalent infarct seen on MRI was 1.25 (95% CI, 0.88 to 1.76) among whites and 1.09 (0.77 to 1.55) in blacks. Similar results were found when the analyses were repeated using clinical categories of 25(OH)D (Supplement [eTables 2–4]).
Table 2.
Adjusted Associations of 25(OH)D With Prevalent WMH Score and Prospective WMH Volume Changea
| Characteristic | Race-Specific 25(OH)D Quartileb | Per 10-ng/mL Decrease in 25(OH)D |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Prevalent WMH score (qualitative score, 0–9) | |||||
| Overall No. | 405 | 408 | 403 | 406 | 1622 |
| β Coefficient (95% CI) | 0.02 (−0.13 to 0.17) | −0.02 (−0.17 to 0.12) | −0.03 (−0.17 to 0.12) | 0 [Reference] | 0.01 (−0.07 to 0.08) |
| Whites, No. | 209 | 209 | 204 | 211 | 833 |
| β Coefficient (95% CI) | 0.02 (−0.17 to 0.21) | 0.00 (−0.19 to 0.18) | 0.00 (−0.18 to 0.19) | 0 [Reference] | 0.04 (−0.05 to 0.12) |
| Blacks, No. | 196 | 199 | 199 | 195 | 789 |
| β Coefficient (95% CI) | 0.08 (−0.15 to 0.32) | 0.00 (−0.23 to 0.23) | −0.02 (−0.25 to 0.21) | 0 [Reference] | −0.01 (−0.14 to 0.12) |
| White matter hyperintensity volume change, cm3 | |||||
| Overall, No. | 201 | 233 | 219 | 235 | 888 |
| β Coefficient (95% CI) | −0.51 (−2.21 to 1.19) | −0.48 (−2.07 to 1.10) | −0.30 (−1.89 to 1.29) | 0 [Reference] | −0.19 (−1.01 to 0.63) |
| Whites, No. | 101 | 118 | 104 | 122 | 445 |
| β Coefficient (95% CI) | 0.20 (−1.94 to 2.35) | −0.53 (−2.54 to 1.47) | 0.26 (−1.76 to 2.27) | 0 [Reference] | 0.12 (−0.80 to 1.05) |
| Blacks, No. | 100 | 115 | 115 | 113 | 443 |
| β Coefficient (95% CI) | −0.65 (−3.34 to 2.04) | 0.08 (−2.43 to 2.58) | −0.45 (−2.94 to 2.03) | 0 [Reference] | −0.38 (−1.89 to 1.12) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; 25(OH)D, 25-hydroxyvitamin D; WMH, white matter hyperintensity.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Prevalent WMH data are from ARIC visit 3 (1993–1994), and prospective WMH volume change data are from ARIC visit 3 to ARIC Brain MRI visit (2004–2006). Model was adjusted for demographic variables (age, sex, and race in overall model) and behavioral and socioeconomic variables (educational level, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation use). Results presented are mean differences in outcome for each quartile compared with the reference quartile or mean change in outcome for a 10-unit decrease in 25(OH)D.
Quartiles are presented for white vs black participants and are described in the footnotes to Table 1.
Table 3.
Adjusted Associations of 25(OH)D With Prevalent High WMH score (≥3) and Risk of Incident High WMH Score Over Follow-upa
| Characteristic | Race-Specific 25(OH)D Quartileb | Per 10-ng/mL Decrease in 25(OH)D |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Prevalent high WMH score (qualitative score ≥3) | |||||
| Overall, No. (No. of cases) | 405 (47) | 408 (44) | 403 (44) | 406 (51) | 1622 (186) |
| OR (95% CI) | 0.95 (0.60–1.50) | 0.83 (0.53–1.30) | 0.86 (0.55–1.35) | 1 [Reference] | 0.92 (0.73–1.17) |
| Whites, No. (No. of cases) | 209 (22) | 209 (21) | 204 (22) | 211 (23) | 833 (88) |
| OR (95% CI) | 0.87 (0.44–1.71) | 0.79 (0.41–1.51) | 0.88 (0.46–1.68) | 1 [Reference] | 0.97 (0.70–1.33) |
| Blacks, No. (No. of cases) | 196 (25) | 199 (23) | 199 (22) | 195 (28) | 789 (98) |
| OR (95% CI) | 1.04 (0.55–1.94) | 0.87 (0.46–1.63) | 0.83 (0.44–1.55) | 1 [Reference] | 0.88 (0.62–1.25) |
| Progression to high WMH score (≥3)c | |||||
| Overall, No. (No. of cases) | 180 (38) | 219 (52) | 202 (59) | 218 (63) | 819 (212) |
| OR (95% CI) | 0.84 (0.50–1.39) | 0.83 (0.53–1.31) | 1.07 (0.68–1.68) | 1 [Reference] | 0.87 (0.68–1.11) |
| Whites, No. (No. of cases) | 92 (18) | 112 (21) | 97 (26) | 112 (27) | 413 (92) |
| OR (95% CI) | 0.93 (0.44–1.96) | 0.71 (0.36–1.40) | 1.11 (0.58–2.15) | 1 [Reference] | 0.93 (0.67–1.29) |
| Blacks, No. (No. of cases) | 88 (20) | 107 (31) | 105 (33) | 106 (36) | 406 (120) |
| OR (95% CI) | 0.83 (0.40–1.69) | 1.06 (0.56–2.01) | 1.10 (0.58–2.06) | 1 [Reference] | 0.82 (0.56–1.21) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio; WMH, white matter hyperintensity.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Prevalent high WMH score data are from ARIC visit 3 (1993–1994), and incident high WMH score data are from ARIC visit 3 to ARIC Brain MRI visit (2004–2006). Model was adjusted for demographic variables (age, sex, and race in overall model) and behavioral and socioeconomic variables (educational level, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation use).
Quartiles are presented for white vs black participants and are described in the footnotes to Table 1.
Participants with a WMH score of 3 or more at ARIC visit 3 were excluded.
Table 4.
Adjusted Associations of 25(OH)D With Prevalent Infarcts and Risk of Incident Infarcts Over Follow-upa
| Characteristic | Race-Specific 25(OH)D Quartileb | Per 10-ng/mL Decrease in 25(OH)D |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Prevalent infarcts, ≥3 mm | |||||
| Overall, No. (No. of cases) | 405 (48) | 408 (45) | 403 (40) | 406 (46) | 1622 (179) |
| OR (95% CI) | 1.25 (0.79–2.00) | 1.09 (0.69–1.73) | 0.98 (0.62–1.57) | 1 [Reference] | 1.13 (0.89–1.44) |
| Whites, No. (No. of cases) | 209 (17) | 209 (18) | 204 (21) | 211 (16) | 833 (72) |
| OR (95% CI) | 1.46 (0.67–3.18) | 1.38 (0.66–2.89) | 1.64 (0.81–3.35) | 1 [Reference] | 1.25 (0.88–1.76) |
| Blacks, No. (No. of cases) | 196 (31) | 199 (27) | 199 (19) | 195 (30) | 789 (107) |
| OR (95% CI) | 1.19 (0.66–2.15) | 0.96 (0.53–1.76) | 0.66 (0.35–1.26) | 1 [Reference] | 1.09 (0.77–1.55) |
| Incident infarcts (≥3 mm)c | |||||
| Overall, No. (No. of cases) | 186 (38) | 225 (50) | 203 (33) | 226 (44) | 840 (165) |
| OR (95% CI) | 1.04 (0.61–1.75) | 1.14 (0.72–1.83) | 0.76 (0.46–1.26) | 1 [Reference] | 1.07 (0.83–1.40) |
| Whites, No. (No. of cases) | 96 (20) | 114 (25) | 96 (12) | 119 (22) | 425 (79) |
| OR (95% CI) | 1.23 (0.59–2.59) | 1.34 (0.68–2.66) | 0.64 (0.30–1.40) | 1 [Reference] | 1.19 (0.85–1.68) |
| Blacks, No. (No. of cases) | 90 (18) | 111 (25) | 107 (21) | 107 (22) | 415 (86) |
| OR (95% CI) | 0.94 (0.44–2.03) | 1.08 (0.54–2.15) | 0.87 (0.43–1.77) | 1 [Reference] | 0.94 (0.62–1.44) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Prevalent infarcts data are from ARIC visit 3 (1993–1994), and incident infarcts data are from ARIC visit 3 to ARIC Brain MRI visit (2004–2006). Model was adjusted for demographics variables (age, sex, and race in overall model) and behavioral and socioeconomic variables (educational level, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation use).
Quartiles are presented for white vs black participants and are described in the footnotes to Table 1.
Participants with prevalent infarcts of 3 mm or more at ARIC visit 3 were excluded.
In adjusted prospective regression analyses, we also did not find any associations of 25(OH)D measured at ARIC visit 3 with changes in WMH volume (Table 2), risk of incident high WMH score 3 or more (Table 3), and risk of incident subclinical brain infarcts (Table 4) between visit 3 and the second brain MRI visit approximately 10 years later (all P > .05). Compared with those in the highest vitamin D quartile, participants in the lowest quartile had nominally more WMH volume progression among whites (β, 0.20 cm3; 95%CI, −1.94 to 2.35) and had less WMH progression among blacks (−0.65; −3.34 to 2.04). We found largely similar results when the analyses were repeated using clinical categories of 25(OH)D (Supplement [eTables 2–4]). In all analyses, additional adjustment for potential mediators and biomarkers related to vitamin D metabolism did not appreciably alter the results (data not shown).
Discussion
In contrast to our hypotheses, we did not find any association of vitamin D with subclinical cerebrovascular abnormalities in any model, including those adjusted and not adjusted for blood pressure and use of hypertensive medications, among predominantly middle-aged white and black adults.
Little is known about the association of vitamin D with cerebrovascular abnormalities. A review22 of the literature on the topic of brain imaging and vitamin D identified only one study15 that evaluated the specific issue. Buell et al15 evaluated a cohort of 318 elderly individuals (23% with dementia) receiving home care services. The authors found a higher prevalence of dementia among participants with 25(OH)D deficiency. Compared with higher vitamin D levels, 25(OH)D levels of 20 ng/mL or less were associated with a higher WMH volume (4.9 vs 2.9 mL; P < .01), higher WHM grade score (3.0 vs 2.2; P = .04), and higher prevalence of large-vessel infarcts (10.1% vs 6.9%; P < .01). Notably, the findings were cross-sectional and were not adjusted for CVD risk factors or other confounding lifestyle factors. In addition, the low vitamin D levels may be a consequence of limited sun exposure and reduced mobility among elderly adults with a high prevalence of dementia.16
To our knowledge, we present for the first time the relationship between 25(OH)D levels and cerebrovascular abnormalities seen on brain MRI among a community-dwelling population free of previous clinical stroke. However, unlike the study of elderly home-care recipients by Buell et al,15 we did not find any cross-sectional association between 25(OH)D and WMH grade or prevalent infarcts after adjustment for age, sex, and race, nor did we identify any association after more extensive adjustment for lifestyle factors, socioeconomic status, established CVD risk factors, or related mineral metabolism biomarkers. Furthermore, we present for the first time the results of the association of 25(OH)D with changes in WMH volume and incident infarcts between 2 brain scans acquired over an approximate 10-year interval. No statistically significant associations were seen regardless of whether 25(OH)D was modeled using race-specific vitamin D quartiles or clinical cut points of 25(OH)D.
Similar to the Cardiovascular Health Study,23 we noted cyclical changes in 25(OH)D levels by month across the population attributed to season. The amplitude of the sine wave for these ARIC Brain MRI participants was somewhat dampened compared with the amplitude in the Cardiovascular Health Study cohort, which is likely because these ARIC Brain MRI participants were recruited from only 2 locations (Forsyth County, North Carolina, and Jackson, Mississippi), both of relatively southern latitude, which is likely less influenced by seasonal variation compared with northern sites. Using monthly adjusted vitamin D levels provides an estimate of mean 25(OH)D level across a 12-month period. However, no significant associations were seen with cerebrovascular disease when the absolute (unadjusted) 25(OH)D value was used instead.
Our study is limited by 25(OH)D levels being measured at only one point in time, but physicians typically recommend therapy based on a single blood level in clinical practice. Although we accounted for annual seasonal variation, a single measurement may not capture lifetime patterns of vitamin D. Also, our sample size might not have been large enough to identify a moderate association between vitamin D and subclinical brain disease. Use of vitamin D supplements was low in this population; therefore, results were unable to be stratified by supplement users. Our study was observational and did not test the effect of vitamin D therapy, although vitamin D supplements contribute to total 25(OH)D levels.
This study also has a number of important strengths. The biethnic ARIC cohort of generally middle-aged to older community-dwelling adults has been well characterized. We had comprehensive measurement of confounders. Additionally, our prospective study design, as well as the measurement of 25(OH)D from blood samples collected in late-middle age among a population of individuals with no history of stroke at baseline, help to avoid the possibility of reverse causation.
Conclusions
A single measure of 25(OH)D was not cross-sectionally associated with WMH grade or prevalent subclinical infarcts and was not prospectively associated with WMH progression or subclinical brain infarcts seen on serial cerebral MRIs obtained approximately 10 years apart. Although the results of our study were largely null, they provide an important contrast to the cross-sectional study by Buell et al15 and the observational studies13 of clinical stroke events. Our findings may dampen the enthusiasm for supplementing vitamin D for the purposes of promoting brain health.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke grant R01NS072243. Dr Schneider was supported by NIH/National Heart, Lung, and Blood Institute (NHLBI) training grant T32HL007024. The Atherosclerosis Risk in Communities (ARIC) study was carried out as a collaborative study supported by NHLBI contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The ARIC Brain MRI study was supported by NIH/NHLBI grant R01-HL70825.
Role of the Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Michos had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Michos, Carson, Schneider, Sharrett, Coker, Gross.
Acquisition, analysis, or interpretation of data: Michos, Carson, Schneider, Lutsey, Xing, Alonso, Coker, Gross, Post, Mosley, Gottesman.
Drafting of the manuscript: Michos.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Carson, Schneider, Xing, Gross.
Obtained funding: Michos, Coker, Mosley.
Administrative, technical, or material support: Coker, Gross.
Study supervision: Michos, Post.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the staff and participants of the ARIC Brain MRI study for their important contributions.
REFERENCES
- 1.Manolio TA, Kronmal RA, Burke GL, et al. Cardiovascular Health Study Collaborative Research Group. Magnetic resonance abnormalities and cardiovascular disease in older adults: the Cardiovascular Health Study. Stroke. 1994;25(2):318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 2.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 3.Silbert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: white matter hyperintensity progression matters. Neurology. 2009;73(2):120–125. doi: 10.1212/WNL.0b013e3181ad53fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64(12):2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 5.Windham BG, Griswold ME, Shibata D, Penman A, Catellier DJ, Mosley TH., Jr Covert neurological symptoms associated with silent infarcts from midlife to older age: the Atherosclerosis Risk in Communities study. Stroke. 2012;43(5):1218–1223. doi: 10.1161/STROKEAHA.111.643379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41(1):3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 9.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11(1):7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annweiler C, Allali G, Allain P, et al. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol. 2009;16(10):1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 12.Buell JS, Dawson-Hughes BVitamin D. and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29(6):415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-Hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol. 2013;73(1):38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 14.Michos ED, Gottesman RF. Vitamin D for the prevention of stroke incidence and disability: promising but too early for prime time. Eur J Neurol. 2013;20(1):3–4. doi: 10.1111/j.1468-1331.2012.03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buell JS, Dawson-Hughes B, Scott TM, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilz S, Dobnig H, Tomaschitz A, et al. Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J Clin Endocrinol Metab. 2012;97(4):653–657. doi: 10.1210/jc.2011-3043. [DOI] [PubMed] [Google Scholar]
- 17.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 18.Hollis BW. Measuring 25-hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutr. 2008;88(2):507S–510S. doi: 10.1093/ajcn/88.2.507S. [DOI] [PubMed] [Google Scholar]
- 19.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33(10):2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Pugliese G, Solini A, Bonora E, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) study formula in subjects with type 2 diabetes. Atherosclerosis. 2011;218(1):194–199. doi: 10.1016/j.atherosclerosis.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Annweiler C, Montero-Odasso M, Muir SW, Beauchet O WALK Team. Vitamin D and brain imaging in the elderly: should we expect some lesions specifically related to hypovitaminosis D? Open Neuroimag J. 2012;6:16–18. doi: 10.2174/1874440001206010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoben AB, Kestenbaum B, Levin G, et al. Seasonal variation in 25-hydroxyvitamin D concentrations in the Cardiovascular Health Study. Am J Epidemiol. 2011;174(12):1363–1372. doi: 10.1093/aje/kwr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.