Abstract
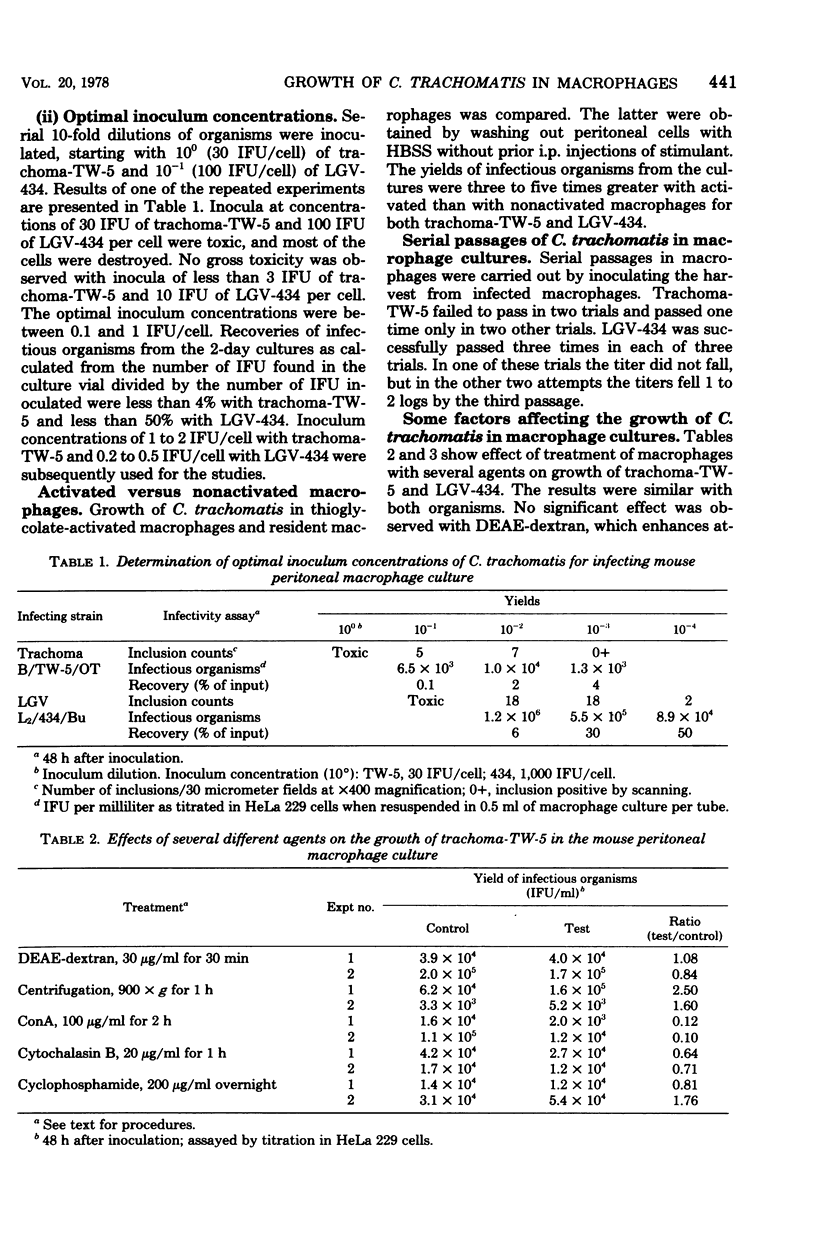
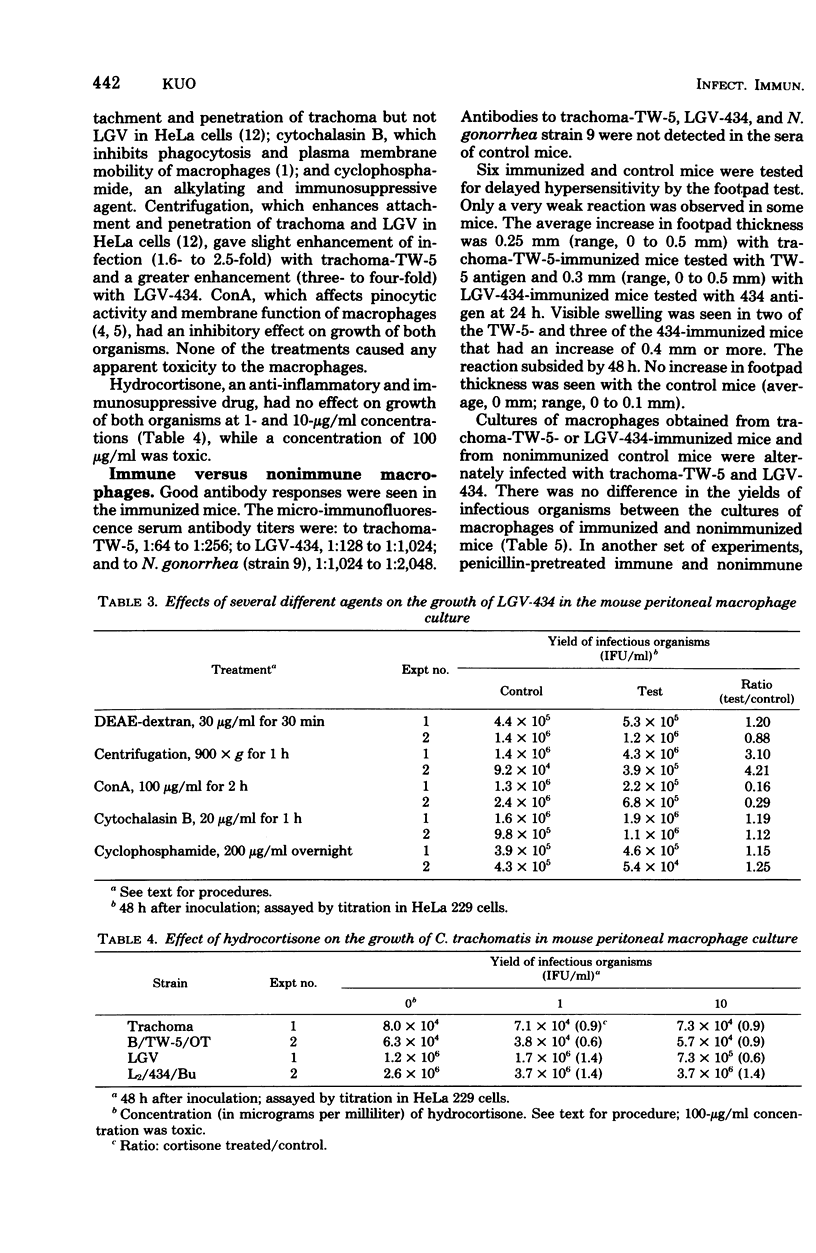
Growth of Chlamydia trachomatis B/TW-5/OT and L2/434/Bu strains in cultures of thioglycolate-activated mouse peritoneal macrophages was studied. Both strains grew to a limited extent in the macrophages, but lymphogranuloma venereum (LGV) grew better than trachoma. Growth was enhanced by centrifugation of the inoculum onto the macrophage cell layer and inhibited by pretreatment of macrophages for 2 h with 100 μg of concanavalin A per ml. No significant effect was observed by pretreatment of macrophages with diethylaminoethyl-dextran (30 μg/ml, 30 min), cytochalasin B (20 μg/ml, 1 h), and cyclophosphamide (200 μg/ml, overnight) or by treatment with hydrocortisone (1 and 10 μg/ml, overnight before inoculation and during a 2-day incubation after inoculation). Resistance to intracellular growth of the two organisms was not increased in macrophages obtained from mice immunized with the organisms compared with macrophages from nonimmunized mice unless they were pretreated in vitro with penicillin (100 U/ml, overnight). The yields of LGV organisms from the penicillin-pretreated macrophages of LGV-immunized mice were 100-fold less than from the pretreated macrophages of nonimmunized control mice. At the same time, the yields of LGV organisms from penicillin-pretreated macrophages of mice immunized with trachoma, gonococcus, and HeLa cells were not different from those obtained in pretreated macrophages of nonimmunized control mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENEDICT A. A., McFARLAND C. Growth of meningopneumonitis virus in normal and immune guinea pig monocytes. Nature. 1958 Jun 21;181(4625):1742–1743. doi: 10.1038/1811742a0. [DOI] [PubMed] [Google Scholar]
- Casazza A. M., Intini C., Verini M. A., Ghione M. Preliminary studies on mouse macrophage cultures infected with trachoma agents. Experientia. 1969 Mar 15;25(3):327–329. doi: 10.1007/BF02034425. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D. F., JENNINGS P. A. Allergy in experimental mouse tuberculosis. Am Rev Tuberc. 1955 Aug;72(2):171–195. doi: 10.1164/artpd.1955.72.2.171. [DOI] [PubMed] [Google Scholar]
- Ginsberg A. H., Monte W. T., Johnson K. P. Effect of cyclophosphamide in vitro and on vaccinia virus replication in tissue culture. J Virol. 1977 Jan;21(1):277–283. doi: 10.1128/jvi.21.1.277-283.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Preservation of structural integrity of liver lysosomes and membrane-stabilizing action of anti-inflammatory drugs, catecholamines and cyclic adenosine monophosphate in isotonic salt media. Biochem Pharmacol. 1973 Jun 1;22(11):1269–1282. doi: 10.1016/0006-2952(73)90301-8. [DOI] [PubMed] [Google Scholar]
- Kordová N., Hoogstraten J., Wilt J. C. Lysosomes and the "toxicity" of Rickettsiales. IV. Ultrastructural studies of macrophages infected with a cytopathic L cell-grown C. psittaci 6BC strain. Can J Microbiol. 1973 Mar;19(3):315–320. doi: 10.1139/m73-052. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Stewart C. C. Colony formation by mouse peritoneal exudate cells in vitro. Nat New Biol. 1973 Jun 6;243(127):176–177. doi: 10.1038/newbio243176a0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBY H. L., THOMPSON G. A., COUSINEAU G. G., LLOYD L. A., HASSARD J. Topical therapy in inclusion conjunctivitis. Am J Ophthalmol. 1952 Dec;35(12):1811–1814. doi: 10.1016/0002-9394(52)92022-9. [DOI] [PubMed] [Google Scholar]
- Orfila J., Lepinay A. Etude du cycle de développement de l'agent de l'ornithose dans les macrophages de souris. Ann Inst Pasteur (Paris) 1967 Jul;113(1):19–27. [PubMed] [Google Scholar]
- Raz A., Goldman R. The in vitro effect of hydrocortisone on endocytosis in cultured mouse peritoneal macrophages. J Reticuloendothel Soc. 1976 Sep;20(3):177–186. [PubMed] [Google Scholar]
- Sladek N. E. Therapeutic efficacy of cyclophosphamide as a function of its metabolism. Cancer Res. 1972 Mar;32(3):535–542. [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]


