Abstract
Purpose:
To describe the technique of cryopreservation of corneal lenticules extracted after small incision refractive lenticule extraction (ReLEx SMILE) and initial results of femtosecond laser intrastromal lenticular implantation for hyperopia.
Methods:
Lenticules were collected from patients undergoing ReLEx SMILE for the correction of myopia and subjected to a tissue processing technique and cryopreservation. These lenticules were subsequently used to treat 8 hyperopic eyes and 1 aphakic eye. A femtosecond laser was used to create a pocket into each patient's cornea, followed by implantation of a cryopreserved lenticule. The patients were monitored through follow-up examinations for a mean 155.4 days (38–310 days).
Results:
The mean interval from storage of lenticules to removal from liquid nitrogen was 96 days (range, 19–178 days). Mean spherical equivalent of hyperopic eyes treated was +4.50 ± 1.1 diopter (D). Mean keratometry and pachymetry changed from preoperative 43.9 D and 531.6 μm to 47.4 D and 605.2 μm, respectively, postoperatively. Mean residual spherical equivalent for hyperopic eyes was +0.6 D and +4.1 D for the aphakic eye. None of the eyes showed evidence of rejection or loss of best-corrected visual acuity at the end of the follow-up period.
Conclusions:
The cryopreservation technique seems to be a safe method of long-term storage of refractive lenticules extracted after ReLEx SMILE for use in allogeneic human subjects. It may potentially be a safe and effective alternative to excimer laser ablation for hyperopia because of the low risks of regression, haze, flap-related complications, postoperative dry eye, and higher-order aberrations.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: CTRI/2014/01/004331.
Key Words: cryopreservation, refractive lenticule extraction, ReLEx SMILE, femtosecond laser intrastromal lenticule implantation
Small incision refractive lenticule extraction (ReLEx SMILE) is a flapless, minimally invasive, first all-in-one femtosecond (FS) laser refractive procedure that treats myopia by creating a refractive intrastromal corneal lenticule using the VisuMax FS laser system (Carl Zeiss Meditec, Jena, Germany), instead of ablation with an excimer laser. Studies suggest that this procedure has better biomechanical stability compared with laser in situ keratomileusis (LASIK) and has other advantages, such as no risk of flap-related complications, fewer aberrations, less postoperative dry eye, faster healing, and better patient comfort.1,2
Another potential advantage of the ReLEx SMILE technique is that it could be a reversible refractive procedure. The removal of the intrastromal lenticule allows the possibility of reimplantation at a future date.3 The implantation of a lenticule in humans was first reported by Pradhan et al,4 who implanted an allogeneic lenticule obtained by SMILE from a myopic donor for correction of high hypermetropia in a young individual. It may also be possible to implant the extracted lenticule into an unrelated individual to treat conditions like aphakia, hypermetropia, keratoconus, and presbyopia.4 To achieve this, the integrity of the extracted stromal lenticule must be maintained after long-term storage. There is enough evidence to suggest the use of cryopreserved corneal tissue for transplantation.5 However, the process of freezing and thawing can damage the corneal endothelium and stroma,6–8 of which only the latter is relevant in ReLEx. Previous studies have confirmed the viability of keratocytes postcryopreservation in primary cultures.9 In a pioneer study by the Tissue Engineering and Stem Cell Group at Singapore Eye Research Institute on human cadaveric eyes, the viability of corneal lenticules extracted following the ReLEx procedure and 1 month after storage was evaluated using a developed cryopreservation technique.10 The results showed a well-preserved and well-aligned collagen structure in the lenticules after cryopreservation, which suggested that the tissue processing technique used was reliable in maintaining the collagen structure of the lenticule. Reimplanting the cryopreserved corneal lenticules at a later date made restoration of the cornea volume possible after the ReLEx FLEX procedure.
In the present study, lenticules from myopic patients undergoing ReLEx SMILE at our center were cryopreserved for use in heterologous individuals to treat hypermetropia using a tissue addition technique. To address the safety issues, the tissue processing technique described by Oh et al8 was modified for suitability in human use.
MATERIALS AND METHODS
The study was approved by the institutional ethics committee, and informed consent was obtained from all patients in accordance with the tenets of the Declaration of Helsinki. All donors were healthy individuals between 21 and 40 years old. Serological tests were conducted in all donors to rule out transmissible viral infections. The donors were selected based on these inclusion criteria: myopic spherical refractive error ranging from −0.5 D to −10.00 D with no astigmatism or astigmatism less than −0.5 D; healthy eyes free from significant systemic and ocular diseases; and ability to understand the content of the consent form and willingness to undergo serological testing.
Eligible candidates underwent the ReLEx SMILE procedure with the standard technique11 by the same surgeon (S.G.) using the VisuMax FS laser. The cap thickness was 100 μm, and the optical zone varied from 6 to 6.5 mm. After dissection of both anterior and posterior planes, the lenticule was extracted through a superior 2-mm incision. A 10-0 nylon suture was applied following lenticule removal. Bites were taken from one end to the diagonally opposite end, with the knot tied on the superior aspect of the lenticule to determine the side at the time of subsequent implantation. No correction for toricity was conducted. The lenticule was immediately transferred to a sterile plastic vial containing phosphate-buffered solution, labeled with the patient's identification number, eye, and date, and sent to the cryobank for processing and cryopreservation.
Tissue Processing and Cryopreservation
Two types of solutions (washing solution and cryopreservation solution) were prepared. The washing solution comprised 10 mL of phosphate-buffered saline (1×), 200 μL of penicillin–streptomycin (100×), and 200 μL of amphotericin B (100×). The cryopreservation solution comprised 9 mL of tissue culture media (Cryobank wash media) and 1 mL of 20% dimethyl sulfoxide (DMSO). DMSO acts as a cryoprotectant agent. The tissue culture medium is a commercially available medium used for semen processing in reproductive biology (Cryocell India Pvt Ltd, New Delhi, India). The medium contains sodium chloride, potassium chloride, magnesium sulfate, anhydrous potassium phosphate monobasic, calcium chloride, anhydrous sodium bicarbonate, HEPES, glucose, sodium pyruvate, sodium lactate, phenol red, and human albumin in company-specified compositions.
Tissue Processing Protocol
Tissue processing was conducted in sterile conditions under a laminar flow hood. Extracted lenticules were washed twice for 10 minutes each in the washing solution (Fig. 1). The lenticules were then transferred into a cryovial and resuspended in 500 μL medium containing 10% tissue culture media. Using a liquid dropper, a stock freezing solution containing 10% human tissue culture medium and 20% DMSO (Sigma-Aldrich, St Louis, MO) was added slowly to make a final volume of 1 mL freezing solution containing 10% human tissue culture medium and 10% DMSO. The cryovials containing the lenticules were transferred in canisters and frozen in liquid nitrogen at a controlled cooling rate that gradually brought down the temperature from 4°C to −196°C and stored in long-term storage containers (IBP; Indian Oil Corporation Limited, Nasik, India).
FIGURE 1.
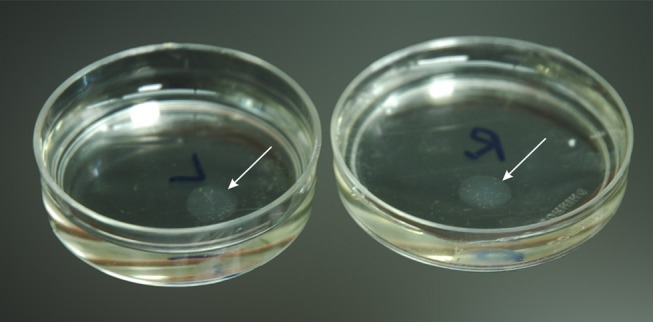
Washing of lenticules 10 minutes each in washing solution (twice). Arrows point to the lenticule.
Preparation of Lenticules for Implantation
On the day of surgery, the tissue was transported from the cryobank to the hospital in a liquid nitrogen container. The vials containing frozen tissue were gradually thawed by rubbing them between the palms or keeping them at room temperature for 5 to 10 minutes until the frozen medium inside the vial was liquefied. The lenticule was then transferred into a petri dish and washed twice with balanced salt solution for 5 minutes each to remove the cryoprotectant agents.
Lenticular Implantation Technique
The technique used was FS laser intrastromal lenticular implantation (FILI). A VisuMax FS laser was used to create a 7.5 mm diameter pocket at a depth of 160 μm and 4 mm superior incision. The incision was opened with a Seibel spatula and the plane of the pocket was dissected. The cryopreserved lenticule (which was matched for the refractive error after correcting for back vertex distance) was placed on the patient's cornea with its center marked with gentian violet dye. The lenticule was held with 2 forceps and inserted into the pocket. After insertion, the lenticule was spread out, and the center was aligned with the pupillary center.
A depth of 160 μm was chosen for implantation in all patients due to uncertainty in refractive outcomes, the novelty of the nomograms, and so the surgeon could have adequate tissue in the cap for later enhancement with surface ablation if required.
Postoperatively, a topical steroid (1% prednisolone acetate; Allergan, Irvine, CA) was prescribed for 3 months in a tapering dosage. In addition, 5% hypertonic saline drops (Hypersol-5; Jawa Pharmaceuticals, India) were prescribed 6 times per day for 1 week to reduce endothelial stress and to aid in lenticule clearing.
Patients were examined postoperatively on days 1 and 15 and at 1, 3, and 6 months. A slit-lamp examination was performed to check for lenticule clarity, position, and wound healing.
Postoperative corneal haze and folds were graded at every follow-up time point using the scale described by Nakamura et al and the Corneal Folds Grading Atlas.12,13
On postoperative day 15 and onward, the following assessments were performed: uncorrected visual acuity, best-corrected visual acuity (BCVA), retinoscopy for residual refractive error, topography using ORBscan(Bausch & Lomb-Technolas, Munchen, Germany) and Sirius (Schwind eye-tech solutions, Kleinostheim, Germany), anterior segment optical coherence tomography (Optovue, Fremont, CA), clinical photography, specular microscopy (Tomey, Japan), dry eye assessment (Schirmer's I), and aberrometry (iTrace; Tracey Technologies, Houston, TX).
RESULTS
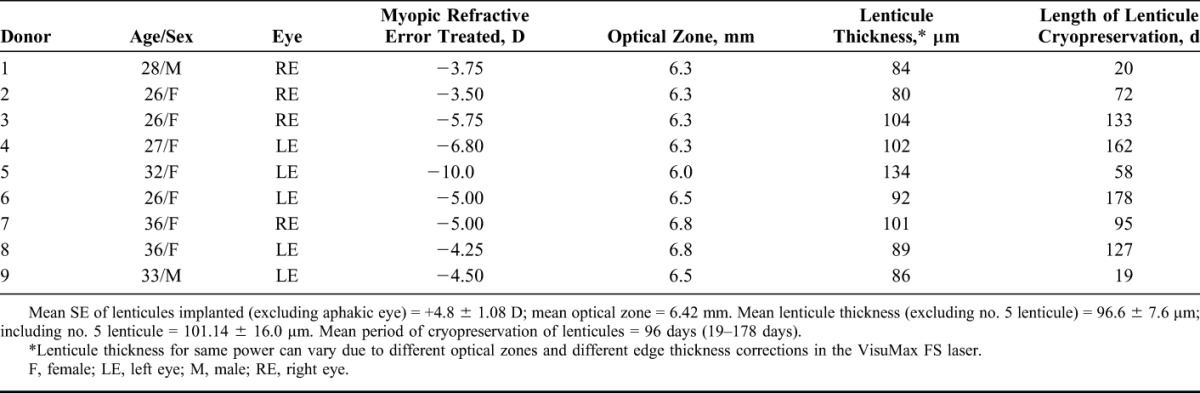
FILI was performed on 9 eyes of 7 patients. Table 1 provides the preoperative data of patients who underwent FILI. Mean follow-up period was 155.4 days (range, 38–310 days). Table 2 provides the details of donors and lenticules used for FILI in the 9 eyes treated. The mean period of cryopreservation of lenticules was 96 days (19–178 days).
TABLE 1.
Demographic Details of Patients Who Underwent FILI (7 Patients, 9 Eyes)
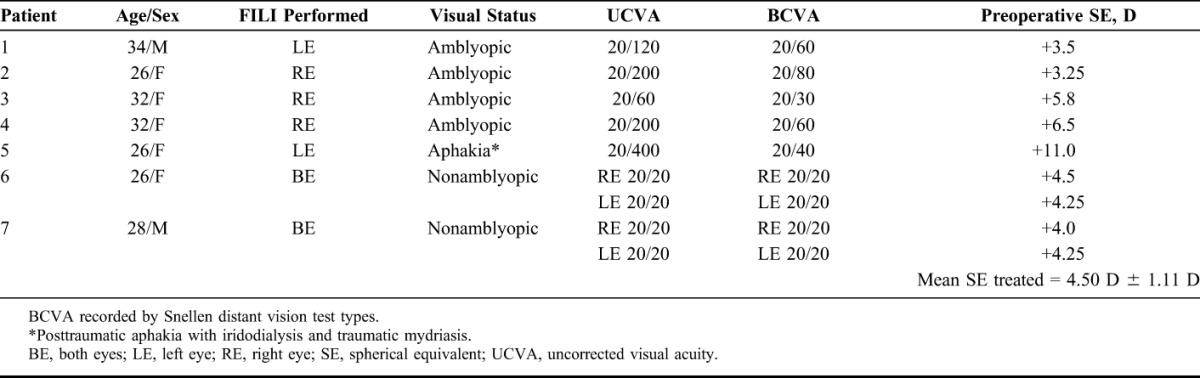
TABLE 2.
Details of Donors and Lenticules Used for FILI (n = 9)
On day 1, in the first 2 consecutive eyes, the implanted lenticules had mild to moderate Descemet membrane folds that resolved spontaneously within 1 week. Subsequently, the treated eyes were prescribed hypertonic saline drops, which showed clear lenticules within 48 hours and until the last follow-up time point. The lenticules were stable and did not show any shift with time. Figure 2 shows serial clinical photographs and anterior segment optical coherence tomography of the eye treated with FILI at day 15 and at 6 months post-FILI showing clear lenticules with good centration.
FIGURE 2.
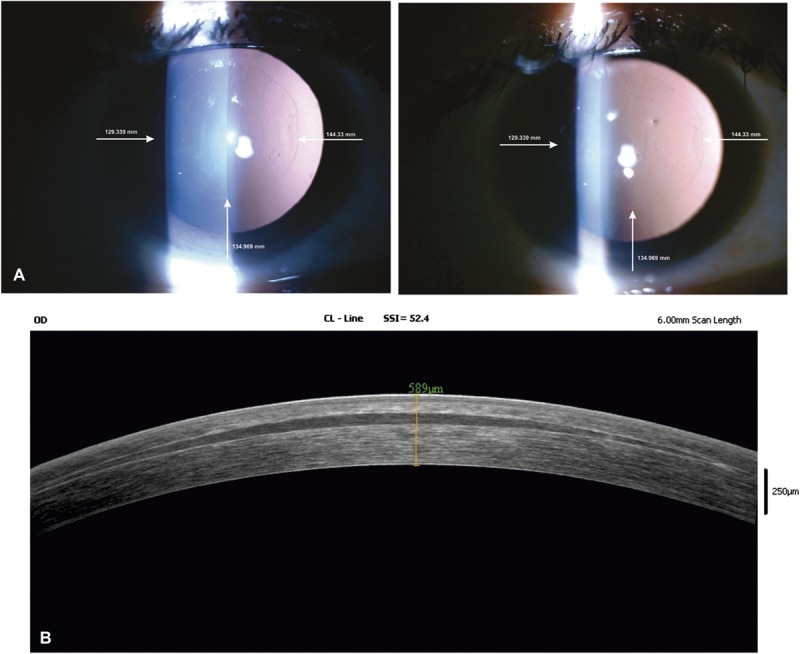
A, Serial digital clinical photographs (16×) of 32-year-old woman operated for +6.5 D hyperopia in the right eye with FILI. Photographs were taken on day 15 and 6 months after the operation. Distance from edge of the lenticule to limbus was measured at 3 points and verified at every visit to check for centering and any shift in position. B, Six-month postoperative anterior segment optical coherence tomography of an eye treated for +6.5 D hyperopia showing a clear and well-centered lenticule.
Table 3 provides the visual and refractive outcomes of the eyes treated with FILI. All eyes had an uncorrected visual acuity equal to or better than the preoperative BCVA. The refractive predictability for hyperopia was fairly accurate, and all eyes had a residual spherical equivalent within ±1.0 diopter (D), although in the aphakic eye, the residual spherical equivalent was +4.1 D. There was an average of 0.75 to 1.00 D against-the-rule astigmatism in all eyes due to the superior 4 mm incision, which reduced and stabilized over time.
TABLE 3.
Postoperative Results of Eyes Treated With FILI (n = 9)
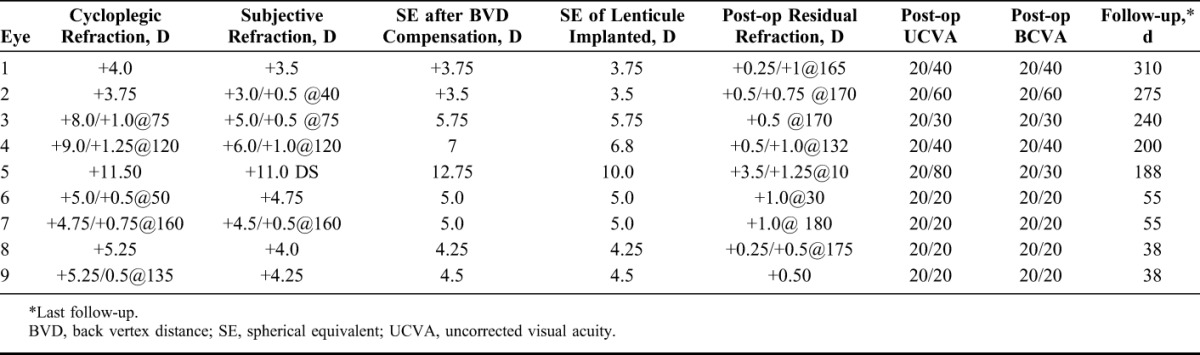
Table 4 provides changes in anterior and posterior curvature, pachymetry and asphericity (Q value) following FILI. All eyes had central corneal steepening with a mean change in anterior keratometry of 3.5 D in the central 3-mm zone. There was an average 0.33 D flattening of posterior corneal curvature in all eyes, which was seen more in thicker lenticules compared with the thinner ones. Figure 3 illustrates changes in anterior and posterior curvature post-FILI in an eye, over time.
TABLE 4.
Changes in Anterior and Posterior Topography, Pachymetry, and Asphericity Post-FILI (n = 9)
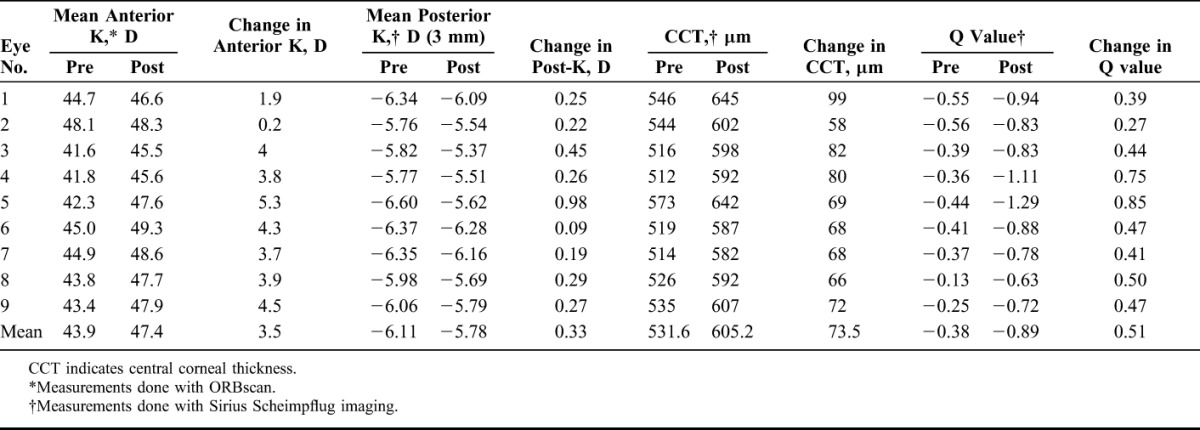
FIGURE 3.
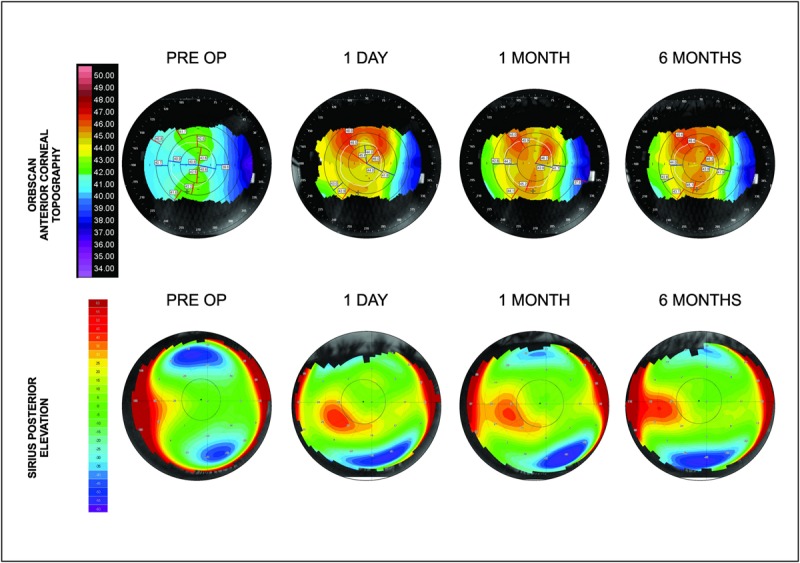
ORBscan anterior corneal surface topography (top) and Sirius posterior elevation (bottom) over the 6-month postoperative course after FILI for eye with +6.5 D hyperopia.
The postoperative Q values in all eyes became more negative, suggesting a hyperprolate shift. The mean Q value changed from −0.38 to −0.89. Table 5 provides analysis of the epithelial thickness profile, which suggested mild epithelial thinning in the 5-mm zone in all hyperopic eyes, except for the aphakic eye, which showed mild epithelial thickening in the corresponding zone. Figure 4 illustrates the epithelial thickness map of an eye treated with FILI at 6 months.
TABLE 5.
Epithelial Thickness Profile on Anterior Segment Optical Coherence Tomography in the Central 5-mm Zone
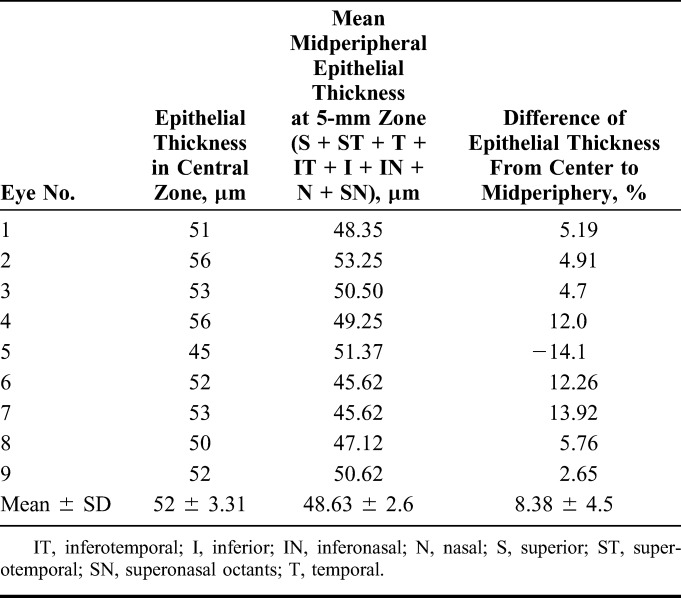
FIGURE 4.
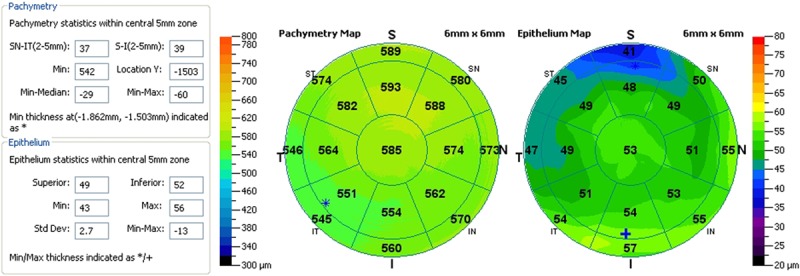
Epithelial thickness profile with anterior segment optical coherence tomography of an eye 6 months post-FILI for +6.5 D hyperopia.
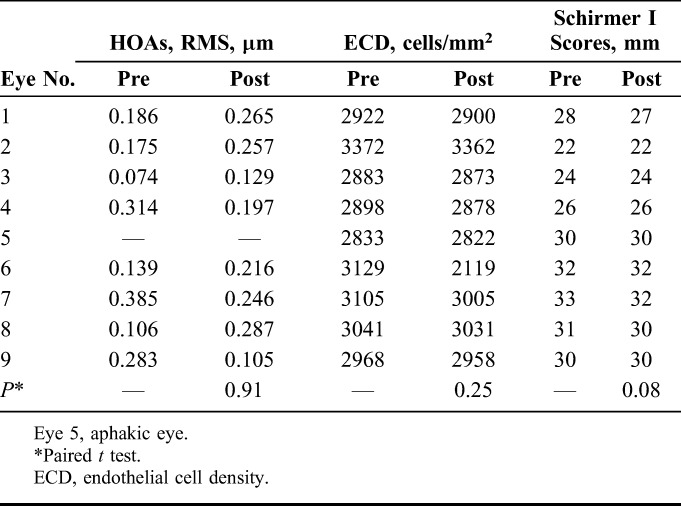
Higher order aberrations (HOA) did not show a significant increase in root mean square values postoperatively in the 8 hyperopic eyes (P > 0.05). Endothelial cell count and Schirmer I test scores were normal and the changes were not significant at the last follow-up (P > 0.05) (Table 6).
TABLE 6.
Total HOAs at 5-mm Pupil Size (n = 8), ECD, and Schirmer's I Scores, Pre- and Post-FILI (n = 9)
DISCUSSION
The potential advantages of cryopreservation of refractive lenticules have been largely described for use in the same patient, in the event of future ectasia or presbyopia by restoring corneal volume.3 With the increasing number of eyes undergoing ReLEx SMILE for myopia, and thus, the extracted lenticules as a by-product, it may be a novel idea to preserve these lenticules on a long-term basis using cryopreservation for potential future use and research. Following establishment of one such tissue bank, we wanted to explore the possibility of use of such cryopreserved refractive lenticules for potential treatment of hyperopia and study the feasibility, safety, efficacy, and reproducibility of this new treatment.
Initially, the amblyopic eyes were studied mainly for changes in refraction and topography following tissue addition. There was also the goal of evaluating any loss of BCVA and complications, such as haze and tissue rejection. Even these patients benefited from the surgery, as their refractive error was corrected to their best spectacle-corrected visual acuity and reduced their dependence on eyeglasses. After reviewing the results for 6 months, the indications were extended to normal eyes and bilateral surgery.
Unlike myopia, the correction of hyperopia involves creation of a furrow-like ring zone in the paracentral zone of the cornea. Both photorefractive keratectomy and LASIK are subtractive procedures that steepen the cornea by ablating the midperipheral tissue.14,15 This creates an abnormal hyperprolate shape of the cornea that is associated with significant regression, especially in higher degrees of refractive errors.16 It potentially leads to the induction of HOAs like coma and spherical aberrations and loss of BCVA.17
FILI is an innovative technique based on Barraquer's law of thickness,18 whereby the cornea is made steeper by addition of a lenticule of known thickness and power into a pocket created in a patient's cornea using the FS laser. The concept is similar to epikeratophakia, used to treat aphakia in the past, which had its own disadvantages.19 A FS laser overcomes these problems by creating precise lenticules and incisions at an accurate depth.
The lenticular tissue processing technique described by Mohamed-Noriega et al10 was modified in this study by replacing bovine serum albumin with human tissue culture media to eliminate the risk of transmission of zoonotic diseases or unexpected adverse reactions due to bovine serum. All other conditions in the procedure remained the same. The protocols of gradual staged thawing, which are essential for the whole cornea to prevent endothelial cell injury due to sudden thawing, were not strictly followed in this study. Microbiological and cellular cultures were not deemed necessary, as the tissue processing and preservation were conducted under strict sterile conditions, and the tissue did not contain vital endothelial cells. Also, previous studies have suggested that stromal keratocytes within the extracted lenticules remain viable and can be stimulated to proliferate in appropriate cell culture conditions, even after a prolonged period of cryopreservation.9,10
The change in asphericity after FILI suggests an increase in the prolateness of the cornea, which is expected after tissue addition, although the observed change was not seen as much as it is after hyperopic LASIK.20 The shape of the cornea is more natural after tissue addition compared with tissue subtraction, which does not lead to significant induction of HOAs.21 The total HOAs after FILI remain within normal acceptable values and may be better than hyperopic LASIK/photorefractive keratectomy.22,23 Also, tissue addition may be more forgiving for mild decentration, which is not the case with hyperopic LASIK. The rate of enhancement following hyperopic LASIK can be up to 10% or more,24 especially in younger patients. After tissue addition, significant changes in topography or regression are not expected; thus, retreatment rates may be lower. Epithelial thickness profile is more favorable, which, unlike hyperopic LASIK, does not cause midperipheral epithelial hyperplasia to compensate for loss of stromal tissue, hence reducing chances of regression.25 Problems of haze and flap-related issues like delayed healing, infection, and dislocation are eliminated, thereby leading to greater patient comfort and faster recovery.
A fairly good refractive predictability was observed with this technique in the treatment of moderate hyperopia, although results were not highly accurate for correcting very high hyperopia (eg, aphakia). In a case study reported by Pradhan et al,4 a 10 D lenticule was implanted to achieve correction of aphakia but had a high residual refraction of +5 D. There are possible explanations for this result. First, the technique of Pradhan et al of pocket creation was different from the technique used in this study. In the said study, a SMILE lenticule was first created, followed by dissection of the anterior plane to create a pocket, thus making 2 planes that could possibly cause weakening of the cornea. Second, the optical zone of the lenticule in the study of Pradhan et al was 5.7 mm compared with 6 mm in the current aphakia case, causing comparatively less volume of tissue addition. The correction is expected to be relatively higher in larger lenticules because there is more volume of the tissue added. In this hyperopic series, the lenticule size ranged from 6.3 to 6.8 mm. Also, the posterior curvature changes as described in the case study of Pradhan et al reduced the effect on anterior curvature. Noncompensation for the back vertex distance could be another reason. This was also probably the cause of the high +4.1 D residual refraction in the current study. After compensation, implantation of lenticule of approximately 12.5 D is required, and this is currently not possible with VisuMax.
The biomechanics of corneas in these patients were not studied due to lack of equipment such as the Corvis (OCULUS Optikgeräte GmbH, Wetzlar, Germany). However, because most of the Bowman layer is preserved and tissue is being added, it is assumed that the biomechanics are more stable than LASIK, where a flap is cut and tissue is subtracted from the periphery.26
The procedure certainly amounts to corneal transplantation and thus, theoretically carries the risk of future rejection. However, unlike traditional full thickness grafts, which have a graft–host junction proximal to host limbus, in this technique, the lenticule was well protected in the host corneal pocket and did not come in direct contact with the host's limbal vasculature and immune system, making the risk of allogeneic graft rejection remotely possible.27 Also, the lenticule was mainly stromal collagen tissue; the incidence of rejection is significantly low in such a scenario compared with endothelial rejection. Because no sutures are applied, there are no suture-related complications such as loosening, vascularization, or infection that would potentially trigger graft rejection.28 Nevertheless, there is a need to monitor for signs of rejection and treat it like allogeneic graft rejection should rejection occur. Because it is a reversible procedure, the lenticule can always be explanted in the event of any adverse effects or rejection in future.
The preliminary results of FILI in hyperopia indicate that this may be a better technique than hyperopic LASIK for moderate hyperopia in terms of stability, regression, aberrations, and postoperative dry eye. Although it is a bit early to exactly predict the refractive outcomes, initial data indicate that the cryopreservation technique used is safe in heterologous individuals.
Further studies with a larger cohort and modification of the surgical technique with a goal of reducing postoperative astigmatism and refining nomograms are suggested to determine the long-term safety and refractive effects on the cornea and establish the technique as a valid option to treat hyperopia.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Ang M, Tan D, Mehta JS. Small incision lenticule extraction (SMILE) versus laser in-situ keratomileusis (LASIK): study protocol for a randomized, non-inferiority trial. Trials. 2012;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol. 2011;95:335–339 [DOI] [PubMed] [Google Scholar]
- 3.Angunawela RI, Riau AK, Chaurasia SS, et al. Refractive lenticule re-implantation after myopic ReLEx: a feasibility study of stromal restoration after refractive surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2012;53:4975–4985 [DOI] [PubMed] [Google Scholar]
- 4.Pradhan KR, Reisntein DZ, Carp GI, et al. Femtosecond laser-assisted keyhole endokeratophakia: correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J Refract Surg. 2013;29:777–782 [DOI] [PubMed] [Google Scholar]
- 5.Eastcott HH, Cross AG, Leigh AG, et al. Preservation of corneal grafts by freezing. Lancet. 1954;266:237–239 [DOI] [PubMed] [Google Scholar]
- 6.Brunette I, Le François M, Tremblay MC, et al. Corneal transplant tolerance of cryopreservation. Cornea. 2001;20:590–596 [DOI] [PubMed] [Google Scholar]
- 7.Komuro A, Hodge DO, Gores GJ, et al. Cell death during corneal storage at 4 degrees C. Invest Ophthalmol Vis Sci. 1999;40:2827–2832 [PubMed] [Google Scholar]
- 8.Oh JY, Kim MK, Lee HJ, et al. Comparative observation of freeze–thaw-induced damage in pig, rabbit, and human corneal stroma. Vet Ophthalmol. 2009;12(suppl 1):50–56 [DOI] [PubMed] [Google Scholar]
- 9.Borderie VM, Laroche L. Ultrastructure of cultured and cryopreserved human corneal keratocytes. Cornea. 1999;18:589–594 [PubMed] [Google Scholar]
- 10.Mohamed-Noriega K, Toh KP, Poh R, et al. Cornea lenticule viability and structural integrity after refractive lenticule extraction (ReLEx) and cryopreservation. Mol Vis. 2011;17:3437–3449 [PMC free article] [PubMed] [Google Scholar]
- 11.Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg. 2011;37:127–137 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Kurosaka D, Bissen-Miyajima H, et al. Intact corneal epithelium is essential for the prevention of stromal haze after laser assisted in situ keratomileusis. Br J Ophthalmol. 2001;85:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenwasser ODG, Nicholson JW. Corneal folds grading atlas. Available at: http://telemedicine.orbis.org/bins/content_page.asp?cid=1-1581-1624. Accessed July 31, 2014
- 14.Juhani P, Petri M, Seppo P. Excimer laser photorefractive keratectomy for hyperopia. J Refract Surg. 1997;13:504–510 [DOI] [PubMed] [Google Scholar]
- 15.Ditzen K, Huschka H, Pieger S. Laser in situ keratomileusis for hyperopia. J Cataract Refract Surg. 1998;24:42–47 [DOI] [PubMed] [Google Scholar]
- 16.Göker S, Kahvecioglu C. Laser in situ keratomileusis to correct hyperopia from +4.25 to +8.00 diopters. J Refract Surg. 1998;14:26–30 [DOI] [PubMed] [Google Scholar]
- 17.Pesudovs K. Wavefront aberration outcomes of LASIK for high myopia and hyperopia. J Refract Surg. 2005;21:S508–S512 [DOI] [PubMed] [Google Scholar]
- 18.Barraquer JI. Refractive corneal surgery. Experience and considerations. An Inst Barraquer 1993;24:113–118 [Google Scholar]
- 19.Ehrlich MI, Nordan LT. Epikeratophakia for the treatment of hyperopia. J Cataract Refract Surg. 1989;15:661–666 [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Izadshenas A, Rana MA, et al. Corneal asphericity after hyperopic laser in situ keratomileusis. J Cataract Refract Surg. 2002;28:1539–1545 [DOI] [PubMed] [Google Scholar]
- 21.Bottos KM, Leite MT, Aventura-Isidro M, et al. Corneal asphericity and spherical aberration after refractive surgery. J Cataract Refract Surg. 2011;37:1109–1115 [DOI] [PubMed] [Google Scholar]
- 22.Alió JL, Piñero DP, Espinosa MJ. Corneal aberrations and objective visual quality after hyperopic laser in situ keratomileusis using the Esiris excimer laser. J Cataract Refract Surg. 2008;34:398–406 [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez A, Alió JL. Corneal aberration changes after hyperopic LASIK: a comparison between the VISX Star S2 and the Asclepion-Meditec MEL 70 G Scan excimer lasers. J Refract Surg. 2006;22:34–42 [DOI] [PubMed] [Google Scholar]
- 24.Randleman JB, White AJ, Jr, Lynn MJ. Incidence, outcomes and risk factors for retreatment after wavefront-optimized ablations with PRK and LASIK. J Refract Surg. 2009;25:273–276 [DOI] [PubMed] [Google Scholar]
- 25.Reinstein DZ, Archer TJ, Gobbe M. Epithelial thickness after hyperopic LASIK: three dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2010;26:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knox Cartwright NE, Tyrer JR, Jaycock PD, et al. Effects of variation in depth and side cut angulation in LASIK and thin-flap LASIK using a femtosecond laser: a biomechanical study. J Refract Surg. 2012;28:419–425 [DOI] [PubMed] [Google Scholar]
- 27.Stulting RD, Sugar A, Beck R, et al. Effect of donor and recipient factors on corneal graft rejection. Cornea. 2012;31:1141–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panda A, Vanathi M, Kumar A, et al. Corneal graft rejection. Surv Ophthalmol. 2007;52:375–396 [DOI] [PubMed] [Google Scholar]


