Abstract
Background:
Therapeutic drug monitoring of adalimumab (ADM) has been introduced recently. When no detectable ADM serum concentrations can be found, the formation of antidrug antibodies (ADA) should be investigated. A variety of assays to measure the occurrence of ADA have been developed. Results are expressed as arbitrary units or as a titration value. The aim was to develop a monoclonal antibody (MA) that could serve as a universal calibrator to quantify the amount of ADA in ADM-treated patients.
Methods:
Hybridoma technology was used to generate a MA toward ADM. The functionality of the MA was tested in a bridging enzyme linked immunosorbent assay (ELISA) setup and in a cell-based assay. Sera from 25 anti–tumor necrosis factor naive patients with inflammatory bowel disease were used to determine the cutoff values. Sera from 9 ADM-treated patients with inflammatory bowel disease, with undetectable serum concentrations of ADM were used to quantify the ADA response.
Results:
In this study, MA-ADM6A10, an IgG1 that can be used as a calibrator in both an ELISA to quantify the amount of binding antibodies and in a cell-based assay to quantify the amount of neutralizing antibodies, was generated. Combining the results of both assays showed that the sera with high concentrations of anti-ADM binding antibodies also had the highest neutralizing capacity.
Conclusions:
The availability of a universal calibrator could facilitate the interlaboratory harmonization of antibody titers in patients who develop anti-adalimumab antibodies.
Key Words: adalimumab, inflammatory bowel disease, antibody standard, ELISA, immunogenicity, therapeutic drug monitoring
INTRODUCTION
The introduction of therapeutic monoclonal antibodies targeting tumor necrosis factor (TNF) has revolutionized the treatment of chronic inflammatory diseases (eg, rheumatoid arthritis and Crohn disease). However, the downside is that patients can develop an antibody response toward foreign elements inherent to the therapeutic antibodies. Binding of both nonneutralizing and neutralizing antibodies result in the formation of immune complexes that are cleared from the circulation. In addition, neutralizing antibodies obstruct the TNF binding site of these therapeutic antibodies, thereby reducing their ability to scavenge TNF, leading to loss of response.
Adalimumab (ADM, NDC:0074-3799, Humira; Abbott Laboratories, Chicago, IL) is a human recombinant IgG1 antibody that is administered subcutaneously to treat patients suffering from either Crohn disease, ulcerative colitis, plaque psoriasis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, or juvenile idiopathic arthritis. The estimated elimination half-life is 15–19 days.1 Because of the full human nature of ADM, the majority of the antibodies developed in response to this antibody are expected to target the hypervariable region of ADM.
Recently, therapeutic drug monitoring of anti-TNF drugs has been introduced. Several retrospective trials have shown that better clinical outcome is associated with detectable trough concentrations.2–4 Prospective studies to evaluate the efficacy of trough concentration–based dosing are ongoing.5,6 When no detectable ADM serum concentrations can be found, the formation of antidrug antibodies (ADA) should be investigated.
A variety of assays (bridging ELISA,7–9 antigen binding assay,10 pH-shift-anti-idiotype binding assay,11 ELISA including an acid-dissociation step,12 cell-based assay (CBA),13 homogeneous mobility shift assay,14 fluid-phase radio immunoassay15) to measure the occurrence of ADA have been developed. Unfortunately, because of the lack of a universal calibrator, results are either defined as arbitrary units toward (purified) (monospecific) polyclonal rabbit antibodies7–12,15 or as some form of titration value.13,16 In this study, we aimed to develop a monoclonal antibody that could serve as a universal calibrator to quantify the amount of ADA in serum samples.
MATERIALS AND METHODS
Through hybridoma technology,17 a mouse MA was generated toward ADM (MA-ADM6A10). MA-ADM6A10 was produced on a large scale using a CL350 CELLine system (Integra Biosciences) and purified using ProSep Ultra Plus Affinity Chromatography (Millipore). The concentration was determined by spectrophotometry using 1.35 (280 nm, 1 mg/mL) as the extinction coefficient. MA-ADM6A10 was stored in phosphate-buffered saline solution pH 7.4 at −20°C in aliquots ready to use. On storage at −20°C for more than 6 months, the concentration was 95%–105% of the original concentration (n = 2 on 2 different batches of MA-ADM6A10). The isotype of MA-ADM6A10 was determined using Isostrip (Roche Applied Science, Indianapolis, IN). Binding of purified MA-ADM6A10 to ADM and lack of binding to infliximab (IFX, Remicade; Janssen Biotech Inc, Great Valley Park Malvern, PA) was confirmed in ELISA setting using ADM- and IFX-coated plates and through affinity measurement using surface plasmon resonance analysis (data not shown).
To determine if MA-ADM6A10 could cross-link ADM coated on a plate with soluble biotinylated ADM, a dose–response curve of the selected MA was applied, and bound biotinylated ADM was quantified using streptavidin-labeled horseradish peroxidase (R&D systems, Minneapolis, MN) (n = 3). The dose–response curve was fitted using “one site-specific binding” nonlinear regression (Graphpad Prism 5.0) (Fig. 1). Two different batches of MA-ADM6A10 were compared (n = 3), revealing a Pearson correlation of 0.99 between batches. To determine the cutoff value of the assay, sera from 25 anti-TNF naive patients with inflammatory bowel disease (IBD) were applied on the ADM/ADM-biotin bridging assay using MA-ADM6A10 as a calibrator (n = 1). All patients gave written consent to participate in the institutional review board–approved Vlaamse Erfelijkheidsstudie Crohn en Colitis ulcerosa (VLECC) registry (B322201213950/S53684).
FIGURE 1.
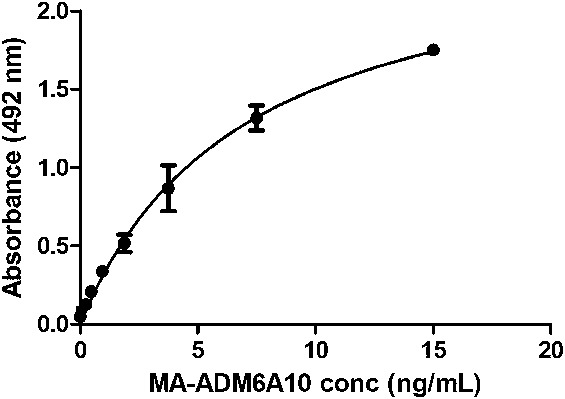
Dose–response curve of MA-ADM6A10 in bridging assay. MA-ADM6A10 is able to cross-link ADM coated on a plate with soluble biotinylated ADM. A dose–response curve (mean ± SD, n = 3) ranging from 0 to 15 ng/mL is shown.
To detect and quantify the amount of neutralizing ADA in sera of ADM-treated patients, we developed a functional CBA. HT1080 (CCL-121; ATCC) is a human fibrosarcoma cell line that expresses interleukin-6 (IL-6), which is stimulated on addition of TNF.18 HT1080 cells were plated in 96-well plates at a density of 4 × 104 cells per well at 5% CO2 and 37°C humidified atmosphere using 200 μL Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum, 2 mM glutamine, 50 mcg/mL streptomycin, and 50 U/mL penicillin per well. After 24 hours, DMEM medium was removed and replaced by 200 μL of supplemented DMEM, to which 2.5% human control serum preincubated for 30 minutes at 37°C with different concentrations (0–120 ng/mL) of TNF (Pepro Tech, London, United Kingdom) was added. IL-6 was measured after an additional 48 hours using the Human IL-6 ELISA Max Deluxe kit (Biolegend, San Diego, CA) according to the manufacturer's protocol. The TNF EC50 and EC80 (n = 2) were determined using nonlinear regression (log agonist versus response; Graphpad Prism 5.0), whereas the ADM IC50 and IC80 (n = 2) and the MA-ADM6A10 IC50 (n = 5) were determined using nonlinear regression (log inhibitor versus response; Graphpad Prism 5.0). Two different batches of MA-ADM6A10 were compared (n = 4), revealing a Pearson correlation of 0.92 between batches. To determine the cutoff value of the CBA, 25 anti-TNF naive serum samples (see above) were applied on the assay under the same conditions as described above (n = 1).
In addition, the ADA response in sera from 9 ADM-treated patients with IBD with undetectable serum concentrations of ADM was determined using both the ADM/ADM-biotin bridging assay (n = 3) and the CBA using 2.5% and 0.5% serum (n = 3) with MA-ADM6A10 for calibration.
The serum concentration of ADM was determined with an ADM assay developed in-house.9 Using anti-TNF naive samples, the cutoff was determined to be 0.34 mcg/mL. The accuracy and precision for 3 spiked serum samples (3, 7, and 11.25 mcg/mL) was determined to be 97%, 97%, 95% and 10%, 12%, 13%, respectively.
RESULTS
The generated MA, MA-ADM6A10, was an IgG1 with a kappa light chain. Binding of purified MA-ADM6A10 to ADM and lack of binding to IFX was confirmed in ELISA setting using an ADM- and IFX-coated plate and through affinity measurement using surface plasmon resonance analysis (data not shown).
Using a bridging assay setup, a nonlinear dose–response curve was obtained in a concentration range between 0.23 and 15 ng/mL MA-ADM6A10 (Fig. 1). Taking into account the 1:20 serum dilution that was applied, the limit of detection was set at 5 ng/mL. To determine the cutoff value of the assay, sera from 25 anti-TNF naive patients with IBD were applied, which all revealed values below the limit of detection. Therefore, the cutoff value of the assay was set at 5 ng/mL.
HT1080 cells, a human fibrosarcoma cell line that expresses IL-6, was used to develop a functional CBA. Basal IL-6 concentrations [2.2 ± 1.5 ng/mL (mean ± SD), n = 6] were set at 0%. IL-6 concentrations increased on addition of TNF (maximum response was set at 100%), revealing an EC50 of 3.78 ± 0.02 ng/mL (217.7 ± 1.2 pmol/L) and an EC80 of 11.1 ± 0.02 ng/mL (637.7 ± 1.3 pmol/L) (Fig. 2A). Based on the obtained TNF EC50/80, a concentration of 7.5 ng/mL TNF (441.2 pmol/L) was chosen to determine the reduced IL-6 production because of the inhibition of TNF by ADM. ADM was able to neutralize 7.5 ng/mL TNF with an IC50 of 8.07 ± 0.18 ng/mL (53.8 ± 1.2 pmol/L) and an IC80 of 21.2 ± 0.2 ng/mL (141.9 ± 1.4 pmol/L) (Fig. 2B). To determine if MA-ADM6A10 is able to inhibit the inhibitory effect of ADM on TNF, different doses of MA-ADM6A10 were added to sera supplemented with 7.5 ng/mL TNF (441.2 pmol/L) and 15 ng/mL ADM (100 pmol/L) to HT1080 cells using a similar setup as described above. On addition of 7.5 ng/mL TNF and 15 ng/mL ADM, an IC50 of 9.1 ± 0.20 ng/mL (61.0 ± 1.3 pmol/L) was determined for MA-ADM6A10 (Fig. 2C).
FIGURE 2.
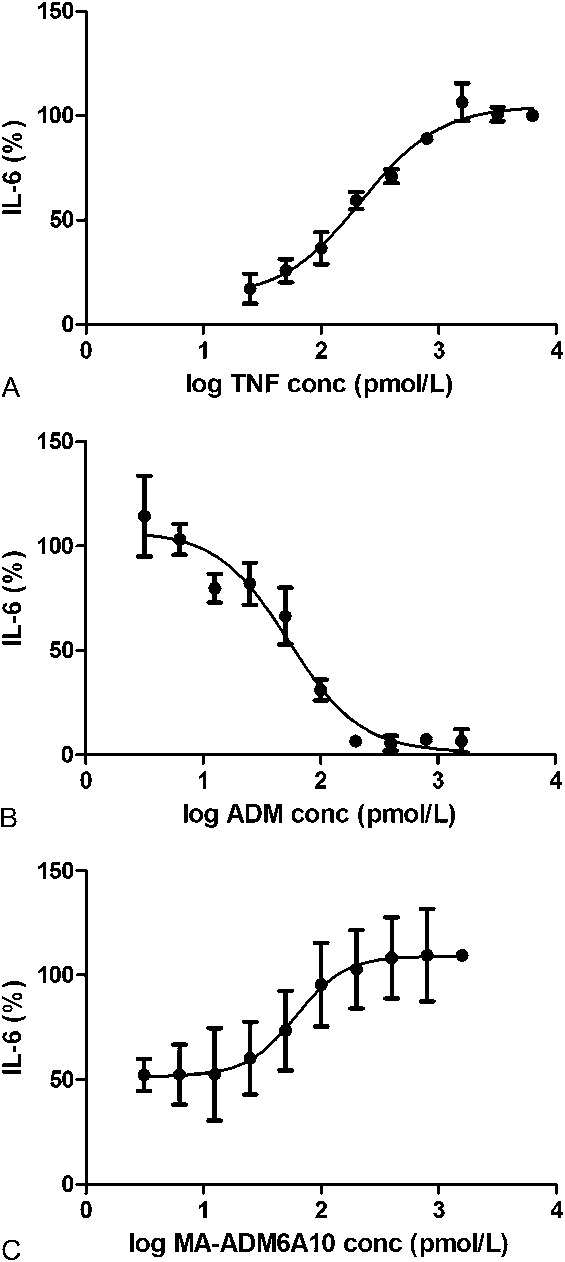
Dose–response curve of TNF (A), ADM (B), and MA-ADM6A10 (C) in CBA. HT1080 cells were incubated with TNF. A, IL-6 was measured and expressed as log TNF concentration versus IL-6 response (mean ± SD, n = 2). B, Inhibition of TNF by ADM was determined using 7.5 ng/mL TNF (441.2 pmol/L) and ADM (0–240 ng/mL) (mean ± SD, n = 2). C, To determine the inhibitory effect of MA-ADM6A10, different doses (0–240 ng/mL) of MA-ADM6A10 were added to sera supplemented with 7.5 ng/mL TNF (441.2 pmol/L) and 15 ng/mL ADM (100 pmol/L) (mean ± SD, n = 5).
The cutoff value of the CBA was determined by applying 25 anti-TNF naive serum samples on the assay under the same conditions as described above at a final serum concentration of 2.5%. Basal IL-6 concentrations (0 ng/mL TNF, 0 ng/mL ADM) were 0.9 ± 0.17 ng/mL. On addition of 7.5 ng/mL TNF to each individual serum sample, the increased IL-6 production was set at 100% for each sample separately, and IL-6 production determined on addition of 15 ng/mL ADM was calculated versus this 100%. For the 25 anti-TNF naive samples, addition of 15 ng/mL ADM reduced the IL-6 response to 58% ± 5.2% (mean ± SD, n = 25). Allowing a 0.1% chance of a type 1 error revealed a cutoff value of 74% corresponding with a 3.3 ng/mL MA-ADM6A10 (22 pmol/L) equivalence. Taking into account the 1:40 dilution, the cutoff of the CBA using MA-ADM6A10 as a calibrator was set at 132 ng/mL (880 pmol/L).
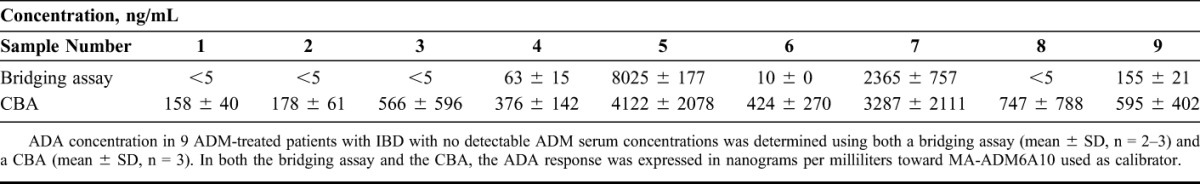
Subsequently, ADA in sera from 9 ADM-treated patients with IBD with undetectable serum concentrations of ADM was quantified using both the ADM/ADM-biotin bridging assay and the CBA. Five of the 9 sera had detectable ADA concentrations in the bridging assay with values between 10 ng/mL and >8 mcg/mL. All sera had detectable ADA concentrations in the CBA with values between 158 ng/mL and 4.1 mcg/mL. The 2 samples that gave the highest ADA values in the bridging assay also showed the highest amount of neutralizing ADA in the CBA with comparable values (8.0 and 2.4 mcg/mL in the bridging assay versus 4.1 and 3.3 mcg/mL in CBA for samples 5 and 7, respectively; Table 1).
TABLE 1.
ADA Concentrations of 9 ADM-Treated Patients With IBD
DISCUSSION
Loss of response to anti-TNF drugs can be because of the production of ADA. Currently, there is a large heterogeneity in the reported outcome measures of ADA assays. The availability of a universal calibrator could facilitate clinical research on the impact of immunogenicity on clinical outcomes.
MA-ADM6A10, generated in this study, was able to cross-link ADM coated on a plate with soluble biotinylated ADM and could be used in a bridging assay format. The bridging assay was very convenient and easy to perform, but it should be noted that it was less sensitive toward low-affinity antibodies, could not detect IgG4, and it could not distinguish neutralizing from nonneutralizing antibodies. In addition, MA-ADM6A10 efficiently inhibited ADM from binding to TNF in a CBA. The CBA was labor intensive and showed a large interassay variability, but the advantage of the technique was that both low- and high-affinity neutralizing antibodies of different subtypes (including IgG4) could be measured in the assay.
The generated MA was used to quantify the ADA response in sera of 9 ADM-treated patients IBD with no detectable ADM trough concentration. Five of the 9 samples were positive for ADA in the bridging assay, and 2 of the 9 revealed a high titer of antibodies. All sera were positive in the CBA assay.
Van Schouwenburg et al19,20 have shown recently that a substantial portion of ADA is of the IgG4 isotype and that at least 98% of the ADM-specific ADA are neutralizing antibodies. The presence of IgG4 and the underestimation of IgG4 in a bridging assay can explain the (higher) ADA values measured in some samples using the CBA. Moreover, because of the washing procedure, the bridging assay is less sensitive to low-affinity antibodies. The 2 samples that revealed the highest values in the CBA also revealed the highest values in the bridging assay, suggesting that in patients with IBD who develop a substantial ADA response, high-affinity antibodies of the IgG1 subtype are generated. From this study, it can also be concluded that when high antibody titers are measured in a bridging assay, this should be considered as a substantial immunogenic reaction with an impact on further treatment.
The affinity of the ADA developed in ADM-treated patients can be of a different nature to that developed in a mouse, and therefore, the affinity of MA-ADM6A10 does not necessarily reflect the affinity of the human ADA. However, the purpose of this study was to generate an antibody that could be produced easily and reproducibly on a large scale and that could be used as a universal calibrator.
CONCLUSIONS
In this study, a highly specific and inhibitory MA toward ADM was generated that could be used as a calibrator in ADA assays to determine the binding and the neutralizing effect of the ADA. The availability of a universal calibrator could contribute to the harmonization of ADA concentrations reported by different laboratories using different methods to analyze the ADA response in ADM-treated patients. Subsequently, this may also facilitate correlations between the magnitude of the ADA response and clinical outcome of ADM therapy.
ACKNOWLEDGMENTS
A. Gils, N. Vande Casteele, and P. Declerck designed the study, supervised the experiments, and wrote the article. R. Poppe and M. Peeters set up and performed the cell-based assay. M. Van de Wouwer and E. Brouwers generated, selected, and purified the monoclonal antibody. G. Compernolle performed the bridging ELISA. S. Vermeire provided the serum samples. N. Geukens supervised the generation of the MA. All authors approved the final version of the article.
Footnotes
A. Gils has served as a speaker for MSD and as an advisory board member for Pfizer and has received an Investigator Initiated Research Grant from Pfizer. N. Vande Casteele has served as a speaker for Abbvie and a consultant for MSD and Janssen Biologics. S. Vermeire has served as a speaker for MSD, Abbvie, UCB Pharma, Pfizer, Centocor, and Ferring and has received research grants from Abbvie, MSD, UCB Pharma, and Centocor. The other authors declare no conflict of interest.
A. Gils and N. Vande Casteele have contributed equally.
Supported in part by the Fund for Scientific Research, Flanders (grant number G.0617.12), and N. Vande Casteele is a Postdoctoral Fellow of the Research Foundation, Flanders (FWO), Belgium.
REFERENCES
- 1.Weisman MH, Moreland LW, Furst DE, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25:1700–1721 [DOI] [PubMed] [Google Scholar]
- 2.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254 [DOI] [PubMed] [Google Scholar]
- 3.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54 [DOI] [PubMed] [Google Scholar]
- 4.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;137:1628–1640 [DOI] [PubMed] [Google Scholar]
- 5.Vermeire S, Gils A. Value of drug level testing and antibody assays in optimising biological therapy. Frontline Gastroenterol. 2013;4:41–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaparro M, Guerra I, Muñoz-Linares P, et al. Systematic review: antibodies and anti-TNF-α levels in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:971–986 [DOI] [PubMed] [Google Scholar]
- 7.Hart MH, de Vrieze H, Wouters D, et al. Differential effect of drug interference in immunogenicity assays. J Immunol Methods. 2011;372:196–203 [DOI] [PubMed] [Google Scholar]
- 8.Vande Casteele N, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36:765–771 [DOI] [PubMed] [Google Scholar]
- 9.Vande Casteele N, Ballet V, Van Assche G, et al. Early serial trough and antidrug antibody level measurements predict clinical outcome of infliximab and adalimumab treatment. Gut. 2012;61:321; author reply 322 [DOI] [PubMed] [Google Scholar]
- 10.van Schouwenburg PA, Bartelds GM, Hart MH, et al. A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J Immunol Methods. 2010;362:82–88 [DOI] [PubMed] [Google Scholar]
- 11.van Schouwenburg PA, Krieckaert CL, Rispens T, et al. Long-term measurement of anti-adalimumab using pH-shift-anti-idiotype antigen binding test shows predictive value and transient antibody formation. Ann Rheum Dis. 2013;72:1680–1686 [DOI] [PubMed] [Google Scholar]
- 12.Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn's disease. J Gastroenterol. 2014;49:100–109 [DOI] [PubMed] [Google Scholar]
- 13.Lallemand C, Kavrochorianou N, Steenholdt C, et al. Reporter gene assay for the quantification of the activity and neutralizing antibody response to TNFα antagonists. J Immunol Methods. 2011;373:229–239 [DOI] [PubMed] [Google Scholar]
- 14.Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies-to-adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal. 2013;78–79:39–44 [DOI] [PubMed] [Google Scholar]
- 15.Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn's disease. Scand J Gastroenterol. 2011;46:310–318 [DOI] [PubMed] [Google Scholar]
- 16.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012;382:177–188 [DOI] [PubMed] [Google Scholar]
- 17.Galfrè G, Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73:3–46 [DOI] [PubMed] [Google Scholar]
- 18.Desai S, Kumar A, Laskar S, et al. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine. 2013;61:54–62 [DOI] [PubMed] [Google Scholar]
- 19.van Schouwenburg PA, van de Stadt LA, de Jong RN, et al. Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann Rheum Dis. 2013;72:104–109 [DOI] [PubMed] [Google Scholar]
- 20.van Schouwenburg PA, Krieckaert CL, Nurmohamed M, et al. IgG4 production against adalimumab during long term treatment of RA patients. J Clin Immunol. 2012;32:1000–1006 [DOI] [PubMed] [Google Scholar]


