Abstract
Background and Objectives
We hypothesized that continuous right thoracic paravertebral block, following bolus initiation, decreases opioid consumption after right-lobe hepatectomy in patients receiving patient-controlled intravenous analgesia with sufentanil.
Methods
Patients undergoing right-lobe hepatectomy with a right thoracic paravertebral catheter placed at T7 30 minutes before surgery were randomly assigned to receive through this catheter either a 10-mL bolus of 0.2% ropivacaine before emergence, followed by a continuous infusion of 6 mL/h for 24 hours (PVB group), or saline at the same scheme of administration (control group). All patients were started on patient-controlled intravenous analgesia with sufentanil in the postanesthesia care unit. The primary outcome measure was total sufentanil consumption during the first 24 postoperative hours. P = 0.05 was considered as significant. For the multiple comparisons of data at 5 different time points, the P value for the 0.05 level of significance was adjusted to 0.01.
Results
Sixty-six patients were assessed for eligibility, and a PVB catheter was successfully placed for 48 patients. Data were analyzed on 22 patients in group PVB and 22 patients in the control group. The cumulative sufentanil consumption in the PVB group (54.3 ± 12.1 μg) at 24 postoperative hours was more than 20% less than that of the control group (68.1 ± 9.9 μg) (P < 0.001). There was also a significant difference in pain scores (numerical rating scale) between groups, where the PVB group had lower scores than did the control group at rest and with coughing for the first 24 hours (P < 0.001).
Conclusions
Continuous right thoracic paravertebral block, following bolus initiation, has an opioid-sparing effect on sufentanil patient-controlled intravenous analgesia for right-lobe hepatectomy patients and reduces numerical rating scale pain scores at rest and with coughing in the first 24 postoperative hours.
Epidural analgesia has commonly been recommended following major upper abdominal surgery in the past,1 but this recommendation is controversial.2 Hemostatic deficiencies after hepatectomy may increase the bleeding risk associated with the use of thoracic epidural analgesia and delay epidural catheter removal.3 Epidural analgesia has been independently associated with increased risk of blood transfusion and administration of significantly greater volumes of intravenous fluid following liver resection.3 Balancing the advantages of optimal analgesia against the risks of current techniques requires additional clinical evidence in patients undergoing hepatectomy.
Paravertebral nerve block (PVB) was first described by Sellheim of Leipzig in 1905 as an alternative to central neural blocks for obstetrics, particularly cesarean delivery. Paravertebral nerve blocks can provide high-quality analgesia for patients undergoing many types of surgery in the thoracic, abdominal, and pelvic regions and also for those suffering from trauma pain and chronic pain. The effect of continuous intraoperative and postoperative paravertebral block after right-lobe hepatectomy has been described in 2 patients, but the study did not include a control group.4 In this prospective, double-blind, randomized, placebo-controlled trial, we tested the hypothesis that continuous thoracic paravertebral block, following bolus initiation, reduces opioid consumption during the first 24 hours after right-lobe hepatectomy in patients receiving patient-controlled intravenous analgesia (PCIA) with sufentanil. The present study is reported according to the CONSORT (Consolidated Standards of Reporting Trials) statement.
METHODS
Patient Recruitment
This is a prospective, randomized, subject- and assessor-blinded, parallel-group, and placebo-controlled trial comparing a continuous right thoracic paravertebral block at T7 versus control group (saline) on postoperative opioid consumption after right-lobe hepatectomy for hepatocellular carcinoma. According to our local standards, the indications for all liver resections include tumor size of 5 to 10 cm; no intrahepatic or distant metastasis; no tumor thrombus in the portal vein, hepatic vein, vena cava, or bile duct; no invasion of the diaphragm or surrounding tissues; no rupture or bleeding of the tumor; indocyanine green retention rate at 15 minutes less than 15%; a remnant liver volume/standard liver volume ratio of greater than 50% in patients with liver cirrhosis and greater than 35% in patients without liver cirrhosis; Child-Pugh class A or B liver function, normal bilirubin concentration; and no previous liver resection. After receiving approval from the local institute’s ethics committee, registration with Clinical Trials (NCT01691937), and written informed consent from the patients, the study was conducted from September 2012 to February 2013 at Tongji Hospital in Wuhan, China. Patients were excluded if any of the following applied: a known allergy to the drugs being used, coagulopathy, on anticoagulants, analgesic intake, history of substance abuse, participation in the investigation of another experimental agent, inability to properly describe postoperative pain to investigators (eg, language barrier, neuropsychiatric disorder), or body mass index of 30 kg/m2 or greater or 15 kg/m2 or less. All patients were informed about the postoperative analgesia routine on the day before surgery and were instructed on how to express pain intensity with use of the numerical rating scale (NRS), where 0 indicates no pain, and 10 indicates the most severe pain. The same surgical team performed all surgical procedures through a subcostal incision.
Randomization
Simple randomization was done by independent research staff using opaque sealed envelopes, 24 for each group, indicating group assignment and describing the anesthetic protocol. A right thoracic paravertebral catheter was placed at the T7 level 30 minutes before surgery by an experienced senior consultant (W.M.). After confirmation of a successful paravertebral block, according to predefined criteria, patients were randomly assigned to the continuous PVB group or control group by research staff, and the PVB catheter was connected to an infusion pump containing either ropivacaine or normal saline accordingly. The infusion pumps are indistinguishable in appearance, and therefore the patients, the intraoperative caregiver (B.N.), the research staff assessing postoperative outcomes (H.C.), the acute pain service team, and the surgical team were blinded to group assignment. Unmasking did not occur until statistical analysis was complete.
Anesthetic Technique
A preoperative pain score at rest was recorded during the preoperative visit. No preoperative medications were administered. Upon arrival in the operating room, standard monitoring, including electrocardiography, noninvasive blood pressure, and pulse oximetry, was established. Surface electrodes for the Narcotrend Monitor (version 4.0; MonitorTechnik, Bad Bramstedt, Germany) were applied to the patient’s forehead, and the Narcotrend index was monitored. After placement of a peripheral venous catheter, a lactated Ringer’s infusion was started.
Before anesthesia, all patients had continuous right thoracic paravertebral block at T7 performed under ultrasound guidance with a SonoSite M-Turbo transportable ultrasound device and a 38-mm linear 6- to 13-MHz ultrasound transducer (SonoSite M-Turbo; SonoSite Inc, Bothell, Washington) in the left lateral position via an in-plane needle insertion approach. Intravenous fentanyl and midazolam were titrated for patient comfort. After standard skin disinfection, the skin and periosteum of the rib were anesthetized with 1% lidocaine. The transducer was positioned in a transverse and partial oblique position to the vertebral column, parallel to the rib. At a point about 6 cm lateral to the midline, a prefilled 18-gauge, 10-cm Tuohy needle (PlexoLong Nanoline; Pajunk Inc, Geisingen, Germany) was inserted and oriented into the T7 paravertebral space between the internal intercostal membrane and the pleura under the real-time ultrasound guidance as described by Renes et al.5 After injection of 5 mL of 0.9% saline, an open-tip, single-orifice, styletted catheter (PlexoLong Nanoline; Pajunk Inc) was advanced 3 to 5 cm medially into the paravertebral space. After gentle and repeated aspiration, 1% lidocaine 15 mL was administered via the catheter over a period of at least 60 seconds. Fifteen minutes after block, sensory assessments were performed with a pinprick test, and the block level was recorded. The PVB was considered successful when subjects experienced a decreased sensation to pinprick in 3 or more adjacent dermatomes corresponding to the site of injection.6 Subjects with a successful catheter placement and nerve block onset per protocol were retained in the study. If the desired block was not achieved, we offered the patient the choice of reinsertion of the PVB catheter or to be excluded from the study. The properly located catheters were connected to a programmable portable, electronic infusion pump immediately before surgery. A nurse who was not otherwise involved in the study prepared and released the infusion pump containing blinded study medication with either 0.2% ropivacaine for the PVB group or 0.9% saline for the control group. In order to avoid inadvertent unmasking of allocation, the extent of sensory block was not tested during the postoperative period.
In both groups, the patient was then turned supine, and general anesthesia was induced with fentanyl 3 to 4 μg/kg and propofol 1.5 to 2 mg/kg. Tracheal intubation was facilitated with 0.6 to 1 mg/kg rocuronium. The patients were mechanically ventilated with oxygen-air mixture maintaining the end-tidal CO2 at 4.5 to 5.0 kPa. Anesthesia was maintained by remifentanil and sevoflurane to keep the Narcotrend index reading between 40 and 60, and the systolic arterial pressure and heart rate within ±20% of baseline values during the procedure. Atracurium was administered for muscle relaxation as needed. The patient’s nasopharyngeal temperature was maintained between 36°C and 37.5°C by WarmTouch.
All patients received a standard right-lobe hepatectomy for hepatocellular carcinoma by a J-shaped right subcostal incision. The J-shaped incision consisted of a right subcostal incision with a mediocranial extension to the xiphoid process. The right lateral extension was variable but comprised transection of the oblique abdominal musculature.7 Twenty minutes before the end of surgery, all patients received 1 to 2 μg/kg fentanyl titration and tropisetron 2 mg intravenously. At the same time, the PVB group had a 10-mL bolus of plain 0.2% ropivacaine via the paravertebral catheter by infusion pump, followed by an infusion of 0.2% ropivacaine at 6 ml/h. The same volume of 0.9% saline was injected and infused in the control group by infusion pump. At the end of surgery, the patient’s trachea was extubated when response to verbal commands, spontaneous respiratory rate exceeding 12 breaths/min, and end-tidal carbon dioxide partial pressure less than 45 mm Hg were observed. They were then transferred to the postanesthesia care unit (PACU).
Postoperative Care and Pain Assessment
Upon PACU arrival, rest pain was evaluated by nurses using NRS (0–10) every 10 minutes. All patients received intravenous sufentanil (2–5 μg) titration at 10-minute intervals for NRS scores greater than 3 in PACU. Once an NRS score of 3 or less had been achieved, spontaneously or after a titration of sufentanil, patients were connected to a patient-controlled analgesia device (BCDB-200; BCM, Shanghai, China) set to deliver 1 μg sufentanil as an intravenous bolus with a 10-minute lockout interval and a continuous background infusion of 1 to 2 μg/h and a maximal permitted dosage of sufentanil 8 μg/h. An acute pain service team was responsible for maintenance of the PCIA pump according to local standard. Transition from PACU to surgical ward was considered safe when the patient had achieved a modified Aldrete score of greater than over equal to 9 for at least 10 minutes and physiological function to near preanesthetic levels.
Pain at rest and on coughing was recorded for each patient using NRS (0–10) at 1, 4, 8, 16, and 24 hours after PACU discharge by the research staff (H.C.). Rescue opioid with intravenous tramadol was provided by acute pain service team when needed. Sedation was recorded using Ramsay Sedation Scale. Excessive sedation was defined as a Ramsay Sedation Scale value of 5 or 6 requiring the administration of naloxone. In the presence of nausea or vomiting, tropisetron 2 mg (maximum dose, 5 mg/d) intravenously was given and repeated if nausea persisted. The data of cumulative dose of sufentanil given postoperatively (titration and patient-controlled analgesia during the 24-hour observation period) and PCIA pressing frequency were recorded. Postoperative nausea and vomiting events during the first 24 hours were also recorded. After 24 hours, patients were asked to rate their overall satisfaction using an NRS, where 0 represents totally unsatisfied, and 10 represents completely satisfied. The infusion of ropivacaine in the PVB group and saline in the control group was stopped 24 hours after the surgery, and the catheter was removed. The PCIA continued until 48 hours after the surgery in both groups.
Statistical Analysis
The primary outcome measure was total sufentanil consumption in the first 24 postoperative hours. Previously unpublished data including 132 patients from April 2012 to July 2012 provided by the acute pain service team in our institution showed a mean and SD of cumulative sufentanil consumption over the first 24 hours after right-lobe hepatectomy to be about 70 and 10 μg, respectively. In order to have 95% power to detect a 20% reduction of sufentanil consumption at an α level of 0.01, we needed 20 patients per study group. To account for any patient dropouts or missing data, we planned to enroll 24 patients per study group. Descriptive statistics were computed for all study variables. Normally distributed data were analyzed using 2-tailed Student t test. Repeated-measures analysis of variance (ANOVA) was used to compare measurements over time (cumulative sufentanil consumption and PCIA pressing frequency). If there was a statistical difference (P < 0.05) between the 2 groups by repeated-measures ANOVA, Student t test was used to compare the data at each time point. The non–normal distributed NRS data are displayed in box-and-whisker plots. The box extends from the upper quartile to the lower quartile and is marked by a quadrate at the median of the data. The whiskers are determined by the upper and lower data point values not including outliers and extremes. Outliers (>1.5 box lengths) and extremes (>3 box lengths) are represented by circles and asterisks, respectively. All NRS data including outliers and extremes were included in final analysis. Differences of NRS scores at each time point between the 2 groups were analyzed using the Mann-Whitney U test. Categorical data were analyzed using the χ2 or Fisher exact test, where appropriate. The data are reported as mean ± SD, mean (range), median (first third quartiles), or percent, as appropriate. P = 0.05 was considered as significant. The Bonferroni correction was performed on the raw P value where applicable. For the multiple comparisons of NRS, sufentanil consumption, and frequency of PCA data at 5 different time points, the P value for the 0.05 level of significance was adjusted to 0.01. All analyses were performed using SPSS (version 12; SPSS Inc, Chicago, Illinois).
RESULTS
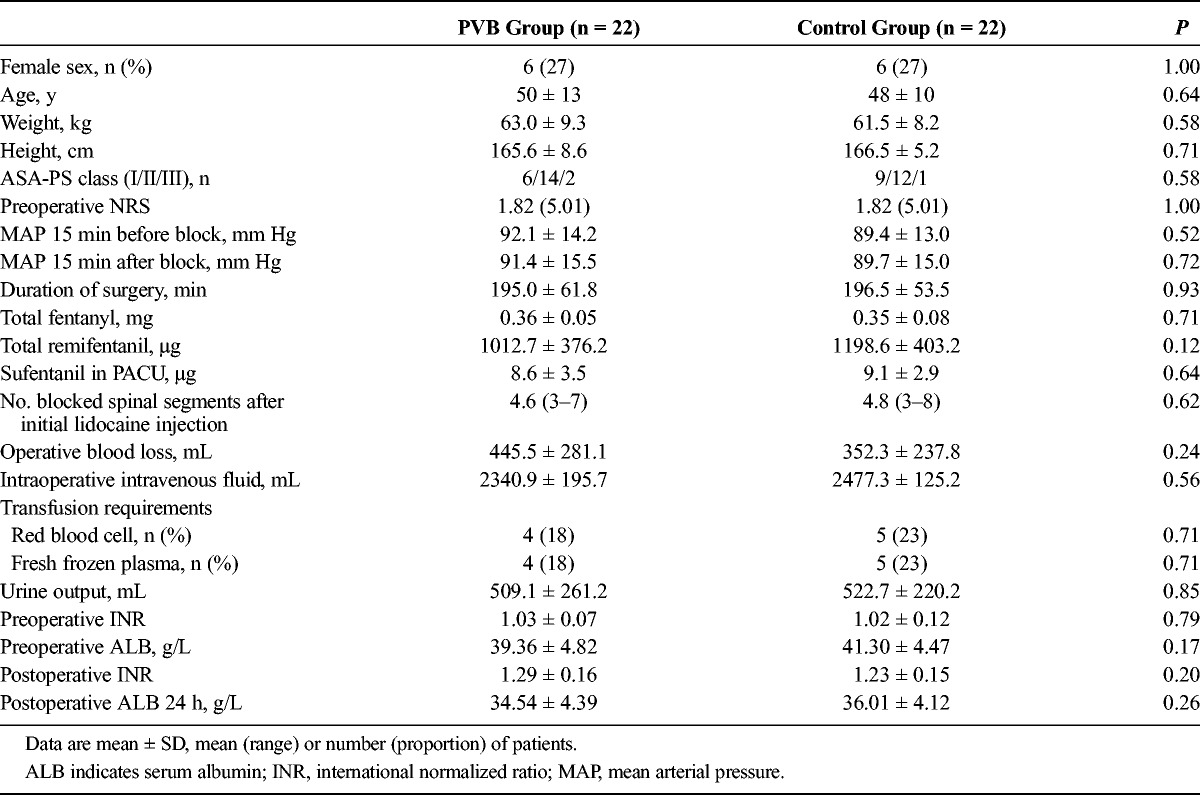
A total of 66 patients were assessed for eligibility, 9 patients did not meet inclusion criteria, and 6 patients refused to participate; PVB failed in 3 patients. A PVB catheter was placed successfully in 48 patients, 4 patients were excluded from final analysis because of catheter obstruction (n = 1), change of operative plan (n = 2), and unplanned postoperative mechanical ventilation (n = 1), respectively. Data were analyzed on 22 patients in each group, as shown in Figure 1. The characteristics of patients, duration of surgery, intraoperative variables, and liver function test indices 24 hours before and after surgery in the paravertebral and control groups are shown in Table 1. The baseline characteristics and intraoperative variables were similar in both groups.
FIGURE 1.
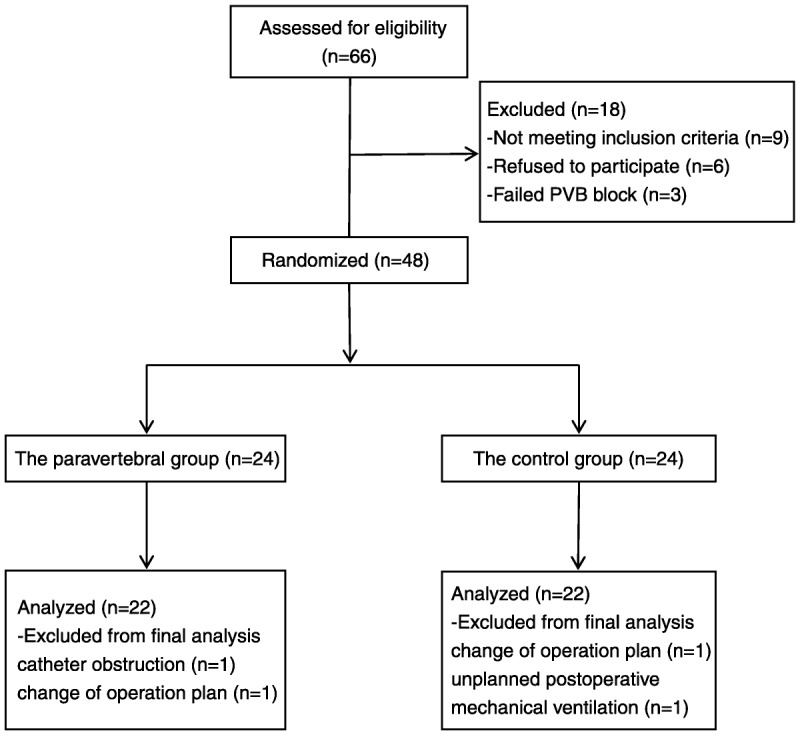
CONSORT patient flow diagram.
TABLE 1.
Patients’ Characteristics, Duration of Surgery, Intraoperative Variables, and Some Indexes of Liver Function 24 Hours Before and After Surgery in the PVB and Control Groups
The time-matched cumulative sufentanil consumption and PCIA pressing frequency after surgery are shown in Figures 2 and 3. The cumulative sufentanil consumption and PCIA pressing frequency at each time point for the control group was significantly higher than the paravertebral group as indicated in Figures 2 and 3. Cumulative sufentanil consumption in the PVB group (54.3 ± 12.1 μg) at 24 postoperative hours was reduced by 20.2% compared with the control group (68.1 ± 9.9 μg), P < 0.001.
FIGURE 2.
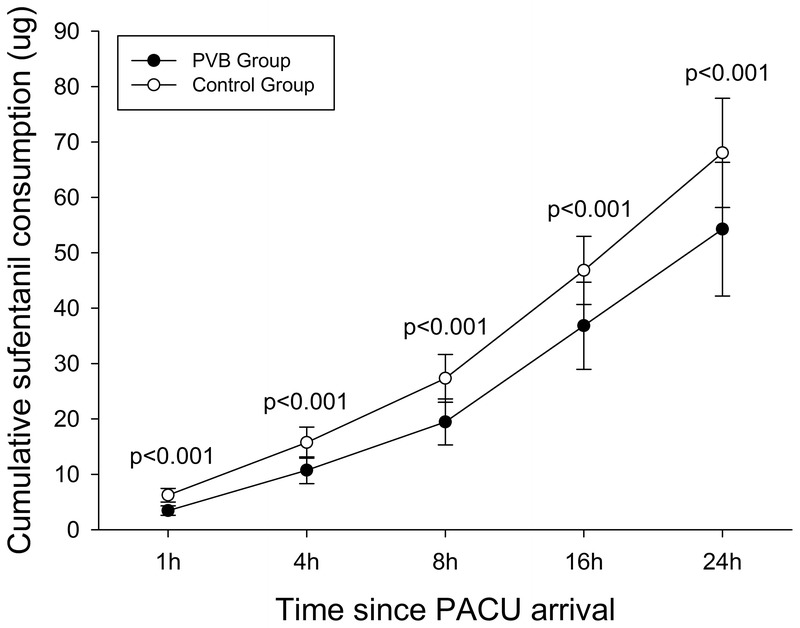
Cumulative sufentanil consumption in the first 24 postoperative hours. Data are presented as mean and SD. Differences of cumulative sufentanil consumption at each time point between the 2 groups were compared with repeated-measures ANOVA followed by Student t test. The Bonferroni correction was performed on the raw P value. Patients in the paravertebral group consumed less sufentanil than did patients in the control group at each time point.
FIGURE 3.
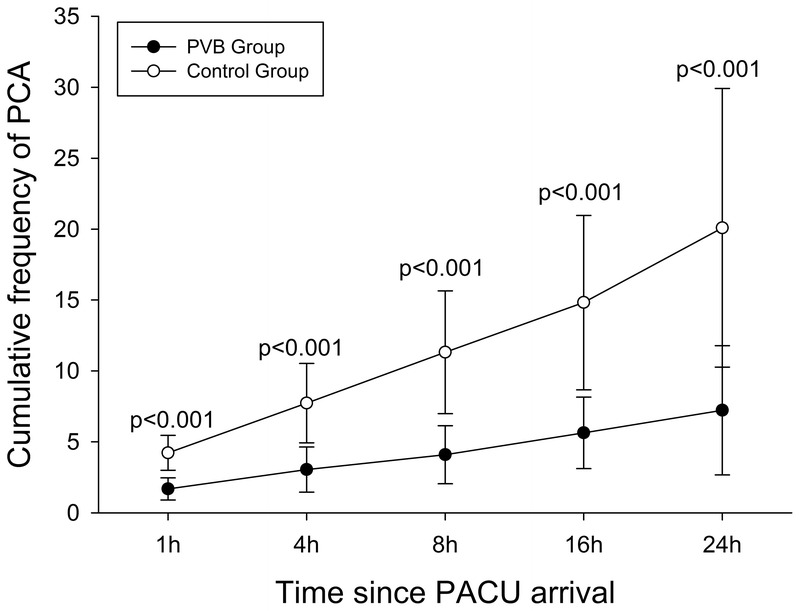
Cumulative frequency of PCIA in the first 24 postoperative hours. Data were presented as mean and SD. Differences of cumulative frequency of PCIA at each time point between the 2 groups were compared with repeated-measures ANOVA followed by Student t test. The Bonferroni correction was performed on the raw P value. The cumulative frequency of PCIA at each time point in the control group was more compared with the paravertebral group.
Pain scores within 24 postoperative hours at rest and with coughing are shown in Figures 4 and 5, respectively. The pain intensity at rest and with coughing reported with the NRS score was significantly lower in the paravertebral group as compared with the control group for each time point. The greatest difference in NRS score was found at 1 hour postoperatively in which the median (first third quartiles) NRS score on coughing was 1 (1–2) in the PVB group and 3 (3–4) in the control group (P < 0.001).
FIGURE 4.
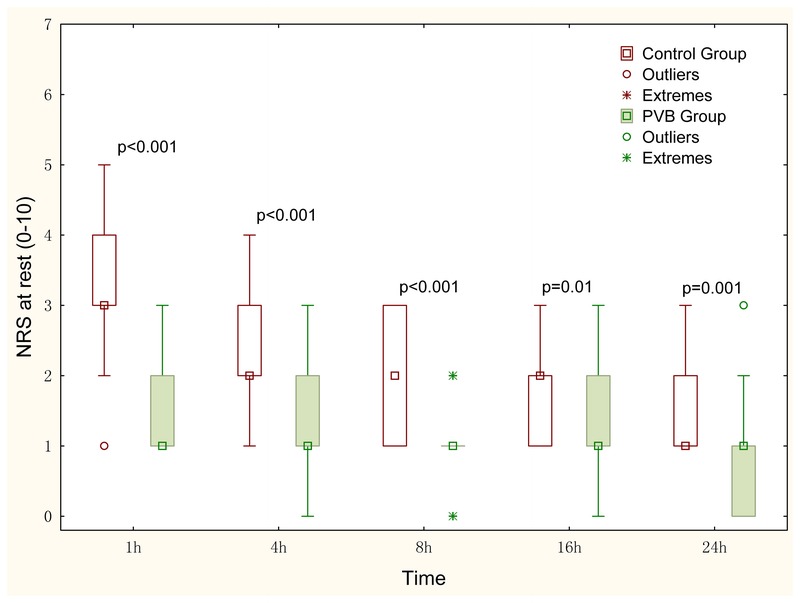
Pain scores at rest in the first 24 postoperative hours. The NRS data are displayed in box-and-whisker plots. Median (quadrate in the box), interquartile range (box), and range not including outliers (error bars) are shown. Outliers (>1.5 box lengths) and extremes (>3 box lengths) are represented by circles and asterisks, respectively. Differences of NRS scores at each time point between the 2 groups were analyzed using the Mann-Whitney U test. The Bonferroni correction was performed on the raw P value. The NRS scores at rest at each time point in the control group were higher compared with the paravertebral group. NRS (0–10), where 0 indicates no pain, and 10 indicates the most severe pain.
FIGURE 5.
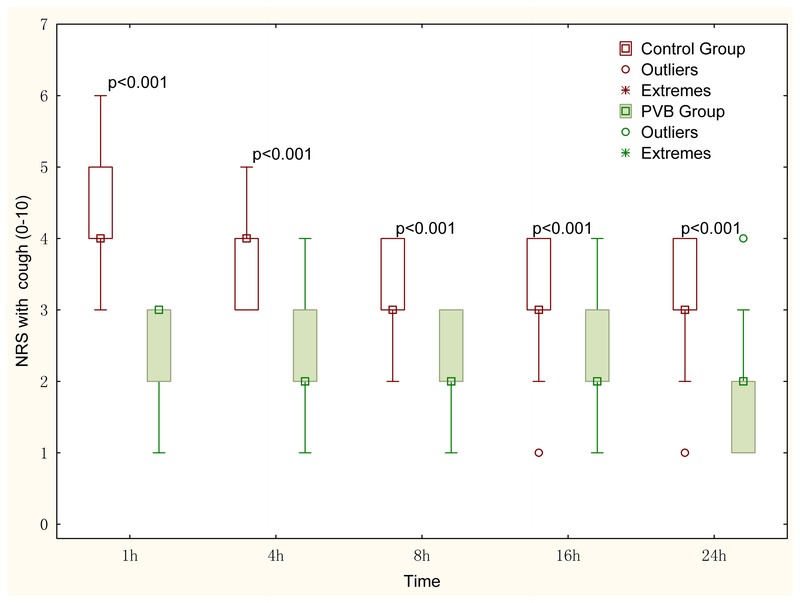
Pain scores on coughing in the first 24 postoperative hours. The NRS data are displayed in box-and-whisker plots. Median (quadrate in the box), interquartile range (box), and range not including outliers (error bars) are shown. Outliers (>1.5 box lengths) and extremes (>3 box lengths) are represented by circles and asterisks, respectively. Differences of NRS scores at each time point between the 2 groups were analyzed using the Mann-Whitney U test. The Bonferroni correction was performed on the raw P value. The NRS scores on coughing at each time point in the control group were higher compared with the paravertebral group. NRS (0–10), where 0 indicates no pain, and 10 indicates the most severe pain.
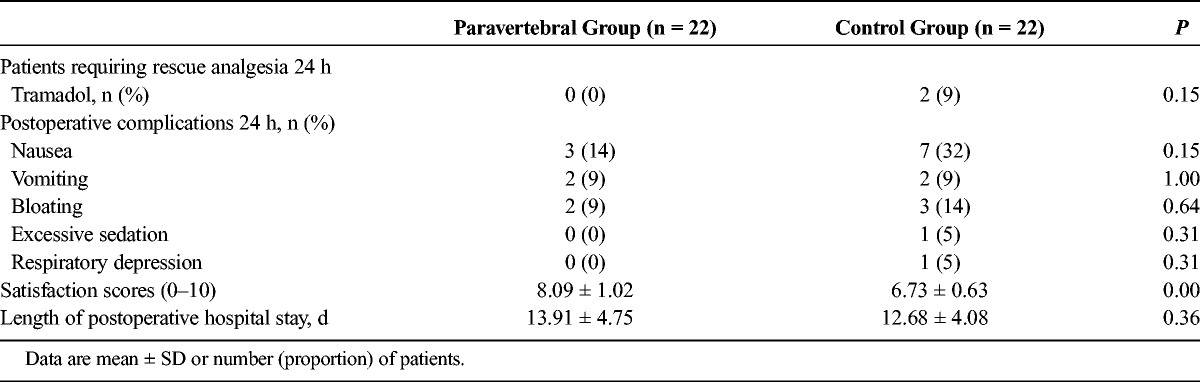
The rescue analgesia, complications, satisfaction scores, and length of postoperative hospital stay after surgery of patients in both groups are shown in Table 2. The incidence of vomiting, bloating, and excessive sedation and the length of hospital stay were comparable between the groups. The patient satisfaction score was 8.1 ± 1.0 in the paravertebral group and 6.7 ± 0.6 in the control group (P < 0.001). One patient in the control group was documented to have an episode of respiratory rate below 8 breaths/min. The PCIA background dose was reduced to 1 μg/h sufentanil, and the respiratory rate returned to an acceptable level.
TABLE 2.
Rescue Analgesia, Complications, Satisfaction, and Length of Hospital Stay After Surgery of Patients in the PVB and Control Groups
Loss of pinprick sensation involved a mean of 4.6 (range, 3–7) dermatomes with upper and lower limits of T4 and T11 in the PVB group. The sensory loss to pinprick was 4.8 (range, 3–8) dermatomes, with upper and lower limits of T4 and T11 in the control group. There was no significant difference in extent of somatic block between the 2 groups following the 15-mL lidocaine testing injection (P = 0.618).
DISCUSSION
In this prospective, randomized, double-blind, placebo-controlled study, a continuous unilateral thoracic PVB, following bolus initiation, produced an opioid-sparing effect within the first 24 hours after right-lobe hepatectomy. Patients receiving continuous thoracic PVB reported significantly lower pain scores than did those with sufentanil PCIA alone. Our results are consistent with previous findings showing that thoracic PVB reduced the severity of postoperative pain or opioid consumption following both minor surgeries such as thoracoscopic surgery,8 breast cancer surgery,9 and major surgeries such as thoracotomy.10
Paravertebral nerve block has been successfully used for pain management for cholecystectomy,11–13 radiofrequency ablation of a metastatic liver lesion,14 percutaneous transhepatic biliary drainage,15 and right-lobe hepatectomy.4 Richardson et al16 have demonstrated that thoracic PVB can abolish the intercostal somatosensory-evoked potentials. Liver capsule injury visceral pain was managed successfully with a continuous infusion of local anesthetic at the right T10 level through a paravertebral catheter,17 whereas common bile duct dilation–associated pain, mediated parasympathetically through the vagus, could not be alleviated by PVB.18 The liver and its capsule are innervated by sympathetic fibers arising from the thoracic sympathetic chain from the T7 to T11 level, and parasympathetic nerves derive from the vagus nerve.19 The right PVB-induced pain reduction following hepatectomy with right-sided subcostal incision may be due to blockade of somatic and sympathetic pain fibers originated from T5 to T11.19 The vagal nerve and contralateral sympathetic fibers are not targeted by unilateral PVB, so total visceral anesthesia may not be achievable. This may be considered a drawback of a unilateral PVB used for pain management of hepatobiliary surgeries. Bilateral PVB technique has been successfully used in thoracic, abdominal, and pelvic regions.20 But bilateral PVB using landmark techniques was found to double the likelihood of inadvertent vascular puncture and to cause an 8-fold increase in pleural puncture and pneumothorax.21 Recent modifications to PVB technique by ultrasound guidance may improve the safety of this technique.
The extent of dermatomal spread of local anesthetics in the paravertebral space is variable and characterized by inconsistent somatic and sympathetic blocks. The mean extent of the somatic block was 4.6 and 4.8 dermatomes in the PVB group and control group, respectively, at 15 minutes after injection of 1% lidocaine 15 mL in the present study. Injections in a multilevel fashion may increase the extent of somatic and sympathetic block but would expose patients to additional risks related to punctures even under the ultrasound guidance. Further studies may be needed to test the clinical safety and efficacy of PVB at multiple levels for pain management after major abdominal surgeries.
There are conflicting results with regard to the use of catheter-based techniques for continuous PVB. Continuous thoracic paravertebral infusion of bupivacaine offered better pain control than a bolus regimen after thoracotomy.22 Ultrasound-guided continuous thoracic PVB has been used for outpatient acute pain management of multilevel unilateral rib fractures.23 However, an unacceptably high misplacement rate of paravertebral catheters has been reported by using a landmark technique recently.24 Catheter displacement may be prevented by the introduction of coiled catheters into clinical practice.25
Because of ethical issues and safety consideration, we have not enrolled patients with impaired coagulation functions that contraindicated epidural analgesia. Common concerns with the PVB are the potential risk for pneumothorax and vascular or neural injury. Paravertebral nerve blocks are technically easy to learn with a high success rate of 89.9% to 93.9% and a relatively low rate of over all complications (5%) and serious complications such as pneumothorax (0.5%).21,26 A recent meta-analysis including 18 randomized controlled trials demonstrated that PVBs, compared with epidural analgesia, are associated with less urinary retention, postoperative nausea and vomiting, and hypotension.27 These results were consistent with previous meta-analysis performed by Davies et al28 in 2006. In addition, the rates of failed technique were also lower in the PVB group than those in the epidural analgesia group.27 Paravertebral nerve block may offer an attractive alternative to the epidural technique for patients with local sepsis, coagulation disorders, preexisting neurological disorders, and abnormal thoracic vertebral anatomy. A comparison between the standard epidural analgesia and ultrasound-guided PVB would be meaningful and warrants further studies.
Patients in the PVB group had longer length of stay, and this may be due to differences in the American Society of Anesthesiologists–Physical Status (ASA-PS) class, age, and blood loss. Although these differences between groups were not statistically significant, together they may have accounted for longer hospitalization.
This study has several limitations. Although a difference in the primary end point was shown, we were unable to demonstrate a difference in secondary outcome measures because of the small sample size. According to previous studies, we arbitrarily defined block success as loss of pinprick sensation in 3 or more ipsilateral vertebral dermatomes.6 After liver resection, the possibility of extending the analgesic benefits of paravertebral analgesia beyond 24 hours with or without a more concentrated solution of local anesthetic warrants further investigation. Further larger-scale studies are required to evaluate the effect of paravertebral block for right-lobe hepatectomy on clinically important outcomes including ropivacaine plasma levels, the incidence of postoperative complications, and chronic pain syndrome. Because sufentanil and ropivacaine are metabolized hepatically, elevated plasma levels can be seen after liver surgery.29,30 For safety concerns and in the absence of measurement of plasma levels of these medications, we limited the PVB infusion to 24 hours. This makes it more difficult to distinguish the effect of the initial ropivacaine PVB bolus from the continuous infusion on reducing pain scores and opioid consumption. It has been reported that single-shot PVB with 0.5% ropivacaine or 0.5% bupivacaine can produce 6- to 8-hour analgesic effects after thoracoscopic procedures.31,32 At the end of surgery, the 10-mL bolus of 0.2% ropivacaine prior to active infusion may have produced a significant opioid-sparing effect for an indeterminate number of hours out of the first 24 postoperative hours. The pain alleviating and opioid-sparing effects of PVBs in the first 24 postoperative hours demonstrated by our study may be the results of both the initiation bolus injection and the following continuous infusion of 0.2% ropivacaine.33,34 The present study aimed to test the hypothesis that a continuous PVB protocol (bolus initiation followed by continuous infusion of 0.2% ropivacaine) can reduce opioid consumption during the first 24 hours after right-lobe hepatectomy. We are unable to separate the effect of the initial bolus from the continuous infusion without having avoided the bolus, or running the infusion long enough to be certain there is no residual effect from the initial bolus. Obviously, keeping PVB catheter after single-dose PVB allows feasible rescue bolus injection or continuous infusion of local anesthetics when needed. Comparing the analgesic effects of bolus PVB, continuous PVB without bolus initiation, and bolus initiation followed by continuous infusion is not the primary interests of the present work. This issue clearly warrants further studies. Compared with patients from Western countries, the patients in our study have smaller body size, younger age, and lower ASA-PS scores. This unique demographic profile may impair the generalizability of our results, and further studies are needed to confirm the reproducibility of our technique in more overweight and/or more debilitated patients.
In conclusion, this is the first prospective randomized, double-blind, placebo-controlled study evaluating the analgesic efficacy of continuous thoracic paravertebral analgesia in patients undergoing right-lobe hepatectomy. Although thoracic epidural analgesia and interpleural analgesia after hepatectomy have been reported, the use of paravertebral analgesia for hepatectomy patients has rarely been reported.4,35 Our data demonstrated that continuous thoracic paravertebral block, following bolus initiation, is an effective procedure to improve pain treatment after right-lobe hepatectomy and could be incorporated into multimodal analgesic regimens for right-lobe hepatectomy with a right-sided subcostal incision.
Footnotes
This work was funded in part by grants from National Natural Science Foundation of People’s Republic of China (no. 31000417 to W.M.) and National Clinical Key Disciplines Construction grant (to Y.T.) from the Ministry of Health of the People’s Republic of China.
The authors declare no conflict of interest.
REFERENCES
- 1. Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001; 87: 47– 61 [DOI] [PubMed] [Google Scholar]
- 2. Rawal N. Epidural technique for postoperative pain: gold standard no more? Reg Anesth Pain Med. 2012; 37: 310– 317 [DOI] [PubMed] [Google Scholar]
- 3. Tzimas P, Prout J, Papadopoulos G, Mallett SV. Epidural anaesthesia and analgesia for liver resection. Anaesthesia. 2013; 68: 628– 635 [DOI] [PubMed] [Google Scholar]
- 4. Ho AM, Karmakar MK, Cheung M, Lam GC. Right thoracic paravertebral analgesia for hepatectomy. Br J Anaesth. 2004; 93: 458– 461 [DOI] [PubMed] [Google Scholar]
- 5. Renes SH, Bruhn J, Gielen MJ, Scheffer GJ, van Geffen GJ. In-plane ultrasound-guided thoracic paravertebral block: a preliminary report of 36 cases with radiologic confirmation of catheter position. Reg Anesth Pain Med. 2010; 35: 212– 216 [DOI] [PubMed] [Google Scholar]
- 6. Lonnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995; 50: 813– 815 [DOI] [PubMed] [Google Scholar]
- 7. Heisterkamp J, Marsman HA, Eker H, et al. A J-shaped subcostal incision reduces the incidence of abdominal wall complications in liver transplantation. Liver Transpl. 2008; 14: 1655– 1658 [DOI] [PubMed] [Google Scholar]
- 8. Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005; 95: 816– 821 [DOI] [PubMed] [Google Scholar]
- 9. Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg. 2004; 99: 1837– 1843 [DOI] [PubMed] [Google Scholar]
- 10. Fortier S, Hanna HA, Bernard A, Girard C. Comparison between systemic analgesia, continuous wound catheter analgesia and continuous thoracic paravertebral block: a randomised, controlled trial of postthoracotomy pain management. Eur J Anaesthesiol. 2012; 29: 524– 530 [DOI] [PubMed] [Google Scholar]
- 11. Giesecke K, Hamberger B, Jarnberg PO, Klingstedt C. Paravertebral block during cholecystectomy: effects on circulatory and hormonal responses. Br J Anaesth. 1988; 61: 652– 656 [DOI] [PubMed] [Google Scholar]
- 12. Kumar CM. Paravertebral block for post-cholecystectomy pain relief. Br J Anaesth. 1989; 63: 129. [DOI] [PubMed] [Google Scholar]
- 13. Naja MZ, Ziade MF, Lonnqvist PA. General anaesthesia combined with bilateral paravertebral blockade (T5-6) vs. general anaesthesia for laparoscopic cholecystectomy: a prospective, randomized clinical trial. Eur J Anaesthesiol. 2004; 21: 489– 495 [DOI] [PubMed] [Google Scholar]
- 14. Culp WC, Payne MN, Montgomery ML. Thoracic paravertebral block for analgesia following liver mass radiofrequency ablation. Br J Radiol. 2008; 81: e23– e25 [DOI] [PubMed] [Google Scholar]
- 15. Culp WC, McCowan TC, DeValdenebro M, et al. Paravertebral block: an improved method of pain control in percutaneous transhepatic biliary drainage. Cardiovasc Intervent Radiol. 2006; 29: 1015– 1021 [DOI] [PubMed] [Google Scholar]
- 16. Richardson J, Jones J, Atkinson R. The effect of thoracic paravertebral blockade on intercostal somatosensory evoked potentials. Anesth Analg. 1998; 87: 373– 376 [DOI] [PubMed] [Google Scholar]
- 17. Hall H, Leach A. Paravertebral block in the management of liver capsule pain after blunt trauma. Br J Anaesth. 1999; 83: 819– 821 [DOI] [PubMed] [Google Scholar]
- 18. Culp WC, Jr, Culp WC. Thoracic paravertebral block for percutaneous transhepatic biliary drainage. J Vasc Interv Radiol. 2005; 16: 1397– 1400 [DOI] [PubMed] [Google Scholar]
- 19. Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004; 280: 827– 835 [DOI] [PubMed] [Google Scholar]
- 20. Richardson J, Lonnqvist PA, Naja Z. Bilateral thoracic paravertebral block: potential and practice. Br J Anaesth. 2011; 106: 164– 171 [DOI] [PubMed] [Google Scholar]
- 21. Naja Z, Lonnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001; 56: 1184– 1188 [DOI] [PubMed] [Google Scholar]
- 22. Catala E, Casas JI, Unzueta MC, et al. Continuous infusion is superior to bolus doses with thoracic paravertebral blocks after thoracotomies. J Cardiothorac Vasc Anesth. 1996; 10: 586– 588 [DOI] [PubMed] [Google Scholar]
- 23. Murata H, Salviz EA, Chen S, Vandepitte C, Hadzic A. Case report: ultrasound-guided continuous thoracic paravertebral block for outpatient acute pain management of multilevel unilateral rib fractures. Anesth Analg. 2013; 116: 255– 257 [DOI] [PubMed] [Google Scholar]
- 24. Luyet C, Siegenthaler A, Szucs-Farkas Z, et al. The location of paravertebral catheters placed using the landmark technique. Anaesthesia. 2012; 67: 132– 1326 [DOI] [PubMed] [Google Scholar]
- 25. Luyet C, Meyer C, Herrmann G, et al. Placement of coiled catheters into the paravertebral space. Anaesthesia. 2012; 67: 250– 255 [DOI] [PubMed] [Google Scholar]
- 26. Richardson J, Lonnqvist PA. Thoracic paravertebral block. Br J Anaesth. 1998; 81: 230– 238 [DOI] [PubMed] [Google Scholar]
- 27. Ding X, Jin S, Niu X, et al. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One. 2014; 9: e96233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006; 96: 418– 426 [DOI] [PubMed] [Google Scholar]
- 29. Rudin A, Lundberg JF, Hammarlund-Udenaes M, Flisberg P, Werner MU. Morphine metabolism after major liver surgery. Anesth Analg. 2007; 104: 1409– 1414 [DOI] [PubMed] [Google Scholar]
- 30. Chan SK, Lai PB, Li PT, et al. The analgesic efficacy of continuous wound instillation with ropivacaine after open hepatic surgery. Anaesthesia. 2010; 65: 1180– 1186 [DOI] [PubMed] [Google Scholar]
- 31. Hill SE, Keller RA, Stafford-Smith M, et al. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology. 2006; 104: 1047– 1053 [DOI] [PubMed] [Google Scholar]
- 32. Zhang W, Fang C, Li J, et al. Single-dose, bilateral paravertebral block plus intravenous sufentanil analgesia in patients with esophageal cancer undergoing combined thoracoscopic-laparoscopic esophagectomy: a safe and effective alternative. J Cardiothorac Vasc Anesth. 2014; 28: 978– 984 [DOI] [PubMed] [Google Scholar]
- 33. Kotze A, Scally A, Howell S. Efficacy and safety of different techniques of paravertebral block for analgesia after thoracotomy: a systematic review and metaregression. Br J Anaesth. 2009; 103: 626– 636 [DOI] [PubMed] [Google Scholar]
- 34. Ilfeld BM, Madison SJ, Suresh PJ, et al. Treatment of postmastectomy pain with ambulatory continuous paravertebral nerve blocks: a randomized, triple-masked, placebo-controlled study. Reg Anesth Pain Med. 2014; 39: 89– 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moussa AA. Opioid saving strategy: bilateral single-site thoracic paravertebral block in right lobe donor hepatectomy. Middle East J Anesthesiol. 2008; 19: 789– 801 [PubMed] [Google Scholar]


