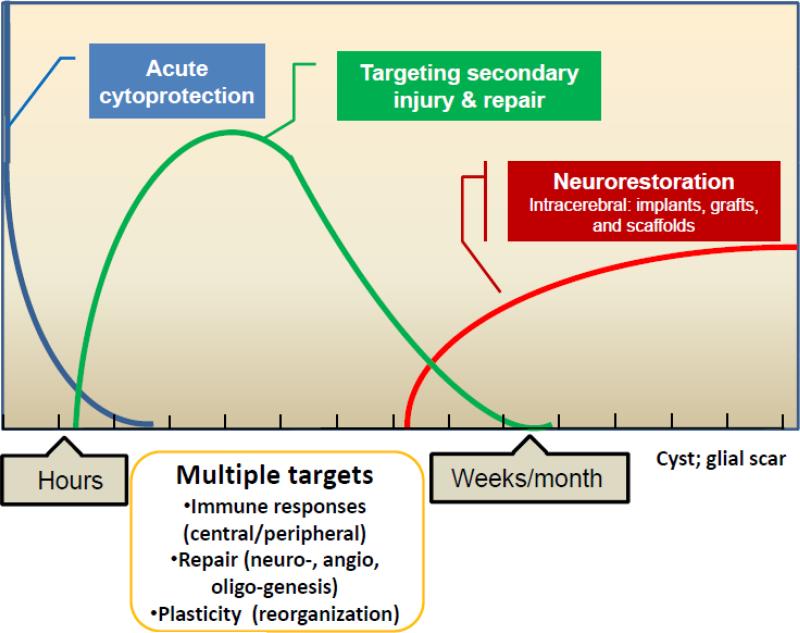
Therapeutic Phases of Stroke: Where do Cell Therapies Theoretically Fit?
During the hyper-acute stage of ischemic stroke, recanalization is the only approved therapeutic approach to improve outcome but is only applicable to the first few hours after symptom onset. There may, however, be other therapeutic windows to consider in the days to weeks after stroke. The inflammatory response after ischemic injury involves multiple interconnected processes both within the brain and in the periphery. During this same time period, there is a cascade of molecular signaling events leading to angiogenesis, neurogenesis, and axonal sprouting 1. Furthermore, neuroplasticity occurs not only at the molecular and cellular level within the peri-infarct zone but also in areas remote from the infarct. Certain types of stem and progenitor cells represent a unique category of therapeutic opportunities because they may attenuate the post-ischemic pro-inflammatory response and may also engage, facilitate, and/or amplify many of the repair mechanisms operating during this acute to subacute stage of stroke (Figure). The temporal window for cell therapies during this time frame is not well-defined and likely depends on the intended targets, cell type, and delivery route.
Figure 1.
Various temporal windows can be envisioned that provide different mechanistic targets for cell therapies in stroke.
In the chronic period of stroke months after symptom onset, the potential therapeutic applications of cell therapies are also being studied in a number of different ways (Figure). The route of delivery has principally involved a stereotactic intracranial administration, with the intent of placing therapeutic cells in the infarct area. In fact, in the 1990s, there were preliminary clinical trials studying intracranial delivery of neural cells in patients with chronic stroke2, 3, studies that were designed in the wake of fetal transplantation approaches for patients with Parkinson's disease. The goal of these pilot trials was to graft neural cells in the infarct cavity but current intracranial clinical studies have been designed with a different intention to implant cells to stimulate local endogenous repair pathways. The concept of grafting cells to restore lost neural circuitry is still in an early stage of pre-clinical development.
Various Cell Types under Investigation for Stroke
While the mechanisms underlying how cell therapies enhance stroke recovery continue to be explored, the number and variety of cell types with therapeutic activity demonstrated in in vitro or in vivo studies has continued to expand in neurological disorders. These cell types can be categorized into embryonic, fetal, birth, and adult tissues. Among the most studied, the investigation of adult derived cell therapies has surged over the past 10 years when it was discovered that the bone marrow harbored mesenchymal stromal cells (MSCs) and hematopoietic stem cells that exert therapeutic effects in rodent stroke models, even when administered by intravenous delivery4, 5. Subsequently, a range of either purified cell types or mixed cell types from bone marrow, umbilical cord, adipose, and other tissues have been shown to improve neurological outcome in rodents with stroke. Our regenerative program has principally focused on bone marrow cells for autologous applications where there are less ethical and practical concerns and less regulatory hurdles compared with embryonic and fetal tissues. However, advances in stem cell biology have recently made it possible to manufacture a number of purified cell types from different tissues, paving the way for the application of allogeneic “off the shelf” cell products that may not require concomitant immunosuppressive drugs.
Bone marrow and stroke repair
The bone marrow is an attractive source to derive or extract cells with therapeutic activity. Several studies have shown that the marrow contains a number of different stem cells involved in tissue homeostasis and repair.6 After stroke, the bone marrow releases different types of stem cells including hematopoietic stem cells as well as mesenchymal and endothelial progenitor cells into the circulation. Once released into the bloodstream, some of these progenitor cells may even home to sites of ischemic injury in the brain.6 Thus, it is hypothesized that the bone marrow may participate in brain repair by mobilizing and releasing stem cells but this concept still requires much further study. It is unclear how exactly stem cells (and in what proportion are they released) enter the brain after injury and stimulate recovery processes.
Bone marrow mononuclear cells for stroke
Our group became interested in the mononuclear fraction of bone marrow because this component of the marrow is enriched with different stem cell populations and by the time we began exploring their therapeutic potential for stroke, a number of trials involving patients with acute myocardial infarction were suggesting that mononuclear cells (MNCs) improve left ventricular function.7 Mononuclear cells can be harvested from the marrow within hours, do not require culture, and can be immediately re-infused by systemic administration. MNCs are therefore suited for autologous applications in the acute setting. In contrast, the manufacture of a more purified progenitor population within the MNCs, for example, MSCs requires several passages in cell culture and cannot be applied in an autologous manner for acute injuries. However, it is important to point out that MNCs are a mixture of different cell types and principally contain immature and mature cells in the myeloid and lymphoid lineages; it is unclear which subpopulations within MNCs contribute to their potential benefits. Among the various pre-clinical studies on MNCs, investigators from different independent laboratories around the world have tested donor derived syngeneic or autologous MNCs in a range of stroke models from mice to rats to sheep.8-12 (Table 1). In rodent models, MNCs, when administered by intravenous or intra-arterial delivery, lead to sustained long term reductions in neurological deficits after stroke8-10, 13. We developed a clinically relevant model to simulate a clinical trial in which MNCs are safely extracted from the bone marrow after stroke without causing limb impairment11, 13 Our studies have shown that intravenous autologous MNC infusion enhances recovery when administered within 3 days after stroke and that MNC-treated animals show a reduction in infarct maturation11. Other laboratories have found that MNCs may reduce neurological deficits in a different model when administered up to 7 days after stroke.14 A similar therapeutic window within 3 days after stroke has been found testing the mononuclear fraction of umbilical cord cells in rodent stroke.15, 16
Table 1.
Summary of preclinical data on bone marrow mononuclear Cells. These studies represent a summary from seven different independent laboratories that are investigator-initiated and not industry sponsored.
| Cells | Autologous or donor-derived (syngeneic). Not allogeneic |
|---|---|
| Species | Mice, rat, sheep |
| Time window | 3 to 7 days, depending on stroke model |
| Types of models | Suture MCAo: photo thrombosis; tandem CCAo/MCAo; endothelin-1; embolic model |
| Routes of Delivery | IV and IA |
| Dose response | 10 million cells/kg optimal hi the CCAo/MCAo model |
| Long term behavioral data | 30 to 60 days after stroke in different models |
| Lesion Size | Reduction in infarct cavity observed in different studies |
| Cytokines/Growth Factors | IGF-1, IL-10, VEGF, bFGF, angiopoeitin, PDGF, SDF-1 |
The mechanisms how any cell therapy exerts therapeutic effects in stroke models still remain poorly understood. After IV administration, MNCs do enter the brain but to a far less extent compared with their presence in the spleen, lungs, and liver11. To assist their entry into the brain, MNCs may release nitric oxide (NO) to dilate vessels and facilitate their passage through the circulation.17 Once in the brain and other organs, MNCs exponentially decrease over the ensuing week because the cells are dying by apoptosis11. These observations suggest that MNCs exert paracrine effects within target organs and then rapidly die off. In support of this hypothesis, we have identified that media derived from cultured MNCs contain a number of bioactive factors and can protect neurons exposed to hypoxia or when placed in contact with activated pro-inflammatory microglia18. Microglia after ischemic stroke, exert both pro and anti-inflammatory effects within the peri-infarct. We have begun to identify that MNCs reduce pro-inflammatory microglia and upregulate anti-inflammatory/neurotrophic microglia (data not shown). Other laboratories have found that MNCs can increase vessel density and neurogenesis19 and protect neurons in the peri-infarct region.8 Overall, the data from multiple laboratories indicate that MNCs, like many other cell therapies such as umbilical cord derived cells, target inflammatory and brain repair mechanisms in the acute to subacute stages of stroke.
Clinical Trials
Because MNCs have been found to exert therapeutic effects in animal stroke models from at least a half dozen independent laboratories, involving three different species (Table), my group has taken forward this particular cell therapy to patients with acute ischemic stroke. Our approach has been to design a trial that closely follows the published animal data including time window, dose response, and delivery route. In our initial pilot study of 10 patients, a limited bone marrow harvest followed by intravenous re-infusion of autologous MNCs within 24 to 72 hrs after stroke onset was feasible and safe. 20 In comparing the 90 day functional outcome of the study patients against historical controls, nearly all the patients fell within or below the range of natural recovery of the historical cohort. We then completed enrollment on a total of 25 patients and have thus far not encountered any definite study related severe adverse events (data not published). Other centers have subsequently reported similar, small studies on the safety of autologous MNCs when administered by intra-arterial injection at later time points after stroke.21
Future
Much remains to be studied with respect to elucidating the key mechanisms underlying how MNCs and other cell therapies enhance stroke recovery. As further safety data are accumulating, more advanced clinical stage trials will be needed to begin testing their potential efficacy for stroke. While MNCs are suited for autologous applications, studies attesting to the safety of manufactured allogeneic cells are escalating and carry the unique advantages of being far more homogenous and immediately available from storage (off the shelf product). The field of cell therapy for stroke is slowly advancing from the bench to the bedside and it is hoped that as more clinical trials are launched, they will be guided by preclinical studies.22
Acknowledgments
Sources of Funding: NIH R-01 NS071127 (SIS), R21 HD060978 (SIS), R21 NS064316 (SIS), T32NS007412, and Howard Hughes Medical Institute.
Footnotes
Disclosures: Received honoraria from Kyorin Pharmaceutical. Consultant to KM Pharmaceutical, Celgene, Mesoblast, and Neuralstem. Conduct sponsored research with Aldagen, Athersys, Celgene, and J&J.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carmichael ST. Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 2008;39:1380–1388. doi: 10.1161/STROKEAHA.107.499962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: A preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20:101–107. doi: 10.1159/000086518. [DOI] [PubMed] [Google Scholar]
- 3.Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. Journal of neurosurgery. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komatsu K, Honmou O, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res. 2010;1334:84–92. doi: 10.1016/j.brainres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Giraldi-Guimaraes A, Rezende-Lima M, Bruno FP, Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.062. (epub) [DOI] [PubMed] [Google Scholar]
- 9.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 10.Baker AH, Sica V, Work LM, Williams-Ignarro S, de Nigris F, Lerman LO, et al. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci U S A. 2007;104:3597–3602. doi: 10.1073/pnas.0611112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boltze J, Nitzsche B, Geiger KD, Schoon H-A. Histopathological investigation of different mcao modalities and impact of autologous bone marrow mononuclear cell administration in an ovine stroke model. Translational Stroke Research. 2012;2:279–293. doi: 10.1007/s12975-011-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr., Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vasconcelos Dos Santos A, da Costa Reis J, Diaz Paredes B, Moraes L, Jasmin, Giraldi-Guimaraes A, et al. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010;1306:149–158. doi: 10.1016/j.brainres.2009.09.094. [DOI] [PubMed] [Google Scholar]
- 15.Boltze J, Schmidt UR, Reich DM, Kranz A, Reymann KG, Strassburger M, et al. Determination of the therapeutic time window for human umbilical cord blood mononuclear cell transplantation following experimental stroke in rats. Cell Transplant. 2012;21:1199–211. doi: 10.3727/096368911X589609. [DOI] [PubMed] [Google Scholar]
- 16.Newcomb JD, Brown WD, Rodriguez AI, Garbuzova-Davis S, Saporta S, Sanberg PR, et al. Behavioral alterations in lewis rats following two-day continuous 3-nitropropionic acid administration. Neurotoxicity research. 2005;8:259–266. doi: 10.1007/BF03033979. [DOI] [PubMed] [Google Scholar]
- 17.Kasam M, Yang B, Strong R, Schaar K, Misra V, Xi X, et al. Nitric oxide facilitates delivery and mediates improved outcome of autologous bone marrow mononuclear cells in a rodent stroke model. PLoS One. 2012;7:e32793. doi: 10.1371/journal.pone.0032793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, et al. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88:2869–2876. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, et al. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010;28:1292–1302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 20.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr., Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich MA, Martins MP, Araujo MD, Klamt C, Vedolin L, Garicochea B, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(Suppl 1):S13–21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- 22.Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem cell therapy as an emerging paradigm for stroke (steps) ii. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]