Abstract
We recently discovered that the antidepressant sertraline is an effective inhibitor of hippocampus presynaptic Na+ channel permeability in vitro and of tonic-clonic seizures in animals in vivo. Several studies indicate that the pro-inflammatory cytokines in the central nervous system are increased in epilepsy and depression. On the other hand inhibition of Na+ channels has been shown to decrease pro-inflammatory cytokines in microglia. Therefore, the possibility that sertraline could overcome the rise in pro-inflammatory cytokine expression induced by seizures has been investigated. For this purpose, IL-1β and TNF-α mRNA expression was determined by RT-PCR in the hippocampus of rats administered once, or for seven consecutive days with sertraline at a low dose (0.75 mg/kg). The effect of sertraline at doses within the range of 0.75 to 25 mg/kg on the increase in IL-1β and TNF-α mRNA expression accompanying generalized tonic-clonic seizures, and increase in the pro-inflammatory cytokines expression induced by lipopolysaccharide was also investigated. We found that under basal conditions, a single 0.75 mg/kg sertraline dose decreased IL-1β mRNA expression, and also TNF-α expression after repeated doses. The increase in IL-1β and TNF-α expression induced by the convulsive agents and by the inoculation of lipopolysaccharide in the hippocampus was markedly reduced by sertraline also. Present results indicate that a reduction of brain inflammatory processes may contribute to the anti-seizure sertraline action, and that sertraline can be safely and successfully used at low doses to treat depression in epileptic patients.
Introduction
Psychiatric disorders, and particularly depression, are known as frequent co-morbidities in patients with epilepsy [1]–[7]. Interestingly, evidence that inflammatory processes take place in both illnesses, namely depression and epilepsy, has been provided. For instance, the hypothesis that inflammation plays an important role in depression, initially suggested by some pioneer studies [8]–[10], was further supported by various meta-analyses indicating that some pro-inflammatory cytokines are increased in patients with major depressive disorders [11]–[13]. The involvement of brain pro-inflammatory cytokines in seizure generation and maintenance, as well as in the establishment of chronic epileptic focuses, has been amply documented [14]–[20].
Voltage sensitive Na+ channels are particularly involved in exacerbating neuronal excitability during seizures. Thus, among the most effective of all anti-epileptic drugs are those capable of decreasing voltage sensitive Na+ channel permeability. In hippocampal isolated nerve endings, sertraline, a drug broadly prescribed for the treatment of depression [21], in addition to its action on the 5-HT transporter, resulted in a potent and effective inhibitor of voltage sensitive Na+ channel permeability [22]. Additionally, in lipopolysaccharide (LPS) stimulated microglia, sertraline inhibited the production of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) [23]. In mixed glial cell cultures the Na+ channel blocker, tetrodotoxin, as well as the classic anti-epileptic drug, phenytoin, whose mechanism of action involves inhibition of Na+ channels, reduced the secretion of the pro-inflammatory cytokines interleukin-1beta (IL-1β) and TNF-α induced by lipopolysaccharide [24]. Therefore, the possible in vivo action of sertraline on the cerebral expression of those inflammatory markers was tested in the hippocampus, which is a highly epileptogenic brain structure. Recently we found that generalized tonic-clonic seizures induced by the pro-convulsive agents pentylenetetrazole (PTZ), and 4-aminopyrydine (4-AP), increased the expression of IL-1β and TNF-α in the rat hippocampus (unpublished results), and that sertraline prevented seizures and the epileptiform EEG activity induced by those pro-convulsive agents [25]. Therefore, we also measured the effect of sertraline on the increase in those pro-inflammatory cytokines induced by seizures.
Materials and Methods
Source of Materials
4-aminopyridine (4-AP) and lipopolysaccharide (LPS, Escherichia coli, serotype 0127:B8) were obtained from Sigma-Aldrich (St. Louis, MO), pentylenetetrazole (PTZ) was obtained from MP Biochemicals Inc. (Aurora, Ohio) and sertraline was kindly donated by Psicofarma S.A. de C.V. (México).
Animal Groups
The present study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Official Mexican Standard (NOM-062-ZOO-1999). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México. All efforts were made to minimize animal suffering.
The 59 male Wistar rats (294±2.5 g initial weight) included in the present study were divided in the 12 groups summarized in Table 1. Briefly, the control Group 1 (G1) was administered either with saline, or with the vehicle used to dissolve sertraline, which consisted of 70% saline and 30% DMSO. No statistical difference in the pro-inflammatory cytokines expression in animals injected with saline or the sertraline vehicle was found. Group 2 (G2) received a single injection of sertraline at a dose of 0.75 mg/kg, and Group 4 (G4) a daily injection of 0.75 mg/kg sertraline for one week. Group 3 (G3) was administered with a daily injection of the sertraline vehicle for one week and used as the control for G4. Group 5 (G5), was administered once with saline followed by 4-AP; Group 6 (G6), was administered once with 0.75 mg/kg sertraline followed by 4-AP, Group 7 (G7) with 0.75 mg/kg sertraline daily for one week, followed by 4-AP, Group 8 (G8) with saline followed by PTZ; Group 9 (G9) with sertraline at a dose of 2.5 mg/kg followed by PTZ, and Group 10 (G10) with 25 mg/kg sertraline followed by PTZ.
Table 1. Experimental Animal Groups.
| Groups | One Injection | Seven Injections | One Injection | ||||||
| Vehicle | Sertraline | Vehicle | Sertraline | 4-AP | PTZ | LPS | |||
| 0.75 mg/kg | 2.5 mg/kg | 25 mg/kg | 0.75 mg/kg | ||||||
| G1 | √ | ||||||||
| G2 | √ | ||||||||
| G3 | √ | ||||||||
| G4 | √ | ||||||||
| G5 | √ | √ | |||||||
| G6 | √ | √ | |||||||
| G7 | √ | √ | |||||||
| G8 | √ | √ | |||||||
| G9 | √ | √ | |||||||
| G10 | √ | √ | |||||||
| G11 | √ | √ | |||||||
| G12 | √ | √ | |||||||
√, indicates injection(s) of the substance(s) specified on the headlines.
4-AP = 2.5 mg/kg 4-aminopyridine; PTZ = 50 mg/kg pentylenetetrazole; LPS = 100 µg/kg lipopolysaccharide.
4-AP and PTZ were both dissolved in saline and injected i.p. at convulsive doses of 2.5 mg/kg and 50 mg/kg, respectively. After injections of 4-AP the animals were observed for 1 h, and after the injection of PTZ for 30 min before euthanasia. These two time points of euthanasia were defined on the basis of our previous study [25].
The effect of sertraline on the changes induced by LPS in the hippocampal IL-1β and TNF-α mRNA expression was tested in two additional groups: Group 11 (G11) that was injected with saline before LPS (100 µg/kg i.p.) inoculation, and Group 12 (G12) that was injected with 2.5 mg/kg sertraline before LPS. The animals in these two groups were sacrificed 1 h following LPS.
In an effort to minimize stress, the animals of all Groups were rapidly decapitated and their hippocampi immediately dissected (see Fig. 1) and frozen.
Figure 1. Hippocampus Dissection.
Following decapitation, the brain was exposed and the hippocampus immediately dissected as shown. The whole process takes less than 3 min.
Sertraline was always administered 4 h before 4-AP, PTZ or LPS. This particular time was chosen because sertraline, after 4 h was reported to reach a high concentration in rat brain tissue [26].
RNA Extraction
For isolating the hippocampal RNA, the brains of the animals who were pre-administered with a sertraline vehicle, or sertraline alone, and in combination with the pro-convulsive agents, or LPS were removed, the hippocampus was dissected, placed in sterile tubes containing 1 ml of the TRIzol Reagent (Invitrogen Life Technologies, USA), and frozen at −80°C until used. Total RNA extraction was performed after hippocampus homogenization (15 strokes with a Thomas Scientific AA Teflon homogenizer) according to the manufactureŕs instructions. The RNA samples were suspended in 50 µl of nuclease-free water.
A nanodrop spectrophotometer (Thermo scientific, Wilmington, Delaware, USA) was used to determine the amount and purity of total RNA in each sample. The integrity of the total RNA was assessed by agarose gel electrophoresis, using ethidium bromide staining.
cDNA Synthesis
The cDNA was obtained by reverse transcription of total RNA, using the kit SuperScript III First-Strand Synthesis SuperMix (Invitrogen Life Technologies, USA). For that purpose a small aliquot containing 2 µg of total RNA, suspended in nuclease-free water was mixed with 0.5 µl oligo (dT)20 (50 µM), 0.5 µl annealing buffer, and brought up to a final volume of 4 µl with nuclease-free water. This mixture was incubated at 65°C for 5 min and then chilled with ice. For reverse transcription, 1 µl of III/RNaseOUT™ enzyme mix and 5 µl of the 2X first-strand reaction mix (10 mM MgCl2, and 1 mM of each dNTP) were added before incubation at 50°C for 50 min. The reaction was stopped by heating the mixture at 85°C for 5 min. The cDNA resulting from this procedure was stored at −20°C until use.
Polymerase Chain Reaction (PCR)
The effect of sertraline on the rise of IL-1β and TNF-α mRNA expression induced by the convulsive agents was evaluated by reverse transcription-polymerase chain reaction (RT-PCR) using the kit GoTaq DNA Polymerase. Briefly, 1.5 µl of cDNA (250 ng/µl) were amplified in a mixture containing 2 µl of 5X green buffer, 0.8 µl of MgCl2 (25 mM), 0.25 µl of PCR nucleotide mix (10 mM), 0.5 µl of the sense primer (10 pM), 0.5 µl of the antisense primer (10 pM), 0.05 µl of DNA Polymerase (5 u/µl) and 4.4 µl of sterile Milli-Q water.
PCR reactions were done in an Eppendorf Mastercycler gradient (USA). The temperature cycling conditions were: initial denaturation at 95°C for 5 min, followed by 34 cycles, including denaturation at 94°C for 30 s, primer annealing for 45 s at 58°C for IL-1β and β-actin or at 66°C for TNF-α, and primer extension at 72°C for 1 min. A final primer extension was performed at 72°C for 10 min after which the samples were immediately cooled at 4°C. The nucleotide sequences for the IL-1β primers were: CCA-GGA-TGA-GGA-CCC-AAG-CA (sense) and TCC-CGA-CCA-TTG-CTG-TTT-CC (antisense); and the expected product size was 519 bp (gen bank accession NM_031512.2). The nucleotide sequences for the TNF-α primers were: AAG-CCC-GTA-GCC-CAC-GTC-GTA (sense) and GCC-CGC-AAT-CCA-GGC-CAC-TAC (antisense); and the expected product size was 663 bp (gen bank accession NM_012675.3). The nucleotide sequences for the β-actin primers were: ATC-GTG-GGC-CGC-CCT-AGG-CA (sense) and ACG-TAC-ATG-GCT-GGG-GTG-TTG (antisense); with the expected product size of 302 bp (gen bank accession NM_031144.2).
β-Actin was used as a control to normalize the relative mRNA amount of the amplified cytokines. A negative control in the absence of sample was run together with each transcript.
PCR products were separated by 1.5% agarose gel electrophoresis at 90 volts, stained with ethidium bromide and the resulting bands were quantified by densitometry using a MiniBIS Pro Gel Documentation System (Bio-America, Miami FL, USA) and ImageJ version 1.42 software (National Institute of Health, USA). Results are expressed as relative mRNA level expression (IL-1β/β-actin or TNF-α/β-actin ratio).
Statistical Analysis
One-way analysis of variance (ANOVA) followed by a post hoc Tukey test was used for the statistical evaluations. Statistical analyses were performed with SigmaPlot version 11.0 (Systat Software, Germany). From P<0.05 the differences between data were considered statistically significant.
Results
Acute and Chronic Effect of Sertraline on IL-1β and TNF-α mRNA Expression in the Hippocampus
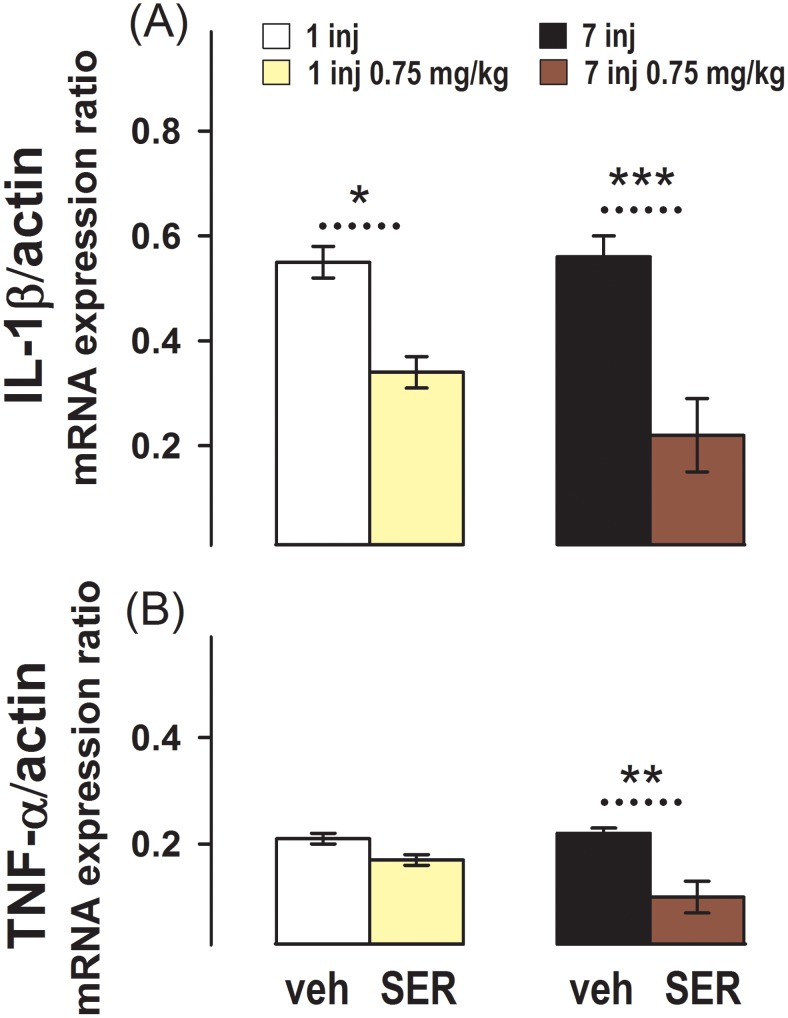
The effect of sertraline injected once or daily for one week on IL-1β and TNF-α messenger expression in the hippocampus is shown in Fig. 2. This figure shows that in the control groups injected with the vehicle once (G1), or 7 times (G3), the baseline mRNA expression of both cytokines is very similar.
Figure 2. Effect of One or Several Sertraline Doses on Pro-inflammatory Cytokines Messengers Expression in the Hippocampus.
Relative IL-1β/β-actin (A) and TNF-α/β-actin (B) mRNA expression measured by RT-PCR in the hippocampi of animals administered with (left bars): a single injection of vehicle (G1) or 0.75 mg/kg sertraline (G2); and in animals administered with (right bars): 7 injections for one week of vehicle (G3) or 0.75 mg/kg sertraline (G4). Results are the mean ± SEM values of 6 (G1 and G3), 4 (G2), or 3 (G4) animals per group. *, p = 0.01 and ***, p<0.001 between the dashed line connecting experimental conditions.
In the animals administered once (G2), or for 7 days (G4) with sertraline 0.75 mg/kg, the expression of IL-1β mRNA was lower than in the respective controls injected once or for 7 days with the vehicle (Fig. 2A).
The expression of TNF-α mRNA was similar in animals injected once with the vehicle (G1), or with 0.75 mg/kg sertraline (G2). Although, in the animals injected daily with 0.75 mg/kg sertraline for one week the TNF-α messenger expression was again lower than in the control animals injected for 7 days with vehicle (Fig. 2B).
No difference between the expression of IL-1β and TNF-α mRNA in the hippocampus from rats administered with saline, or with the vehicle used to dissolve sertraline, namely 70% saline and 30% DMSO, was found. For instance IL-1β expression in animals injected once with saline and with 70% saline and 30% DMSO was 0.57±0.05 (mean ± SEM) and 0.53±0.05, respectively; and in animals injected for 7 days with saline and saline 70%/DMSO 30%, 0.60±0.06 and 0.52±0.05, respectively. In the case of TNF-α mRNA expression in animals injected once with saline, and with the sertraline vehicle was 0.20±0.01 and 0.23±0.01, respectively; and in the animals injected for 7 days, 0.21±0.01 and 0.22±0.02, respectively. Thus, all control animals, namely those injected with saline or the sertraline vehicle, were pooled and referred as “vehicle” in the figures.
Effect of Acute and Chronic Sertraline at a Low Dose on Seizures Induced by 4-AP
Administering 4-AP i.p. at the dose of 2.5 mg/kg 4 h following the injection of the sertraline vehicle induced clonic-tonic seizures with limb extensions in all the rats of Group 5 (G5). The latency and duration of the first tonic-clonic seizure induced by 2.5 mg/kg 4-AP is shown in the first row of Table 2. In the group pre-administered with a single sertraline dose of 0.75 mg/kg 4 h before 4-AP (G6), the latency and duration of the first tonic-clonic seizure induced by 4-AP was similar to the control animals administered with vehicle before 4-AP (second row of Table 2). However, in G7, the group pre-administered daily with 0.75 mg/kg sertraline for one week the administration of 4-AP was unable to induce tonic-clonic seizures at all (bottom row in Table 2).
Table 2. Effect of One and Seven Sertraline Low Doses on 4-AP-Induced Seizures.
| AnimalGroup | AdministeredSubstances | Animalsper Group | Latency § to the 1stTonic-Clonic Seizure | Duration § of the 1stTonic-Clonic Seizure | % of Rats PresentingSeizures |
| G5 | Vehicle followed by 4-AP | (6) | 20.5±2.0 | 1.2±0.2 | 100 |
| G6 | One Sertraline 0.75 mg/kg Dose followed by 4-AP | (4) | 20.5±2.0 | 0.9±0.2 | 100 |
| G7 | Seven Sertraline 0.75 mg/kg Daily Doses followed by 4-AP | (4) | NO Tonic-Clonic Seizures | NO Tonic-Clonic Seizures | 0 |
in min.
4-AP = 2.5 mg/kg 4-aminopyridine.
Results are the mean ± SEM values of the indicated number of animals.
No conspicuous behavioral changes were observed in the groups administered with vehicle once (G1) or 7 times (G3), or with 0.75 mg/kg sertraline once (G2) or 7 times (G4). These groups were not included in Table 2, but the hippocampal mRNA expression of the pro-inflammatory cytokines in these groups was determined in parallel with the groups exposed to the convulsive agent.
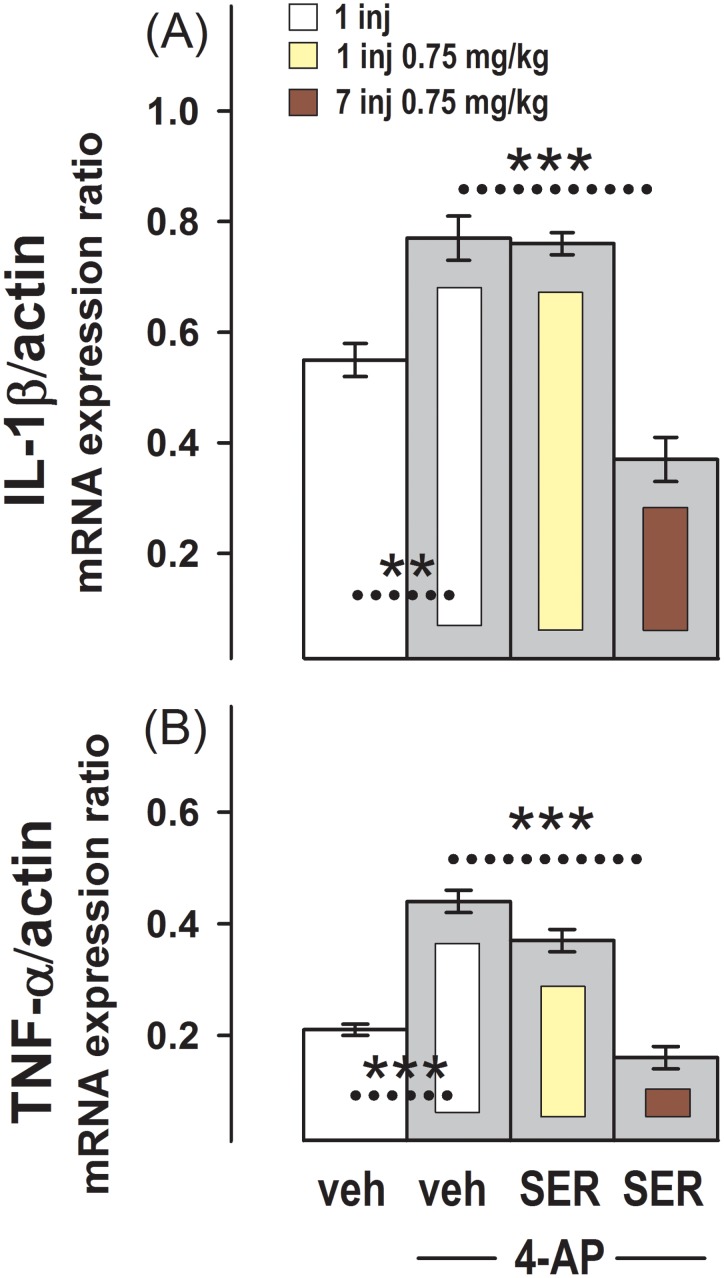
Effect of Sertraline on the Increase in IL-1β and TNF-α mRNA Expression Induced by 4-AP in the Hippocampus
Fig 3 shows that in the group of animals administered with the convulsing agent, 4-AP (G5), the expression of IL-1β and TNF-α mRNA observed in the control group administered once with vehicle (G1) was increased. This increase induced by 4-AP on the expression of both cytokines was also observed in the group administered with the single dose of 0.75 mg/kg sertraline (G6). However, in the group administered for 7 consecutive days with 0.75 mg/kg sertraline (G7), 4-AP was unable to increase IL-1β or TNF-α mRNA expression above the control values observed in animals injected with vehicle.
Figure 3. Effect of One or Several Sertraline Doses on the Increase in IL-1β and TNF-α mRNA Expression Induced by 4-AP in the Hippocampus.
Relative IL-1β/β-actin (A) and TNF-α/β-actin (B) mRNA expression in the groups administered with a single injection of vehicle (G1) or with one daily injection of vehicle for one week (G3), and in groups injected with 2.5 mg/kg 4-AP and pre-administered with: a single injection of vehicle (G5), a single injection of 0.75 mg/kg sertraline (G6), or one daily injection of 0.75 mg/kg sertraline for one week (G7). Results are the mean ± SEM values of 6 (G5) or 4 (G6 and G7) animals per group.*, p = 0.01 and ***, p<0.001 between the dashed line connecting experimental conditions.
Effect of Sertraline on Seizures Induced by PTZ
The convulsive agent PTZ administered i.p. at the dose of 50 mg/kg 4 h after the injection of vehicle also induced clonic-tonic seizures with limb extensions in all the rats of Group 8 (G8). The latency and duration of the first tonic-clonic seizure induced by PTZ in this group is shown in the first row of Table 3. It is notable that 50 mg/kg PTZ induces the first tonic-clonic seizure very rapidly. A single injection of sertraline at a dose of 2.5 mg/kg 4 h before PTZ slightly increased the latency to the first tonic-clonic seizure induced by PTZ. At a dose of 2.5 mg/kg, however, sertraline was unable to prevent the tonic-clonic seizures induced by PTZ in most animals (second row in Table 3). The latency of this group (G9) was only assessed for the animals that had seizures. On the other hand, seizures induced by PTZ were completely suppressed in the group pre-administered with the single injection of 25 mg/kg sertraline 4 h before PTZ (third row in Table 3). No noticeable changes were observed for the 4 h after the single injection of 25 mg/kg sertraline.
Table 3. Effect of Sertraline at Two Doses on PTZ-Induced Seizures.
| Animal Group | AdministeredSubstances | Animals perGroup | Latency § to the 1stTonic-Clonic Seizure | Duration § of the 1stTonic-Clonic Seizure | % of Rats Presenting Seizures |
| G8 | Vehicle + PTZ | (7) | 1.5±0.2 | 1.2±0.2 | 100 |
| G9 | Sertraline 2.5 mg/kg + PTZ | (5) | 3.2±0.6* | 0.8±0.3 | 80 |
| G10 | Sertraline 25 mg/kg + PTZ | (5) | NO Tonic-Clonic Seizures | NO Tonic-Clonic Seizures | 0 |
in min.
PTZ = 50 mg/kg pentylenetetrazole.
Results are the mean ± SEM values of the indicated number of animals.
*, P<0.05 between the animals pre-administered with vehicle and with 2.5 mg/kg sertraline before PTZ.
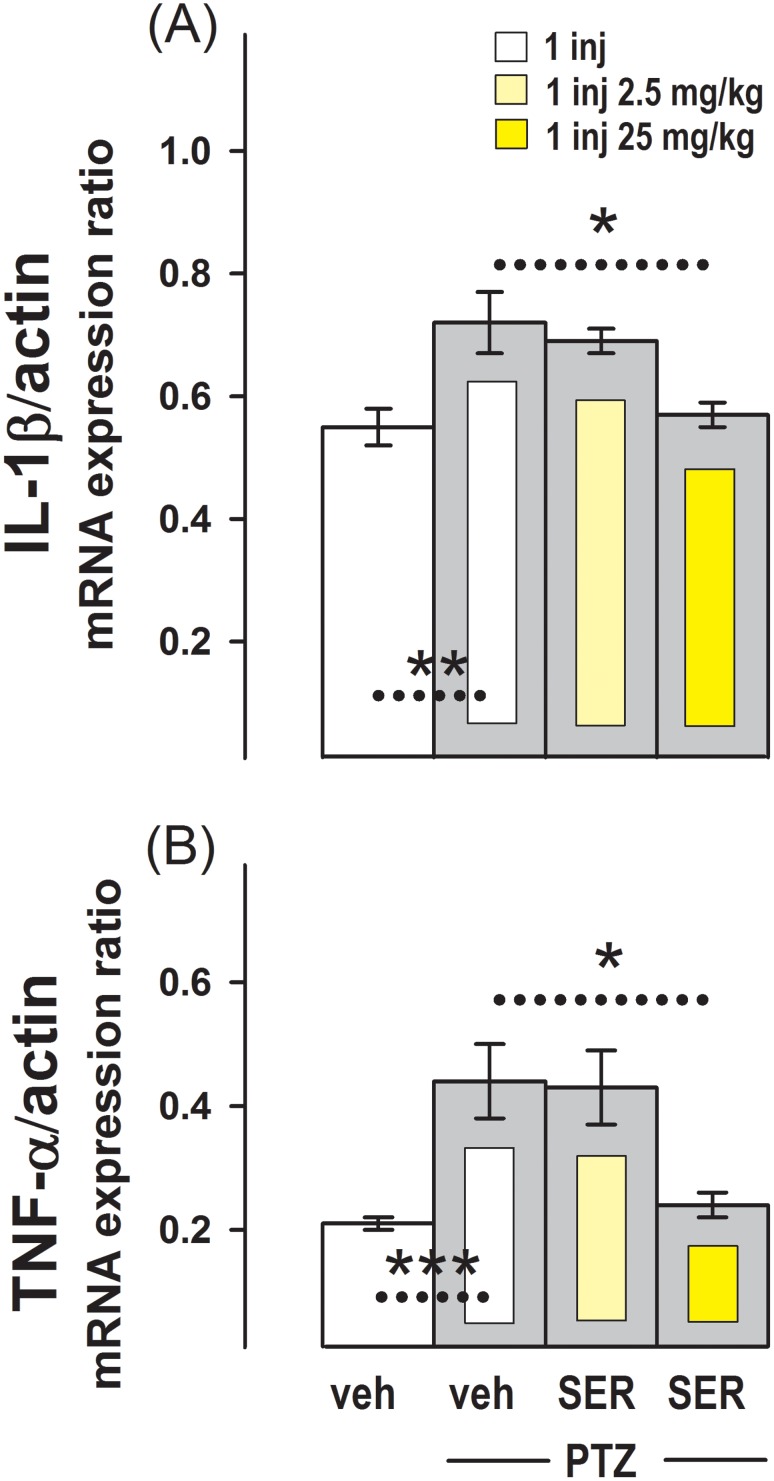
Effect of the Convulsive Agent PTZ on Hippocampal IL-1β and TNF-α mRNA Expression Levels in the Vehicle and in the Sertraline Pre-administered Animals
Fig 4 shows that in the group of animals administered with PTZ (G8), the basal expression of IL-1β and TNF-α mRNA observed in the control group administered once with vehicle (G1) was increased. This increase induced by PTZ on the basal expression of both cytokines was also observed in the group pre-administered with sertraline at a dose of 2.5 mg/kg 4 h before PTZ (G9). However, in the group administered with 25 mg/kg of sertraline the typical increase in the IL-1β and TNF-α mRNA basal expression caused by PTZ was prevented.
Figure 4. Effect of Sertraline on the Increase in IL-1β and TNF-α mRNA Expression Induced by PTZ in the Hippocampus.
Relative IL-1β/β-actin (A) and TNF-α/β-actin (B) mRNA expression in the group administered with the single injection of vehicle (G1), and in groups injected with 50 mg/kg PTZ and pre-administered with single injections of: vehicle (G8), 2.5 mg/kg sertraline (G9), or 25 mg/kg sertraline (G10). Results are the mean ± SEM values of 7 (G8) or 5 (G9 and G10) animals per group. *, p<0.04; **, p<0.01 and ***, p<0.001 between the dashed line connecting experimental conditions.
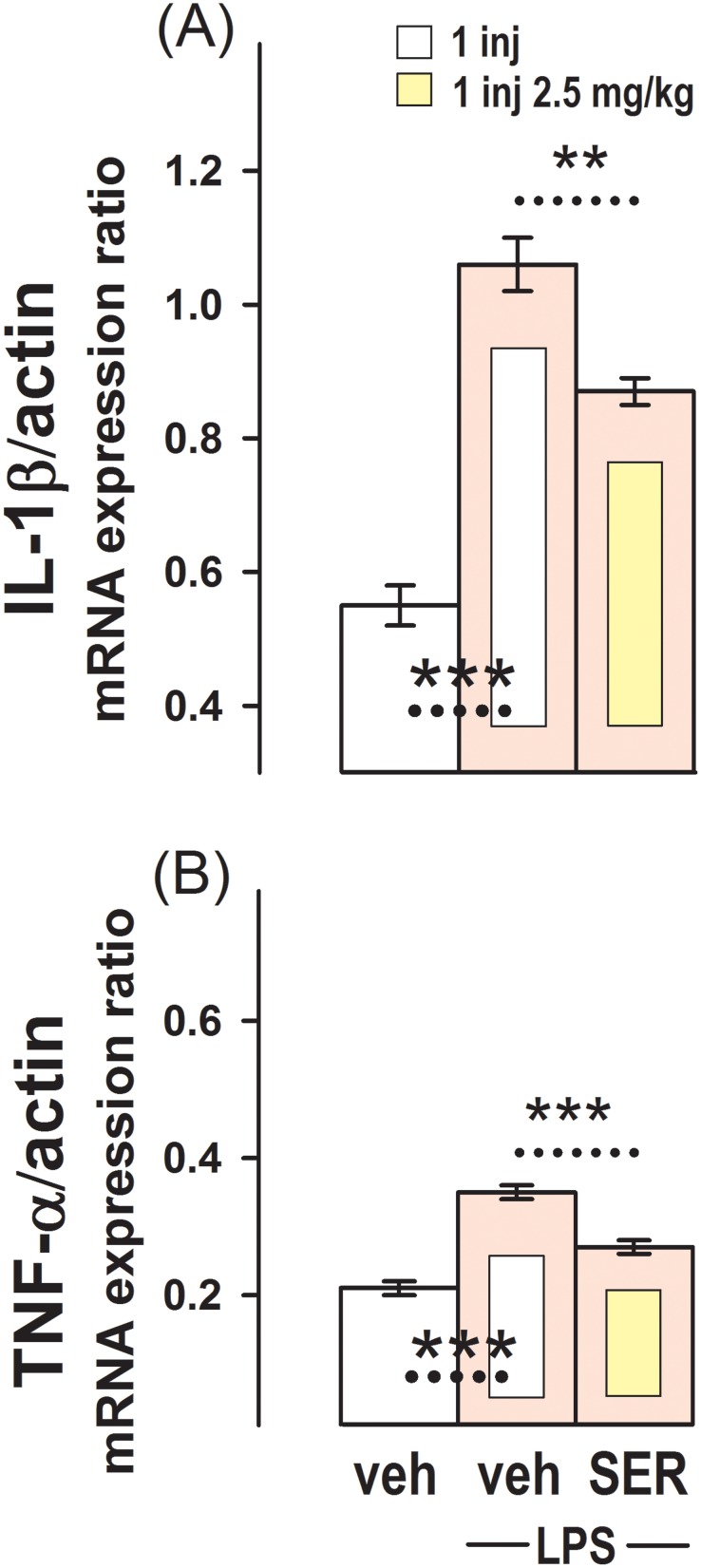
Effect of Sertraline on the Increase in IL-1β and TNF-α mRNA Basal Expression Induced by LPS
The effect of a single dose of 2.5 mg/kg sertraline on the rise in pro-inflammatory cytokines induced by LPS in the hippocampus is shown in Fig. 5. This figure shows that 1 h after the inoculation of LPS, at a dose of 100 µg/kg, the hippocampal mRNA expression of IL-1β and TNF-α in G11 was much higher than in the control group injected once with vehicle (G1). In the group pre-injected once with 2.5 mg/kg sertraline, the increase in the pro-inflammatory cytokines mRNA basal expression induced by the administration of LPS was less noticeable (G12).
Figure 5. Effect of Sertraline on the Increase in IL-1β and TNF-α mRNA Expression Induced by LPS in the Hippocampus.
Relative IL-1β/β-actin (A) and TNF-α/β-actin (B) mRNA expression in the group injected with vehicles (G1) and in groups inoculated with 100 µg/kg LPS and pre-administered vehicles (G11), or 2.5 mg/kg sertraline (G12). Results are the mean ± SEM values of 5 (G11) or 4 (G12) animals per group. **, p<0.01 and ***, p<0.001 between the dashed line connecting experimental conditions.
Discussion
In the present study the capability of the antidepressant sertraline to decrease the basal expression of the pro-inflammatory cytokines IL-1β and TNF-α from basal conditions in the hippocampus, was demonstrated. This finding along with the sensitivity to sertraline of the rise in IL-1β and TNF-α expression induced by LPS, strongly suggest a cerebral anti-inflammatory action of sertraline. In line there is evidence that pro-inflammatory cytokines can be modulated by some anti-depressants [27].
Mechanistic hypothesis of how immune-mediated changes and serotonin levels are involved in depression, as well as critical reviews of the effect of antidepressants on serotonin levels and inflammation, have been provided [28], [29]. However, our recent findings show that besides its action as a serotonin reuptake inhibitor, sertraline is an effective inhibitor of presynaptic Na+ channel permeability [22], and secretion of IL-1β and TNF-α in mixed glial cell cultures, was shown to be inhibited by the Na+ channel blockade [24]. Thus, another mechanistic explanation of how antidepressants, or at least sertraline, can affect inflammation is possible. For instance, it is probable that sertraline can reduce the baseline level of pro-inflammatory cytokines by decreasing the permeability Na+ channels controlling the release of inflammatory markers such as IL-1β and TNF-α.
Among the most effective antiepileptic drugs are those that reduce cerebral excitability, and stop the brain paroxysmal neuronal activity that accompany seizures, by blocking Na+ channels. Although, the potential anti-seizure capability of sertraline has not been explored before, probably because of reports suggesting that some selective serotonin reuptake inhibitors act as pro-convulsive drugs [30]. In fact, there used to be a black box warning in the Physicians Desk Reference (PDR) against their use in patients with epilepsy due to the possibility that they could reduce the seizure threshold, particularly in adolescents.
Although the selective serotonin reuptake inhibitor, sertraline, was needed in a higher dose to prevent the PTZ, than the 4-AP-induced changes, the capability of sertraline to prevent the increase of IL-1β and TNF-α expression, accompanying the generalized tonic-clonic seizures induced by both, 4-AP and PTZ, was also clearly demonstrated in the present study. This different efficacy might again be related with the capability of sertraline to directly decrease Na+ channel permeability [22], because the 4-AP mechanism of action involves several brain presynaptic ion channels, including Na+ channels [31], [32], but the PTZ mechanism of action is primarily due to a decrease in GABAergic transmission [33], [34].
The acute administration of 0.75 mg/kg sertraline to the rat reduced the expression of IL-1β in the hippocampus below basal conditions, but repeated doses were required to reduce TNF-α expression. It is amply recognized that both, anti-depressive and anti-epileptic drugs, are needed to be administered for a certain time before being able to produce their therapeutic action in patients. Administration of 0.75 mg/kg sertraline for several days was required to overcome the generalized tonic-clonic seizures induced by 4-AP, and the increase in IL-1β and TNF-α mRNA expression to 4-AP.
The single sertraline dose of 2.5 mg/kg given to the rat did not prevented the tonic-clonic seizures, and the rise in pro-inflammatory cytokines mRNA expression induced by PTZ, while the single sertraline dose of 25 mg/kg completely overcame the generalized tonic-clonic seizures as well as the increase in IL-1β and TNF-α mRNA expression induced by PTZ. In a previous study [25] we tested the effects of single sertraline doses in ranges from 2.5 to 25 mg/kg on the EEG epileptiform activity induced in the anaesthetized rat by PTZ, and found that a single sertraline dose of 5 mg/kg already prevented the epileptiform activity induced by PTZ in 75% of the animals, and single doses of 10, 15 and 20 mg/kg, completely prevented the EEG epileptiform activity induced by PTZ. Therefore, it is very possible that at doses below 25 mg/kg, sertraline will also be able to prevent the increase in IL-1β and TNF-α mRNA expression induced by PTZ. Moreover, bearing in mind that the higher effectiveness of sertraline after its repeated administration on the 4-AP induced changes, it might be expected that sertraline repeatedly administered will also prevent the increase in pro-inflammatory cytokines mRNA expression induced by PTZ at lower doses.
Several studies strongly suggest that brain pro-inflammatory cytokines may play a significant role in the generation and/or maintenance of seizures, and also in the establishment of chronic epileptic focuses [15], [17], [20]. Therefore, the putative brain anti-inflammatory action of sertraline suggested by present findings, might also contribute importantly to its anti-seizure effect.
Serotonin-reuptake inhibitors are known to decrease TNF-α and IL-1β serum levels in patients with major depressive disorders [35]. Seizures on the other side are known to increase BBB permeability [36]–[38]. The possibility that the drop in pro-inflammatory cytokines exerted by sertraline in the hippocampus was only due to its action on plasma levels, is unlikely. Present findings also show that sertraline decreases IL-1β and TNF-α expression from basal conditions in animals that were not exposed to the pro-convulsive agents, and did not experience seizures.
In summary, we conclude that the decrease in brain excitability and inflammation exerted by sertraline, makes this drug a highly potential alternative to control seizures.
Acknowledgments
The authors thank Araceli Guarneros and Luz María Chiu for their excellent technical assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are contained within the paper.
Funding Statement
This study was financially supported by project IN200614 from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Universidad Nacional Autónoma de México. Carlos D. Gómez and Blanca I. Aldana are PhD students in the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México, and were awarded with a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACYT), México. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Danzer SC (2011) Depression, stress, epilepsy and adult neurogenesis. Exp Neurol 233: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanner AM (2008) Depression in epilepsy: a complex relation with unexpected consequences. Curr Opin Neurol 21: 190–194. [DOI] [PubMed] [Google Scholar]
- 3. Kanner AM, Trimble M, Schmitz B (2010) Postictal affective episodes. Epilepsy Behav 19: 156–158. [DOI] [PubMed] [Google Scholar]
- 4. Mula M (2012) Epilepsy: Bidirectional link between epilepsy and psychiatric disorders. Nat Rev Neurol 8: 252–253. [DOI] [PubMed] [Google Scholar]
- 5. Noe KH, Locke DE, Sirven JI (2011) Treatment of Depression in Patients with Epilepsy. Curr Treat Options Neurol 13: 371–379. [DOI] [PubMed] [Google Scholar]
- 6. Stevanovic D, Jancic J, Lakic A (2011) The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia 52: e75–e78. [DOI] [PubMed] [Google Scholar]
- 7. Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S (2007) Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia 48: 2336–2344. [DOI] [PubMed] [Google Scholar]
- 8. Maes M (1993) A review on the acute phase response in major depression. Rev Neurosci 4: 407–416. [DOI] [PubMed] [Google Scholar]
- 9. Maes M (1995) Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 19: 11–38. [DOI] [PubMed] [Google Scholar]
- 10. Smith RS (1991) The macrophage theory of depression. Med Hypotheses 35: 298–306. [DOI] [PubMed] [Google Scholar]
- 11. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 12. Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Ho RC, Mak A (2011) Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 139: 230–239. [DOI] [PubMed] [Google Scholar]
- 14. De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, et al. (2000) Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 12: 2623–2633. [DOI] [PubMed] [Google Scholar]
- 15. Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ (2005) Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 57: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, et al. (2000) Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res 75: 248–258. [DOI] [PubMed] [Google Scholar]
- 17. Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, et al. (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29: 142–160. [DOI] [PubMed] [Google Scholar]
- 18. Sinha S, Patil SA, Jayalekshmy V, Satishchandra P (2008) Do cytokines have any role in epilepsy? Epilepsy Res 82: 171–176. [DOI] [PubMed] [Google Scholar]
- 19. Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22: 797–803. [DOI] [PubMed] [Google Scholar]
- 20. Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheehan DV, Kamijima K (2009) An evidence-based review of the clinical use of sertraline in mood and anxiety disorders. Int Clin Psychopharmacol 24: 43–60. [DOI] [PubMed] [Google Scholar]
- 22.Aldana BI, Sitges M (2012) Sertraline inhibits presynaptic Na+ channel-mediated responses in hippocampus-isolated nerve endings. J Neurochem 121, 197–205. [DOI] [PubMed]
- 23. Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, et al. (2012) A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun 26: 469–479. [DOI] [PubMed] [Google Scholar]
- 24. Black JA, Liu S, Waxman SG (2009) Sodium channel activity modulates multiple functions in microglia. Glia 57: 1072–1081. [DOI] [PubMed] [Google Scholar]
- 25. Sitges M, Aldana BI, Gomez CD, Nekrassov V (2012) The antidepressant sertraline prevents the behavioral and EEG changes induced in two animal models of seizures. Epilepsy Behav 25: 511–516. [DOI] [PubMed] [Google Scholar]
- 26. Tremaine LM, Welch WM, Ronfeld RA (1989) Metabolism and disposition of the 5-hydroxytryptamine uptake blocker sertraline in the rat and dog. Drug Metab Dispos 17: 542–550. [PubMed] [Google Scholar]
- 27. Janssen DG, Caniato RN, Verster JC, Baune BT (2010) A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol 25: 201–215. [DOI] [PubMed] [Google Scholar]
- 28. Leonard B, Maes M (2012) Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36: 764–785. [DOI] [PubMed] [Google Scholar]
- 29. Walker FR (2013) A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: Do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 67: 304–317. [DOI] [PubMed] [Google Scholar]
- 30. Hamid H, Kanner AM (2013) Should antidepressant drugs of the selective serotonin reuptake inhibitor family be tested as antiepileptic drugs? Epilepsy Behav 26: 261–265. [DOI] [PubMed] [Google Scholar]
- 31. Galvan E, Sitges M (2004) Characterization of the participation of sodium channels on the rise in Na+ induced by 4-aminopyridine (4-AP) in synaptosomes. Neurochem Res 29: 347–355. [DOI] [PubMed] [Google Scholar]
- 32. Sitges M, Sanchez-Tafolla BM, Chiu LM, Aldana BI, Guarneros A (2011) Vinpocetine inhibits glutamate release induced by the convulsive agent 4-aminopyridine more potently than several antiepileptic drugs. Epilepsy Res 98: 257–288. [DOI] [PubMed] [Google Scholar]
- 33. Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, et al. (2001) Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: mechanism and site of action. J Pharmacol Exp Ther 298: 986–995. [PubMed] [Google Scholar]
- 34. Macdonald RL, Barker JL (1977) Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature 267: 720–721. [DOI] [PubMed] [Google Scholar]
- 35. Hannestad J, DellaGioia N, Bloch M (2011) The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36: 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fieschi C, Lenzi GL, Zanette E, Orzi F, Passero S (1980) Effects on EEG of the osmotic opening of the blood-brain barrier in rats. Life Sci 27: 239–243. [DOI] [PubMed] [Google Scholar]
- 37. Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, et al. (2007) Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 48: 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, et al. (2009) Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 33: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are contained within the paper.