Abstract
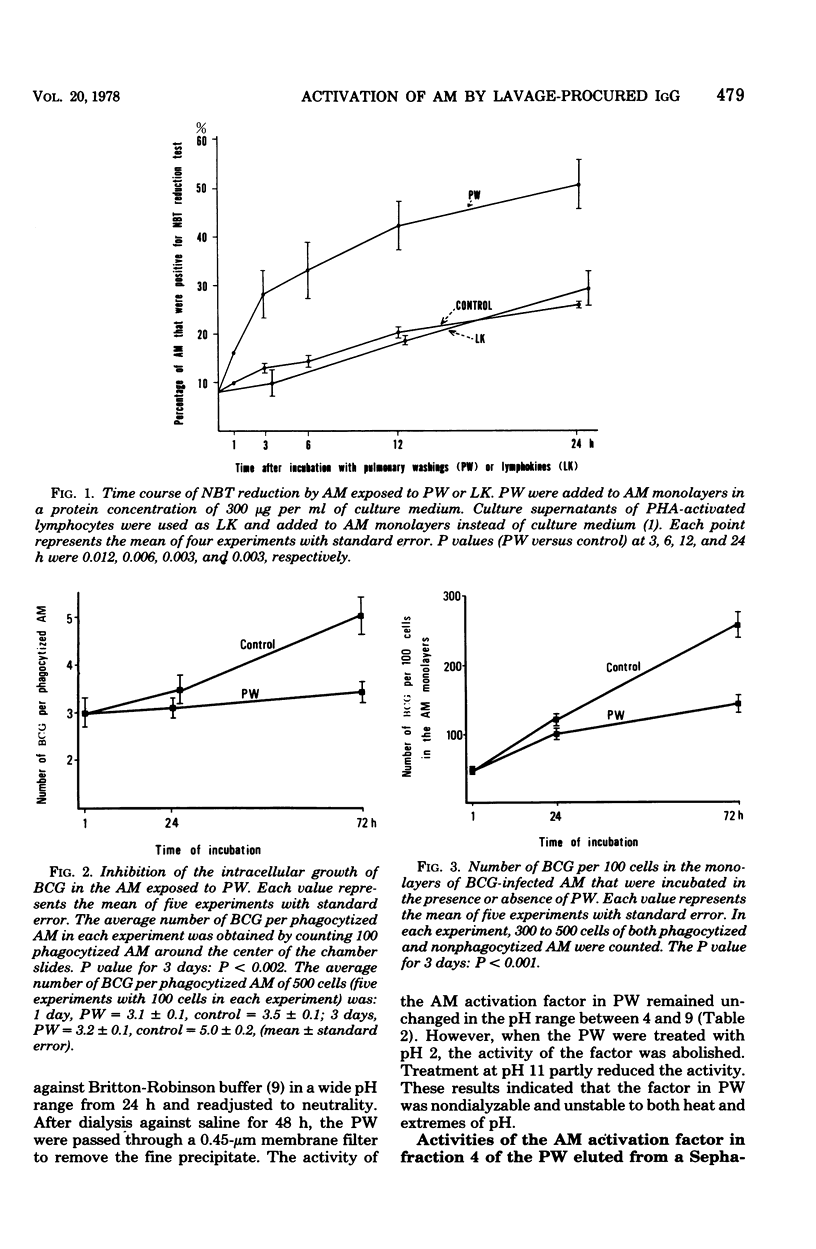
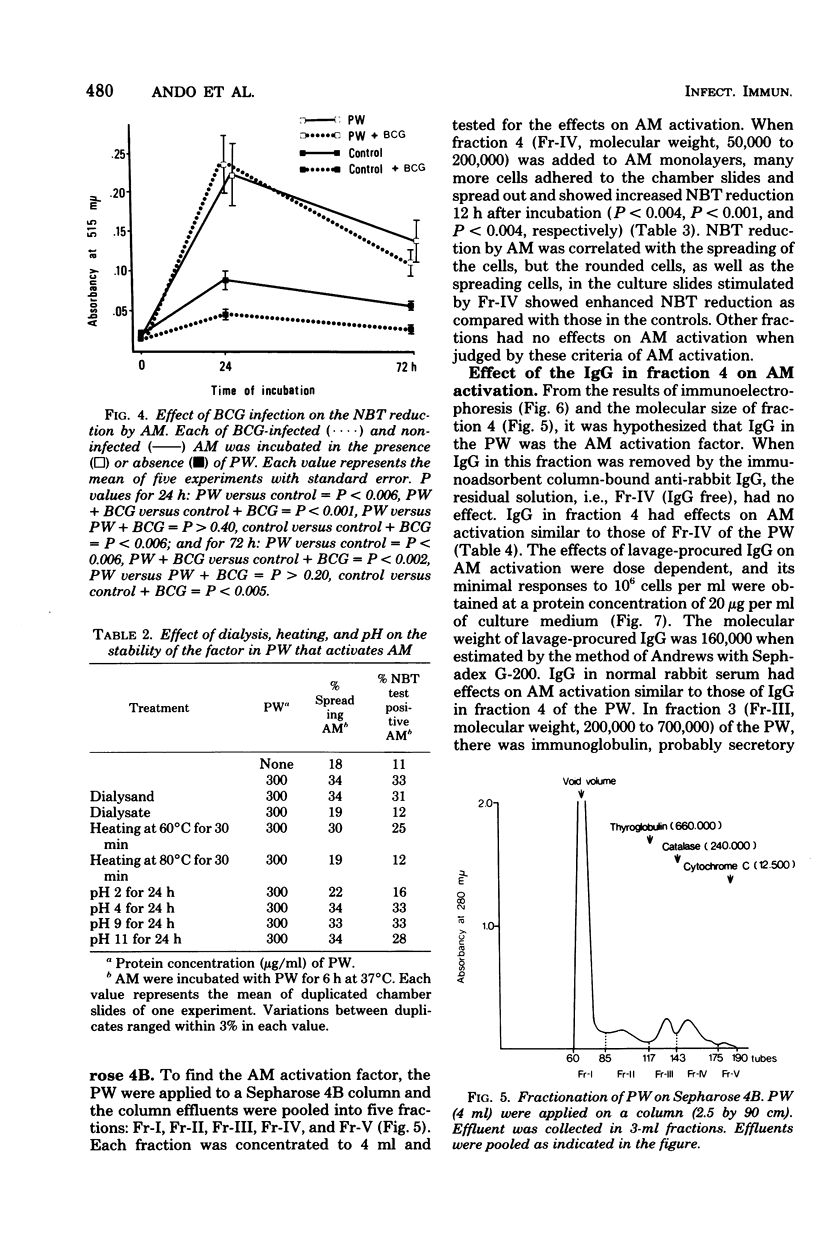
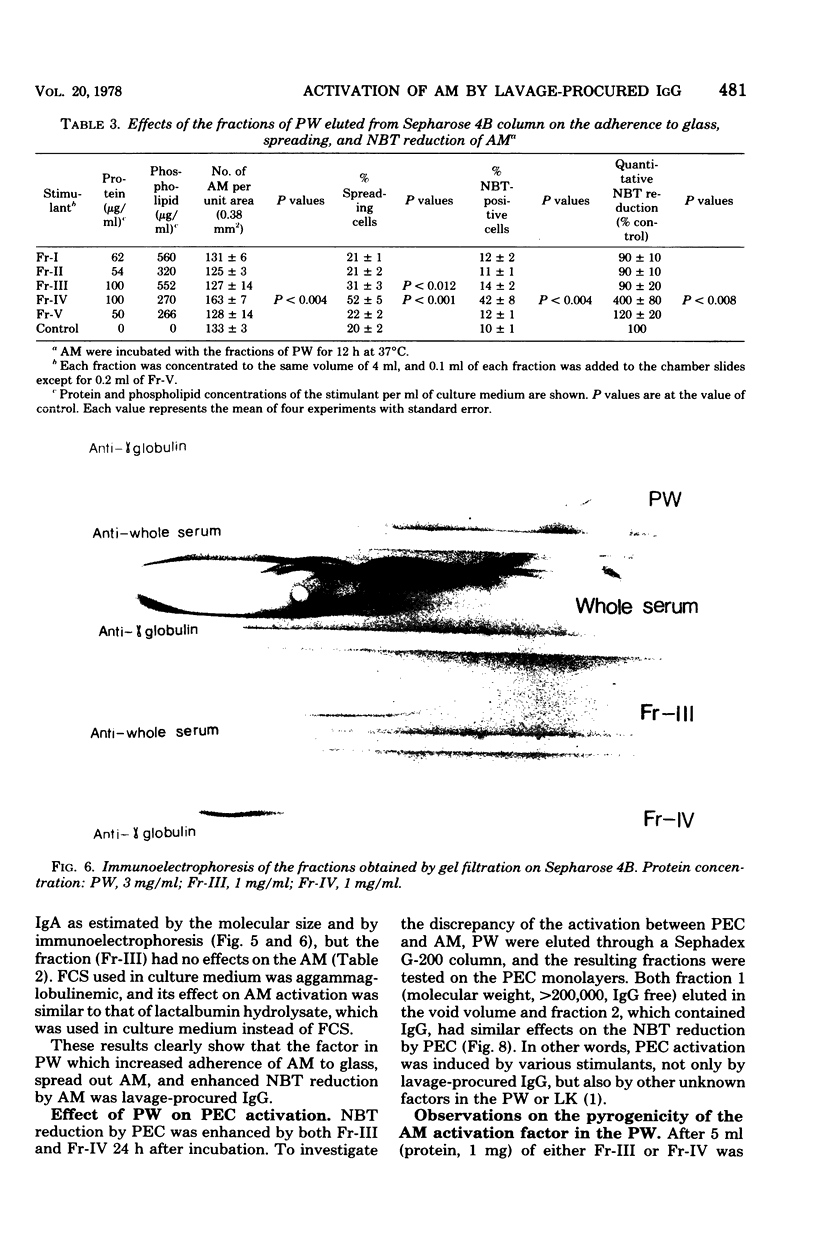
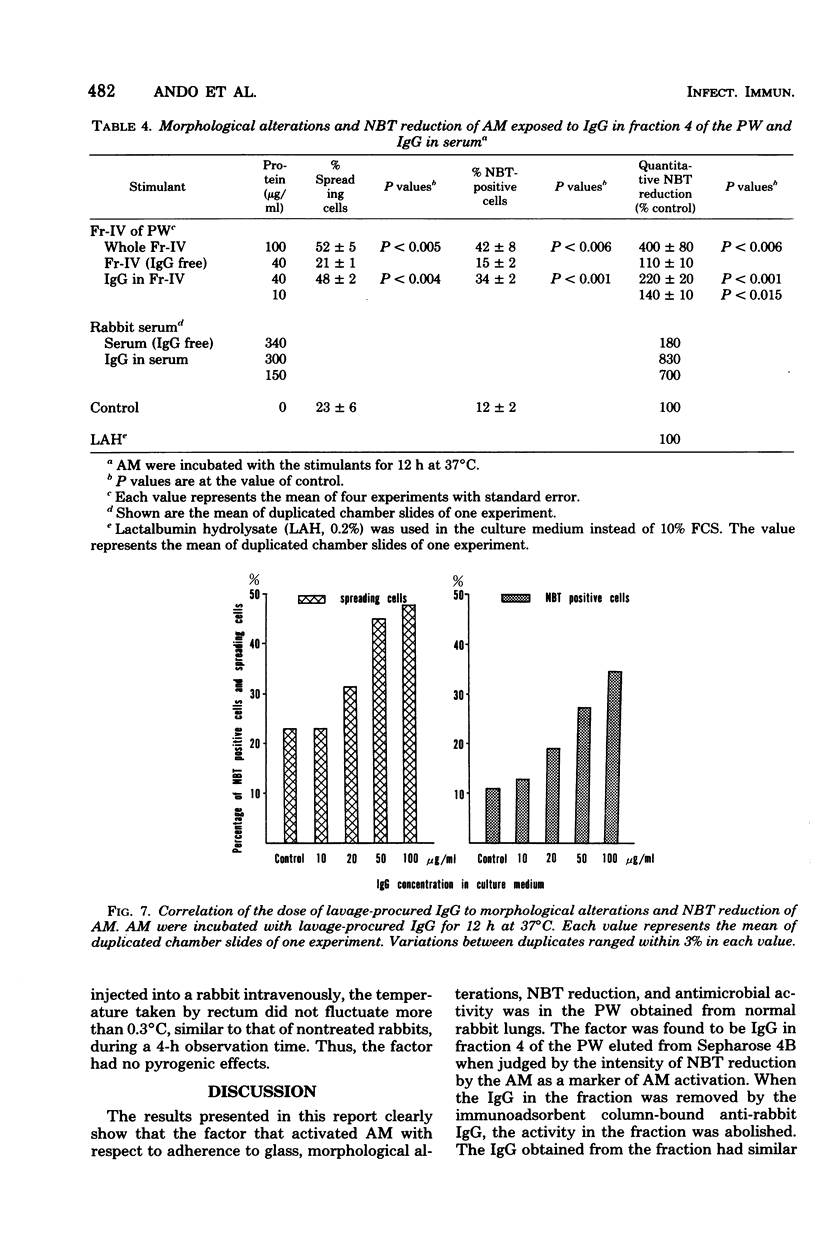
Pulmonary washings from rabbits were freed of cells and added to the monolayers of homologous alveolar macrophages (AM). At 1 h after incubation with the pulmonary washings, many more cells adhered to glass, spread out, and showed enhanced Nitro Blue Tetrazolium reduction. The maximal effect of the pulmonary washings on AM activation was obtained 12 h after incubation. The AM activated by the pulmonary washings showed a higher capacity to inhibit the growth of intracellular BCG, and that capacity was correlated with the intensity of Nitro Blue Tetrazolium reduction by the AM. Gel filtration of the pulmonary washings through Sepharose 4B yielded five fractions. The factor that activated the AM functions was in fraction 4. When the immunoglobulin G in the fraction was removed by an immunoadsorbent column, AM activity was abolished. The effect of the immunoglobulin G was dose dependent, and minimal responses to 106 cells per ml were obtained at a protein concentration of 20 μg/ml. Lymphokines had no effect on AM activation with respect to the morphological alterations and Nitro Blue Tetrazolium reduction during the 24-h observation time. In summary, AM from normal rabbits were soon activated markedly by lavage-procured immunoglobulin G, but not by lymphokines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Suga M., Shima K., Sugimoto M., Higuchi S. Different effects of phytohemagglutinin-activated lymphocytes and their culture supernatants on macrophage function. Infect Immun. 1976 May;13(5):1442–1448. doi: 10.1128/iai.13.5.1442-1448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Murrmann S. K., Davis J., Johnston R. B., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Studies on the migration inhibitory factor associated with delayed-type hypersensitivity: cytodynamics and specificity. Transplantation. 1967 Jul;5(4 Suppl):996–1000. [PubMed] [Google Scholar]
- Bignon J., Jaurand M. C., Pinchon M. C., Sapin C., Warnet J. M. Immunoelectron microscopic and immunochemical demonstrations of serum proteins in the alveolar lining material of the rat lung. Am Rev Respir Dis. 1976 Feb;113(2):109–120. doi: 10.1164/arrd.1976.113.2.109. [DOI] [PubMed] [Google Scholar]
- Colacicco G., Buckelew A. R., Jr, Scarpelli E. M. Protein and lipid-protein fractions of lung washings: chemical characterization. J Appl Physiol. 1973 Jun;34(6):743–749. doi: 10.1152/jappl.1973.34.6.743. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., King N. L., Hughes C. G. Activation of alveolar macrophages after lower respiratory tract infection. J Immunol. 1975 Jul;115(1):80–84. [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENZI G. A., BERMAN L., FIRST M., KASS E. H. A QUANTITATIVE STUDY OF THE DEPOSITION AND CLEARANCE OF BACTERIA IN THE MURINE LUNG. J Clin Invest. 1964 Apr;43:759–768. doi: 10.1172/JCI104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaForce F. M. Effect of alveolar lining material on phagocytic and bactericidal activity of lung macrophages against Staphylococcus aureau. J Lab Clin Med. 1976 Nov;88(5):691–699. [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. H., Fikrig S. M., Smithwick E. M. Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid. Lancet. 1968 Sep 7;2(7567):532–534. doi: 10.1016/s0140-6736(68)92406-9. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- Tamerius J., Nepom J., Hellström I., Hellström K. E. Tumor-associated blocking factors: isolation from sera of tumor-bearing mice. J Immunol. 1976 Mar;116(3):724–730. [PubMed] [Google Scholar]
- Tizard I. R. Macrophage-cytophilic antibodies and the functions of macrophage-bound immunoglobulins. Bacteriol Rev. 1971 Dec;35(4):365–378. doi: 10.1128/br.35.4.365-378.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy L., Burr B. The use of affinity chromatography for the specific purification of antibodies and antigens. J Immunol. 1969 Aug;103(2):380–382. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]



