Abstract
The ahpC (MSMEG_4891) gene encodes alkyl hydroperoxide reductase C in Mycobacterium smegmatis mc2155 and its expression is induced under oxidative stress conditions. Two well-defined inverted repeat sequences (IR1 and IR2) were identified in the upstream region of ahpC. Using a crp (cAMP receptor protein: MSMEG_6189) mutant and in vitro DNA-binding assay, it was demonstrated that the IR1 sequence serves as a Crp-binding site and that Crp functions as an activator in the regulation of ahpC expression. The expression level of ahpC was shown to be proportional to intracellular cAMP levels. Intracellular levels of cAMP were increased in M. smegmatis, when it was treated with oxidative stress inducers. The IR2 sequence is very similar to the known consensus sequence of FurA-binding sites and involved in the negative regulation of ahpC expression. Taken together, these results suggest that the induction of ahpC expression under oxidative stress conditions probably results from a combinatory effect of both inactivation of FurA by oxidative stress and activation of Crp in response to increased levels of cAMP.
Introduction
Alkyl hydroperoxide reductase C is a member of the peroxiredoxin family that reduce organic peroxides to their corresponding organic alcohols [1]. These enzymes from mycobacteria possess peroxinitrite reductase activity as well as peroxidase activity reducing both organic peroxides and hydrogen peroxide [2], [3], [4], [5]. Alkyl hydroperoxide reductase C is encoded by the ahpC gene in mycobacteria and contains two catalytically important cysteine residues, one of which (peroxidatic cysteine) is used to reduce the substrates (peroxides or peroxinitrite) with its concomitant oxidation to cysteine sulfenic acid. The sulfhydryl group of the other cysteine residue (resolving cysteine) attacks the peroxidatic cysteine sulfenic acid to form a disulfide bond [6], [7]. The mycobacterial AhpC forms a homodimer as a minimum functional unit in which the resolving cysteine from one subunit acts on the peroxidatic cysteine in the other subunit [2], [8]. X-ray diffraction analysis of crystallized AhpC revealed that AhpC has the structure of a ring-shaped hexamer of dimers [7]. The disulfide bond formed between the peroxidatic and resolving cysteine residues in AhpC is reduced for the next catalytic cycle by the AhpD peroxiredoxin reductase [2], [6], [7]. The reducing equivalents for the reduction of the oxidized AhpC are transferred to AhpD from NADH via dihydrolipoamide succinyltransferase (SucB) and dihydrolipoamide dehydrogenase (Lpd) [9]. It was reported that AhpC can be also reduced by thioredoxin C (TrxC) and NADPH-dependent thioredoxin reductase [10]. The ahpC gene forms an operon with its downstream gene, ahpD [11]. Genes encoding the OxyR homologs, which are LysR family regulators and involved in peroxide stress response, are divergently located upstream of the ahpCD operons in most mycobacteria [12]. However, the oxyR genes identified in Mycobacterium tuberculosis and other members of the M. tuberculosis complex (Mycobacterium bovis, Mycobacterium africanum, and Mycobacterium microti) are inactivated by numerous mutations and Mycobacterium smegmatis does not have the oxyR gene [12], [13]. Despite the lack of the functional oxyR genes, expression of ahpC was reported to be induced in M. bovis BCG in the presence of diamide and synthesis of AhpC in M. smegmatis was shown to be inducible by both hydrogen peroxide and organic hydroperoxides such as cumene hydroperoxide (CHP) and tert-butyl hydroperoxide (BHP) [14], [15], indicating that these bacteria possess other regulatory system(s) responding to oxidative stress and regulating ahpC expression. An ahpC mutant of M. tuberculosis is more susceptible to CHP than the wild type [16]. It was also demonstrated that ahpC expression was derepressed in a virulent M. tuberculosis strain grown under static growth conditions, suggesting the possibility that depletion of oxygen might lead to derepression of ahpC [16]. Overexpression of the oxyS gene was demonstrated to reduce the level of AhpC in M. tuberculosis, and microarray analyses revealed that expression of ahpC was downregulated in Crp (cAMP receptor protein; Rv3676) and SenX3-RegX3 two-component system mutants and upregulated in a WhiB4 mutant, compared with the wild-type strain of M. tuberculosis [17], [18], [19], [20]. These findings indicate the possible involvement of OxyS, Crp, WhiB4, and SenX3-RegX3 TCS in the regulation of ahpC expression. It was also reported that expression of ahpC was not changed in a SigF mutant of M. smegmatis, ruling out the involvement of SigF in the regulation of ahpC expression [21]. Despite a number of reports regarding ahpC expression, detailed regulatory mechanisms by which expression of ahpC is regulated in response to oxidative stress still remains elusive.
The Crp protein is a transcriptional regulator that responds to intracellular fluctuation of the cAMP level [22]. M. tuberculosis Crp (CrpMtb) consists of the N-terminal cAMP-binding domain (residues 1–114) and the C-terminal DNA-binding domain (residues 146–233) that are connected by a hinge region (residues 117–144) [23]. Three-dimensional structures of the cAMP-bound and cAMP-free CrpMtb revealed that CrpMtb forms homodimer like Escherichia coli Crp and it undergoes allosteric conformational changes by cAMP binding [24], [25]. The binding affinity of CrpMtb for cAMP is lower than that of E. coli Crp and cAMP binding to CrpMtb is not cooperative [26], [27]. These properties were suggested to render CrpMtb responsive to changes in the cAMP level in the background of high cAMP concentrations within mycobacterial cells [27]. Conformational changes of CrpMtb by cAMP binding were proposed to lead to a small increase (∼2 fold) in its binding affinity for the target DNA sequence (TGTGA-N6-TCACA) [26]. Growth of M. tuberculosis was shown to be compromised in both macrophages and a mouse infection model by the inactivation of the crp gene [18]. Internalization of mycobacteria into macrophages resulted in a surge in cAMP production by mycobacteria [28], [29]. Both findings imply that some genes involved in the enhanced survival of mycobacteria in hostile environments such as oxidative and nitrosative stress conditions within macrophages might be regulated by Crp.
The FurA proteins in mycobacteria are members of the ferric uptake regulator (Fur) family. FurA is composed of the N-terminal domain containing a helix-turn-helix motif for DNA binding and the C-terminal metal-binding domain. The Fe2+-binding form of FurA functions as an active transcriptional regulator that control expression of genes involved in iron homeostasis and oxidative stress response [30], [31] In mycobacteria there are also FurB proteins belonging to the Fur family. FurB is a Zn2+-dependent regulator that regulates genes related to zinc homeostasis [32].
Here, we report that expression of the ahpC gene, whose product is implicated in detoxification of peroxides and peroxinitrite, is regulated by Crp and probably FurA in M. smegmatis.
Materials and Methods
Strains, plasmids, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were grown in Luria-Bertani (LB) medium at 37°C as described elsewhere [33]. M. smegmatis strains were grown aerobically at 37°C in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 0.2% (wt/vol) glucose as a carbon source and 0.02% (vol/vol) Tween 80 as an anticlumping agent. For iron-depleting growth conditions of mycobacterial cultures, MOPS-defined medium was used in place of 7H9 medium. The MOPS medium is composed of 25 mM MOPS (pH 7.2), 25 mM KCl, 10 mM Na2SO4, 20 mM NH4Cl, 10 mM K2HPO4, 2 mM MgSO4, and 0.1 mM CaCl2. When antibiotics were required, ampicillin (100 µg/ml for E. coli), kanamycin (50 µg/ml for E. coli and 15 µg/ml for M. smegmatis) and hygromycin (200 µg/ml for E. coli and 50 µg/ml for M. smegmatis) were added to the medium. For treatment of M. smegmatis cultures with various oxidative and nitrosative stress conditions, M. smegmatis strains were grown until an optical density at 600 nm (OD600) reached 0.4 to 0.5 on a gyratory shaker (200 rpm). Following the addition of stress-inducing reagents to the cultures, the strains were further grown for 1 h. The working concentrations of the reagents are as follows: 100 µM cumene hydroperoxide (CHP), 100 µM plumbagin (PB), 5 mM diamide, 15 mM hydrogen peroxide (H2O2), 10 mM sodium ascorbate (VC), and 10 mM sodium nitroprusside (SNP).
Table 1. Bacterial strains and plasmids used in this study.
| Strain or plasmid | Relevant phenotype or genotype | Reference or source |
| Strains | ||
| E. coli DH5α | φ80dlacZΔM15 ΔlacU169 recA1 endA1 hsdR17 supE44 thi1 gyrA96 relA1 | [59] |
| E. coli HB101 | F supE44 ara14 galK2 _(gpt-proA)62 lacY1 hsdS20 rpsL20 xyl-5 mtl-1 recA13 _(mcrC-mrr) | Stratagene |
| E. coli BL21 (DE3) | F− ompT hsd S B(rB −mB −) dcm galλ (DE3) | Promega |
| M. smegmatis mc2155 | High-transformation-efficiency mutant of M. smegmatis ATCC 607 | |
| M. smegmatis crp | crp (MSMEG_6189) insertion mutant derived from M. smegmatis mc2155 | This study |
| Plasmids | ||
| pGEM-T Easy | Ampr; linear plasmid derived from pGEM5-Zf | Promega |
| pMH702 | Hygr; a derivative of pYUB572, its ampicillin-resistance gene is replaced by ahygromycin-resistance gene and the gene is flanked by two 34-bp loxP sequences | Yang JY, unpublished |
| phAE159 | Deletion mutant of mycobacteriophage TM4 | [60] |
| pKOTs | Hygr; pKO-based vector containing a temperature-sensitive replication origin (pAL500Ts) and pUC ori | [61] |
| pMV306 | Kmr; integrative vector containing the int and attP sites of mycobacteriophageL5 for integration into the mycobacterial genome | [62], [63] |
| pBluescript II KS + | Ampr; lacPOZ’ | Stratagene |
| pNC | Hygr; promoterless lacZ | [64] |
| pProEX HTa | Ampr; Trc promoter, instrinsic 6 His tag | Invitrogen |
| pMH201 | Kmr; acetamide-inducible promoter, derivative of pMV306 | [65] |
| pProcrpHis | pProEX HTa with 681-bp EcoRI-XhoI fragment containing crp of M. smegmatis mc2155 with 6 His codons | This study |
| pMV306crp | pMV306 with 1,004-bp NotI-HindIII fragment containing crp of M. smegmatis mc2155 | This study |
| pBSahpC | pBluescript II KS+with 836-bp XbaI-ClaI fragment containing the ahpC promoter region | This study |
| pBSM1 | pBSahpC in which the nucleotide G within the Crp-binding site is substituted with C | This study |
| pBSM2 | pBSahpC in which the nucleotide C within the Crp-binding site is substituted with G | This study |
| pBSM3 | pBSahpC derivative that containing the 12-bp-deleted IR2 sequence in the ahpC control region | This study |
| pNCahpC | pNC with 836-bp XbaI-ClaI fragment from pBSahpC | This study |
| pNCM1 | pNC with 836-bp XbaI-ClaI fragment from pBSM1 | This study |
| pNCM2 | pNC with 836-bp XbaI-ClaI fragment from pBSM2 | This study |
| pNCM3 | pNC with 830-bp XbaI-ClaI fragment from pBSM3 | This study |
| pMHpdeHis | pMH201 with 1,011–bp NdeI-XbaI fragment containing the rv0805 gene ofM. tuberculosis with 6 His codons before its stop codon | This study |
DNA manipulation and electroporation
Standard protocols and manufacturers’ instructions were followed for recombinant DNA manipulations. The transformation of M. smegmatis with plasmids was carried out by electroporation as described elsewhere [34].
Construction of plasmids
(i) pProcrpHis: A 681-bp DNA fragment including the crp (MSMEG_6189) gene was amplified with F_crp (5′- GAATTCATGGACGAGATCCTGGCCAG-3′) and R_crp (5′- CTCGAGCTAGCGGGCGCGGCGGGCCA-3′) using M. smegmatis genomic DNA as a template and Pfu DNA polymerase. The PCR product was restricted with EcoRI and XhoI and inserted into pProEX HTa digested with the same enzymes, yielding pProcrpHis that was used to overexpress the N-terminally His6-tagged Crp protein. (ii) pMV306crp: pMV306crp was constructed for complementation of the crp mutant with the intact crp gene. A 1,004-bp DNA sequence containing the crp gene was amplified with the primer set, F_pMV306crp (5′-AATTGCGGCCGCCCCGCGAGCAGGCACCAC-3′) and R_pMV306crp (5′-CCGGAAGCTTTTCGGCGAACGGGGCGAG-3′) using M. smegmatis genomic DNA as a template. The DNA fragment obtained from the PCR reaction was digested with NotI and HindIII and ligated with pMV306 restricted with the same restriction enzymes, resulting in pMV306crp. (iii) pNCahpC, pNCM1, pNCM2, and pNCM3: pNCahpC, pNCM1, pNCM2, and pNCM3 are ahpC::lacZ transcriptional fusion plasmids. For the construction of pNCahpC, a 836-bp DNA fragment comprising the 5′ portion (99 bp) of ahpC and the 737-bp DNA sequence upstream of ahpC was amplified with the primers, F_ahpC (5′-AAAATCTAGACATCGACGTCGCCCGCCC-3′) and R_ahpC (5′-GACAATCGATGTCATCGGGCTGCTTGG-3′). The PCR product was digested with ClaI and XbaI and cloned into pBluescript II KS+, resulting in pBSahpC. pBSahpC was restricted with ClaI and XbaI and the 836-bp fragment was cloned into pNC, yielding the pNCahpC. To construct pNCM1 and pNCM2, PCR-based site-directed mutagenesis was carried out using pBSahpC as a template. Synthetic oligonucleotides 33 bases long and containing substituted nucleotides in the middle of their sequences were used to mutagenize the Crp-binding site (IR1). Mutations were verified by DNA sequencing. The 836-bp XbaI and ClaI fragments from the mutated pBSM1 and pBSM2 were cloned into pNC, resulting in the plasmids pNCM1 and pNCM2, respectively. In order to construct pNCM3, inverse PCR was conducted with the primers, L_M3 (5′-ATATGGATCCCCAGATTTACACCACGATTCTGGTC-3′, the BamHI site is underlined) and R_M3 (5′-ATATGGATCCAAACAAGAACACGTAGATGGGATGC-3′), and pBSahpC as a template to amplify the linear pBSahpC with BamHI sites at both ends. The amplified products were restricted with BamHI and self-ligated, resulting in the plasmid pBSM3 containing a BamHI site in place of IR2. pNCM3 was constructed by cloning the ClaI-XbaI DNA fragment from pBSM3 into pNC. (iv) pMHpdeHis: To construct pMHpdeHis for expression of cyclic nucleotide phosphodiesterase (Rv0805) of M. tuberculosis, PCR reaction was performed with the primers, F_rv0805 (5′-ATATCATATGCAATGGAGAGGGTTGGCACCTCAG-3′) and R_rv0805 (5′-ATATTCTAGATCAGTGATGGTGATGGTGATGGTCGACGGGACTTCGCGG-3′) and M. tuberculosis H37Rv genomic DNA as a template. The PCR product containing the rv0805 gene with 6 His codons immediately before its stop codon was digested with NdeI and XbaI and cloned into pMH201, yielding pMHpdeHis.
Construction of a crp mutant
A crp mutant in which the crp (MSMEG_6189) gene is disrupted by the insertion of a hygromycin-resistance gene, was constructed by one-step homologous recombination using the conditionally replicating shuttle phasmid vector phAE159 as previously described [35], [36]. Briefly, a 970-bp DNA fragment containing the 5′ portion (60 bp) of crp flanked with the 910-bp crp upstream sequence and a 963-bp DNA fragment containing the 3′ portion (60 bp) of crp flanked with the 903-bp crp downstream sequence (left and right arms, respectively) were amplified by PCR using M. smegmatis genomic DNA as a template with the primer sets, CrpL_F_BglII (5′-AGATCTGTCGAAGCGCTCGACGAGTTCCTGG-3′) and CrpL_R_SpeI (5′-ACTAGTCGCAACGGCGGTGGGTTCGA-3′) for the left arm and CrpR_F_NcoI (5′- CCATGGCTGGAGGGCAAGAGCGTGCT-3′) and CrpR_R_NcoI (5′-CCATGGCGTCGAGGTCGAGATCATCG-3′) for the right arm. Both PCR products were cloned into pGEM-T (Promega, Madison, WI), resulting in pGcrpL and pGcrpR. The plasmid pGcrpL was restricted with BglII and SpeI, and pGcrpR with NcoI. The DNA fragments were cloned into the cosmid pMH702 to flank the hygromycin-resistance gene cassette on both sides. The resulting cosmid pMH702crp was linearized with PacI and ligated with the PacI-digested shuttle phasmid phAE159. The ligation mixture was packaged using MaxPlax lambda packaging extracts (Epicentre Biotechnologies, Madison, WI) and transfection of E. coli HB101 was performed. Recombinant phasmids were isolated from hygromycin-resistant clones of E. coli. Transformation of M. smegmatis with the isolated phasmid at 30°C resulted in the generation of recombinant TM4 phages carrying the recombinant phasmid. The crp mutant was selected on hygromycin-containing 7H9 plates at 37°C following transfection of M. smegmatis with the recombinant TM4 phages. The mutation was confirmed by PCR.
Reverse-transcription PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR)
RNA isolation from M. smegmatis strains, preparation of cDNA, RT-PCR, and qRT-PCR were performed as described elsewhere [37]. The primers used in RT-PCR and qRT-PCR were listed in Table 2.
Table 2. The primers used for RT-PCR and qRT-PCR in this study.
| Primer | Sequence (5′ to 3′) |
| 16S rRNA_Forward | CTGGGACTGAGATACGGC |
| 16S rRNA_Reverse | ACAACGCTCGGACCCTAC |
| ahpC_Forward | GTGTGTCGGTGGACA ACGAG |
| ahpC_Reverse | GGTCACCGACACGAACTGGA |
| pNClacZ_Forward | GGCGTTACCCAACTTAATCG |
| pNClacZ_Reverse | ACGACGACAGTATCGGCCTC |
Purification of Crp protein
N-terminally His6-tagged Crp protein was overexpressed in the E. coli BL21 (DE3) strain harboring pProcrpHis. The strain was grown aerobically at 37°C in LB medium containing 100 µg/ml ampicillin to an OD600 of 0.4 to 0.6. Expression of the crp gene was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM, and cells were further grown at 30°C for 4 h. Harvested cells from 300 ml culture were resuspended in 5 ml buffer A [20 mM Tris-HCl (pH 8.0) and 100 mM NaCl] and disrupted by two passages through a French pressure cell. Following DNase Ι treatment (10 units/ml) in the presence of 10 mM MgCl2 for 30 min on ice, cell-free crude extracts were obtained by centrifugation twice at 20,000 × g for 10 min. 0.5 ml of the 80% (vol/vol) slurry of Ni-Sepharose high-performance resin (GE Healthcare, Piscataway, NJ) was added to the crude extracts and mixed gently by shaking for 2 h on ice. The protein-resin mixture was packed into a column. The resin was washed with 40 bed volumes of buffer A containing 5 mM imidazole, 40 bed volumes of buffer A containing 10 mM imidazole, 20 bed volumes of buffer A containing 50 mM imidazole, and then His6-tagged Crp was finally eluted with 13 bed volumes of buffer A containing 200 mM imidazole. Fractions from the elution step were collected and desalted by means of a PD-10 desalting column (GE Healthcare) equilibrated with 20 mM Tris-HCl (pH 8.0).
Determination of the protein concentration
The protein concentration was determined by using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard protein.
Western blotting analysis
To determine the amount of AhpC protein in cells, Western blotting analysis was performed as described elsewhere [38]. Rabbit polyclonal antibodies against AhpC were used at a 1∶2,000 dilution. Alkaline phosphatase-conjugated anti-rabbit IgG (Sigma, St. Louis, MO) was used at a 1∶5,000 dilution for the detection of the primary antibody.
Zone inhibition assay
M. smegmatis strains were cultivated in 7H9 medium aerobically until OD600 reached 0.45. 5 ml of cultures were poured onto 7H9 plates. The plate surfaces were spread uniformly with the cultures and then the rest of the cultures were drained off. The plates were tapped on a paper towel to remove the remaining culture liquid. The plates were dried at room temperature for 3 to 4 h. The paper discs soaked with 15 µl of 1 and 2% (wt/vol) of CHP were placed onto the dried plates. The plates were incubated at 37°C for 3 days to observe zones of growth inhibition.
Electrophoretic mobility shift assay (EMSA)
EMSA was carried out by using the Electrophoretic Mobility Shift Assay (EMSA) kit (Invitrogen, Carlsbad, NJ) according to the manufacturer’s instruction. 150-bp DNA fragments containing the wild-type or mutated IR1 sites were used in the assay. The DNA fragments were generated by PCR using the primer set, EMSA_150_F (5′-TCTGGTCGCGCCCTCTTAC-3′) and EMSA_150_R (5′-GGCAGACCGCATCCGCGG-3′) and pBSahpC, pBSM1, and pBSM2 as templates to obtain the corresponding DNA fragments. Reaction mixtures for DNA-protein binding were composed of appropriate amounts of DNA (2 µl), purified Crp (5 µl), distilled H2O (1 µ), and 5× binding buffer included in the kit (2 µl). cAMP was added to a final concentration of 100 µM. The binding reaction mixtures were incubated for 20 min at room temperature. After the addition of 2 µl of 6× loading buffer (included in the kit), the mixtures were subject to nondenaturing PAGE [8% (wt/vol) acrylamide] in 0.5× TBE buffer (41.5 mM Tris-borate and 0.5 mM EDTA, pH 8.3) at 14 V/cm for 2 h 20 min at 4°C. The gels were stained with the SYBR green staining solution (Invitrogen).
β-galactosidase assay
The β-galactosidase activity was measured spectrophotometrically as described previously [39].
Determination of the intracellular cAMP concentration
M. smegmatis cells corresponding to 1 ml of cultures at OD600 of 0.4 were harvested. Cell pellets were resuspended in 1 ml of 0.1 M HCl and then incubated for 10 min. Cells were disrupted once by using a Fastprep 120 beadbeater (Thermo, Milford, MA) at 6.5 m/sec for 45 sec. Cell-free supernatants were obtained by centrifugation at 13,400×g for 10 min. Using the prepared supernatants, the concentration of intracellular cAMP was determined by using the DetectX Direct Cyclic AMP Enzyme Immunoassay kit (Arbor Assays, Ann Arbor, MI) and a Microplate Reader (Bio-Rad) following the manufacturers’ instructions.
Results
Identification of cis-acting elements in the upstream region of ahpC
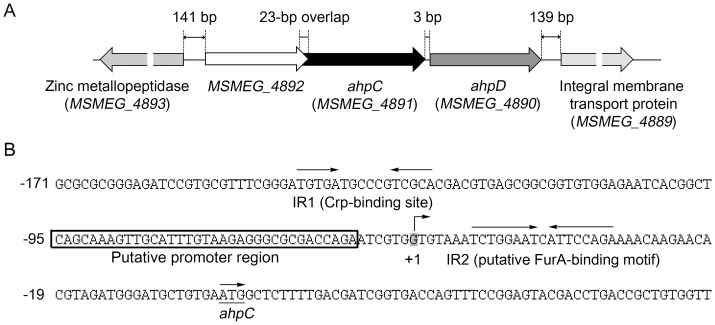
The ahpC (MSMEG_4891) gene forms an operon together with ahpD (MSMEG_4890) in M. smegmatis [11]. The MSMEG_4892 gene annotated as a hypothetical protein gene is located upstream of ahpC with a 23-bp overlap (Figure 1A). MSMEG_4892 differs in its codon preference from other genes of M. smegmatis and its deduced protein product has no obvious similarity to other known proteins. When RT-PCR was performed with the primers that can detect mRNA encompassing both MSMEG_4892 and ahpC, no PCR product was obtained (data not shown), indicating that MSMEG_4892 does not form the same transcriptional unit with ahpCD.
Figure 1. Genetic organization of the ahpC locus in M. smegmatis mc2155 (A) and the upstream sequence of ahpC encompassing its promoter region and the putative cis-acting elements involved in the regulation of ahpC expression (B).
The lengths of the overlapping region between MSMEG_4892 and ahpC and the intergenic regions are given as the nucleotide numbers above the schematic diagram. The two inverted repeats, IR1 (Crp-binding site) and IR2 (putative FurA-binding site), are marked by the two head-facing arrows above their sequences. The putative promoter region of ahpC is boxed. The nucleotide reported to be the transcriptional start point (+1) of ahpC is shaded in gray [15]. The start codon of ahpC is underlined and the arrow above it indicates the transcriptional direction. The numbers on the left of the sequences indicate the positions of the leftmost nucleotides relative to the ahpC gene.
The DNA sequence upstream of ahpC was analyzed to identify cis-acting elements involved in expression of ahpC (Figure 1B). The transcriptional start point of ahpC was reported previously [15]. The promoter region of ahpC around 10 and 35 bp upstream of the transcriptional start point does not have sequences similar to the consensus sequence of the mycobacterial −10 and −35 regions for the SigA sigma factor, indicating that ahpC has either a weak promoter or a promoter recognized by alternative sigma factors [40], [41]. As an initial attempt to identify regulatory systems that are responsible for the regulation of ahpC expression, we searched for inverted repeat sequences in the upstream sequence of ahpC under the presumption that multimeric regulatory proteins normally bind to their target DNA sequences with a dyad symmetry. Two well-defined inverted repeat sequences (IR1 and IR2) were identified and one of them (IR1) almost perfectly matched the consensus sequence of E. coli’s Crp-binding sites (TGTGA-N6-TCACA) [42]. A perfect inverted repeat sequence (IR2: TCTGGAAT-C-ATTCCAGA) was identified immediately downstream of the transcriptional start point. The IR2 sequence is highly similar to the sequence of a “FurA box” serving as the FurA-binding sequence in mycobacteria [43].
Positive regulation of ahpC by Crp
There are two genes encoding Crp homologs (MSMEG_6189 and MSMEG_0539) in M. smegmatis genome. Since MSMEG_6189 showed a higher identity (98%) to M. tuberculosis Crp than MSMEG_0539 (78%), a crp (MSMEG_6189) mutant of M. smegmatis was first constructed to examine whether Crp is involved in the regulation of ahpC expression. Growth of the mutant was slower and reached the stationary phase at a lower cell density than that of the wild type, when both strains were grown aerobically in 7H9 medium supplemented with glucose (the doubling times of the wild type and the mutant were 4.2 and 4.9 h, respectively).
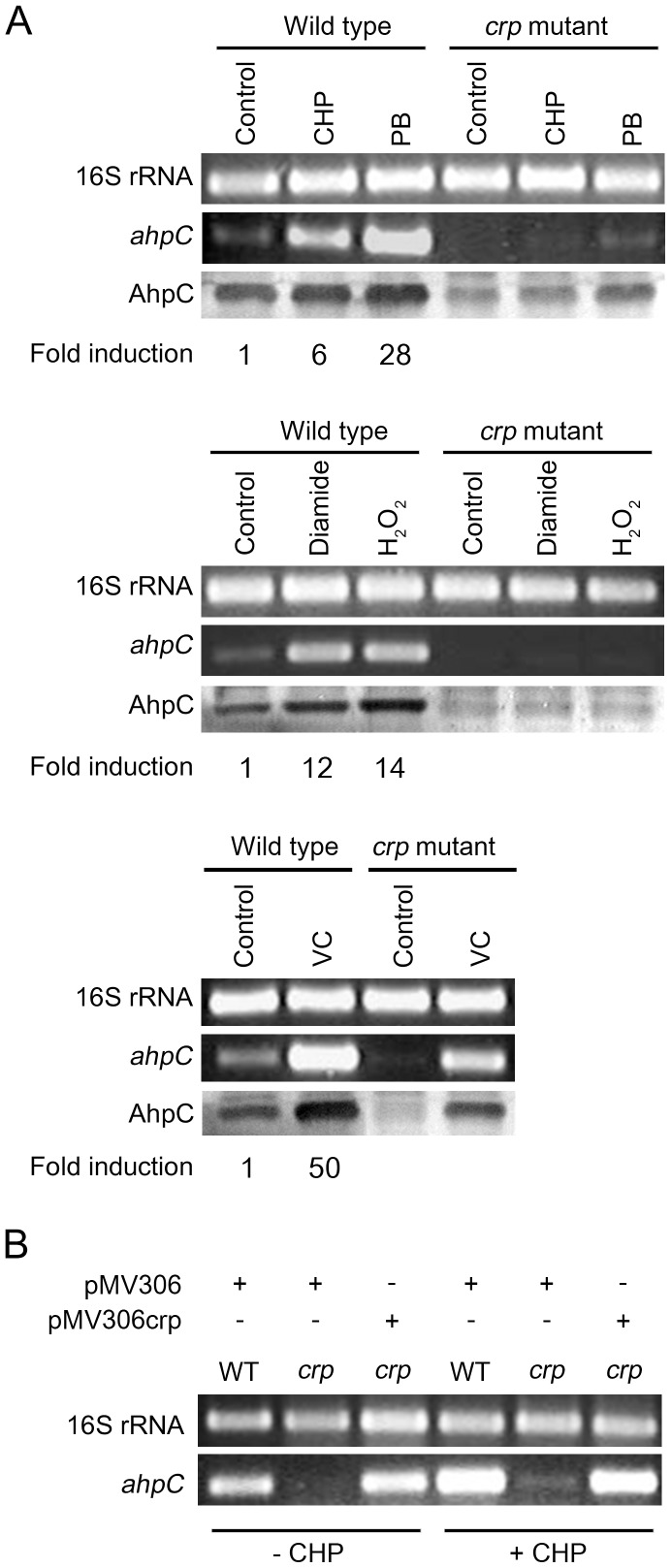
Expression levels of ahpC in the crp mutant were compared with those in the wild-type strain by means of RT-PCR, qRT-PCR, and Western blotting after both strains were subject to oxidative stress generated by CHP (organic peroxide), H2O2, plumbagin (PB: superoxide generator), diamide (thiol-specific oxidant), and sodium ascorbate (VC: intracellular oxidative stress inducer). As controls, the crp mutant and wild-type strains without treatment of the oxidative stress reagents were included in the experiment (Figure 2A). When the wild-type strain was subject to CHP, H2O2, and diamide treatment, transcript levels of ahpC were increased 6-, 14-, and 12-fold, respectively, when compared with those determined for the untreated control strain. Treatment of the wild-type strain with plumbagin and ascorbate led to 28- and 50-fold induction of ahpC expression, respectively. In contrast, induction of ahpC expression by CHP, H2O2, and diamide was almost abolished in the crp mutant. When the crp mutant was treated with the strong inducers, plumbagin and ascorbate, induction of ahpC expression was significantly reduced but still observed. Western blotting analysis using polyclonal antibodies against AhpC also confirmed induction of ahpC by oxidative stress and requirement of Crp for the optimal expression of ahpC, although the extent of ahpC induction detected by Western blotting did not quantitatively well correlate with that determined by RT-PCR and qRT-PCR. The discrepancy in the induction fold of ahpC expression at transcriptional and translational levels might be due to posttranscriptional regulation or oxidative damages of translational machinery in the presence of the oxidative stress inducers. Expression of ahpC in the crp mutant in the presence and absence of CHP was restored by the introduction of the intact crp (MSMEG_6189) gene into the crp mutant (Figure 2B), indicating that a defect in ahpC expression observed for the crp mutant resulted from the inactivation of the crp (MSMEG_6189) gene.
Figure 2. Expression of ahpC in the wild-type and crp mutant strains of M. smegmatis in response to various oxidative stresses and complementation of the crp mutant.
(A) Transcript levels of ahpC were determined by RT-PCR and qRT-PCR. RT-PCR for 16S ribosomal RNA was performed to ensure that the same amounts of total RNA were employed for RT-PCR. Fold induction of ahpC expression determined by qRT-PCR indicates levels of ahpC mRNA in the strains treated with the oxidative-stress inducers relative to those in the untreated strains (control). Protein levels of AhpC were detected by means of Western blotting with polyclonal AhpC antibodies, and the results are presented below the RT-PCR results. Abbreviations: CHP, cumene hydroperoxide; PB, plumbagin; VC, sodium ascorbate. (B) The crp mutant (crp) was complemented by introducing pMV306crp. The wild-type (WT) and crp mutant strains harboring the pMV306 empty vector were used as controls. RT-PCR was performed using total RNAs isolated from the strains treated with CHP (+CHP) and untreated strains (−CHP).
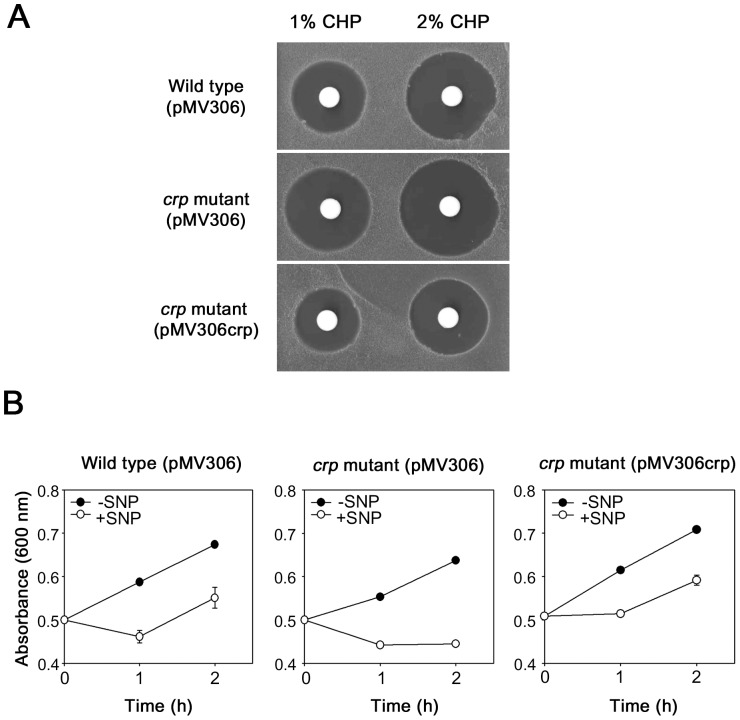
AhpC is known to have the catalytic activity that reduces organic peroxides and peroxynitrite, thereby detoxifying them [2], [3], [4], [5]. Peroxynitrite is a reactive nitrogen intermediate (RNI) produced from the reaction of nitric oxide (NO) with superoxide that is a byproduct of aerobic metabolism [44]. To investigate whether the disruption of crp in M. smegmatis affects its susceptibility to CHP and NO, zone inhibition assay with CHP and growth inhibition assay with SNP (NO generator) were performed. As shown in Figure 3A, the crp mutant with the empty vector pMV306 gave rise to larger clear zones around the discs where 1% and 2% of CHP were applied than the wild type containing pMV306. The crp mutant complemented with pMV306crp resulted in even smaller growth-inhibitory zones than the wild type with pMV306. The result indicates that the inactivation of crp renders M. smegmatis more susceptible to CHP. To examine NO susceptibility of the crp mutant, the crp mutant with pMV306 grown to an OD600 of 0.5 was treated with SNP and growth of SNP-treated (+SNP) and untreated control (−SNP) strains was compared for 2 h by measuring the optical density of the cultures (Figure 3B). As controls, the wild-type strain with pMV306 and the complemented crp mutant were included in the experiment. The addition of 10 mM of SNP to the cultures had a bactericidal effect on both the wild-type and crp mutant strains with pMV306 and a bacteriostatic effect on the complemented crp mutant during the first hour of NO exposure. While growth resumed for the wild type with pMV306 and the complemented crp mutant 1 h after SNP treatment, that of the crp mutant with pMV306 did not, indicating that the crp mutant is more sensitive to NO than the wild type and the complemented crp mutant.
Figure 3. Susceptibility of the wild-type and crp mutant strains of M. smegmatis to CHP and NO.
(A) Zone inhibition assay. The wild-type and crp mutant strains harboring pMV306 as well as the crp mutant complemented with pMV306crp were used. (B) Effect of NO treatment on growth of the wild-type and crp mutant strains of M. smegmatis. When the strains were grown to an OD600 of 0.5, SNP was added to the cultures with a final concentration of 10 mM and the cultures were further grown under the illumination of light (+SNP). As controls the strains without SNP treatment were included in the experiment (−SNP). The absorbance of the cultures was measured at 600 nm at intervals of 1 h. Growth of the cultures was monitored for only 2 h due to instability of SNP. The error bars indicate the deviations from the averages of two independent measurements.
To assess the role of IR1 on ahpC expression, point mutations were introduced into the IR1 sequence by means of site-directed mutagenesis (Figure 4A). The shaded G and C nucleotides of the IR1 sequence corresponding to the strictly conserved nucleotides in the mycobacterial Crp-binding sites [45] were substituted with C and G, respectively. To ascertain whether the IR1 sequence is required for Crp binding in vitro, we performed EMSA with purified Crp (MSMEG_6189) of M. smegmatis and three types of 150-bp DNA fragments containing the wild-type or mutated IR1 sequences (mutation 1 and mutation 2) in the presence of 100 µM cAMP. When the wild-type DNA fragment was employed, the increasing amounts of Crp-DNA complexes were formed in proportion to the amounts of Crp protein. In contrast, the formation of Crp-DNA complexes was abolished when the DNA fragment containing mutation 1 (G to C mutation) was used in EMSA assay. The DNA fragment containing mutation 2 (C to G mutation) exhibited weak retarded bands that were smeared and closely migrated to free DNA bands, compared with the wild-type DNA fragment, indicative of weak interactions between Crp and the DNA fragment containing mutation 2 (Figure 4B).
Figure 4. Effect of mutations in the IR1 sequence on Crp binding and ahpC expression.
(A) Base substitution mutations of the conserved nucleotides within IR1. The consensus sequence of the Crp-binding sites is given at the bottom line. The mutated nucleotides are marked with the asterisks above the sequences. The numbers above the sequences indicate the positions of the mutated nucleotides relative to ahpC. (B) The 150-bp DNA fragments (9.3 ng, 100 fmol) containing the wild-type or mutated IR1 sequence (mutation 1 or 2) were incubated with various amounts of purified Crp in the presence of 100 µM cAMP. The amounts of Crp used are given above the lanes. The Crp-DNA reaction mixtures were subject to native PAGE. After electrophoresis, gels were stained with SYBR green EMSA gel staining solution. (C) Effect of the mutations within the IR1 sequence on the promoter activity of ahpC. The ahpC promoter activity was measured by determining β-galactosidase activity. M. smegmatis wild-type strains harboring pNCahpC, pNCM1, and pNCM2 were grown to an OD600 of 0.45 to 0.5 and treated with CHP or DMSO (the solvent for CHP stock solution: control). The cultures were further grown for 1 h. Cell-free crude extracts were used to measure β-galactosidase activity. All values are the means of two independent experiments. The error bars indicate the deviations from the means.
We next examined the effect of IR1 mutations on ahpC expression using ahpC::lacZ transcriptional fusions. pNCahpC is a pNC-based ahpC::lacZ transcriptional fusion plasmid. pNCM1 and pNCM2 have the same constructs as pNCahpC except for mutation 1 and mutation 2 within IR1, respectively. The wild-type strains of M. smegmatis harboring pNC, pNCahpC, pNCM1, and pNCM2 were aerobically grown and treated with CHP. Promoter activities of ahpC were determined by β-galactosidase assay using cell-free crude extracts. As controls, the same strains without CHP treatment were included in the experiment. Here we chose CHP, the mildest inducer used in Figure 2A, as an inducer of ahpC expression, since treatment of cell crude extracts for 1 h with plumbagin, H2O2, and ascorbate led to a significant decrease in β-galactosidase activity (data not shown). The wild-type strain harboring the empty pNC vector showed virtually no β-galactosidase activity regardless of CHP treatment. In the case of the wild-type strain containing pNCahpC, 2.3-fold induction of ahpC expression by CHP treatment was observed relative to the control without CHP treatment. The CHP-untreated strains containing pNCM1 and pNCM2 showed basal levels of β-galactosidase activity that amounted to approximately 30% of those detected in the untreated strain with pNCahpC. Expression of ahpC was not induced in the wild-type strain with pNCM1 by CHP treatment and the strain with pNCM2 showed a marginal increase in ahpC expression by CHP treatment (Figure 4C). Taken together, the results obtained from both EMSA and promoter activity assay indicate that IR1 serves as an activator-binding site for Crp and that the conserved nucleotides G and C within the IR1 sequence are important for Crp binding and activation of ahpC expression.
Cellular levels of cAMP affect ahpC expression
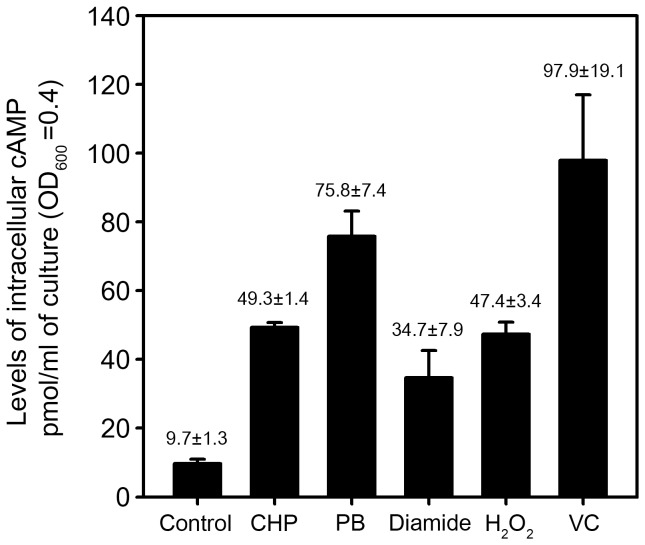
As shown in Figure 5, intracellular levels of cAMP were determined for the wild-type strain of M. smegmatis treated with CHP, PB, diamide, H2O2, and ascorbate. The untreated wild-type strain was included as a control. When the strain was treated with CHP, PB, diamide, H2O2, and ascorbate, intracellular levels of cAMP were increased 5.1-, 7.8-, 3.6-, 4.9- and 10.1-fold, respectively. This result suggests the possibility that increased levels of cAMP under oxidative stress conditions might contribute to enhancement of ahpC expression via Crp. We next examined whether expression of ahpC in M. smegmatis was affected by changes in the cellular level of cAMP. For this experiment, we employed two M. smegmatis strains: one is the wild-type strain containing pMHpdeHis where the cyclic nucleotide phosphodiesterase (PDE, Rv0805) gene of M. tuberculosis is under the control of an acetamide-inducible promoter and the other is the wild-type strain containing pMH201, the empty vector of pMHpdeHis. Both strains were grown aerobically to an OD600 of 0.45 to 0.5 in the presence of 0.2% acetamide and further grown for 1 h either with or without CHP treatment. Under both CHP-treated and untreated conditions a decrease in intracellular cAMP levels was observed in the strains carrying pMHpdeHis, when compared with the strains carrying pMH201 (Figure 6), indicating that the expressed PDE of M. tuberculosis can hydrolyze cAMP in M. smegmatis cells. The determination of ahpC expression by means of RT-PCR revealed that ahpC expression was significantly reduced in the CHP-treated strain with pMHpdeHis relative to the CHP-treated strain with pMH201. We performed this experiment three times independently and the results were reproducible. These results strongly indicate that cellular levels of cAMP are reflected to control ahpC expression in M. smegmatis.
Figure 5. Determination of intracellular cAMP levels.
The wild-type strain of M. smegmatis was grown to an OD600 of 0.45 to 0.5 and treated with various oxidative stress inducers. The untreated wild-type strain was included in the experiment as a control. Levels of intracellular cAMP were determined by using the DetectX Direct Cyclic AMP Enzyme Immunoassay kit. All values are the means of two independent experiments. The error bars indicate the deviations from the means.
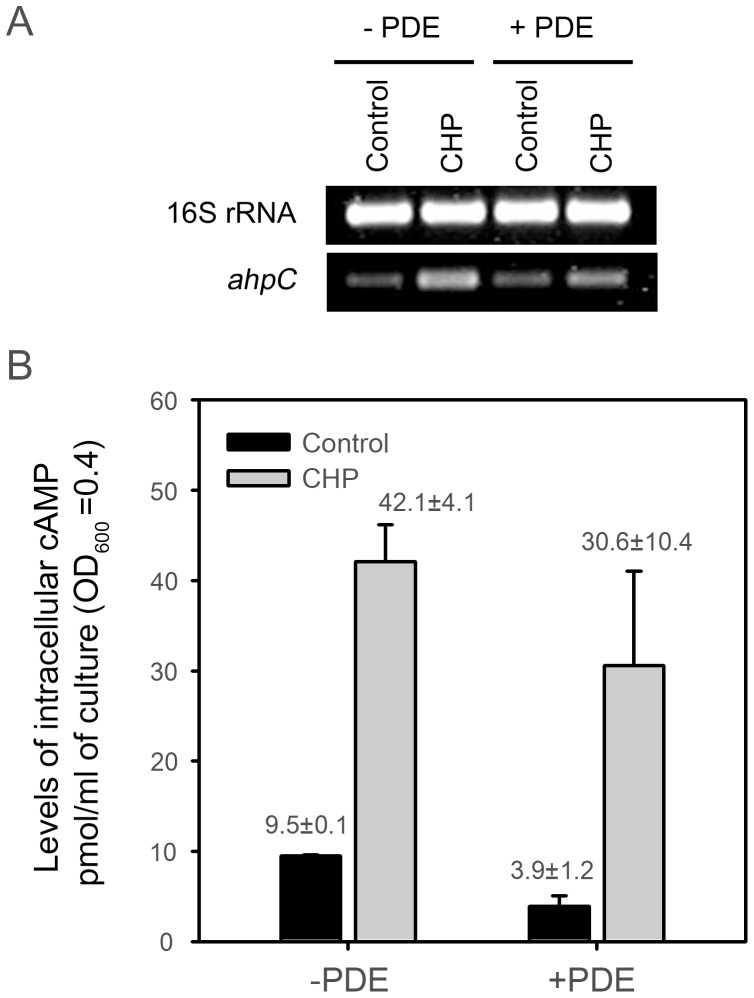
Figure 6. Effect of cAMP phosphodiesterase (PDE) overexpression on ahpC expression in M. smegmatis.
(A) Expression levels of ahpC were determined by means of RT-PCR. For induction of rv0805 encoding PDE of M. tuberculosis, the wild-type strain of M. smegmatis with pMHpdeHis (+PDE) was grown to an OD600 of 0.45 to 0.5 in the presence of 0.2% acetamide. The cultures were treated with CHP or DMSO (control) and further grown for 1 h to induce ahpC expression. The wild-type strain harboring pMH201 (−PDE) was included in the experiment. (B) Intracellular cAMP levels in the strains described in the panel A were determined. All values are the means of three independent experiments. The error bars indicate the standard deviations.
Role of IR2 as a cis-regulatory element in the regulation of ahpC expression
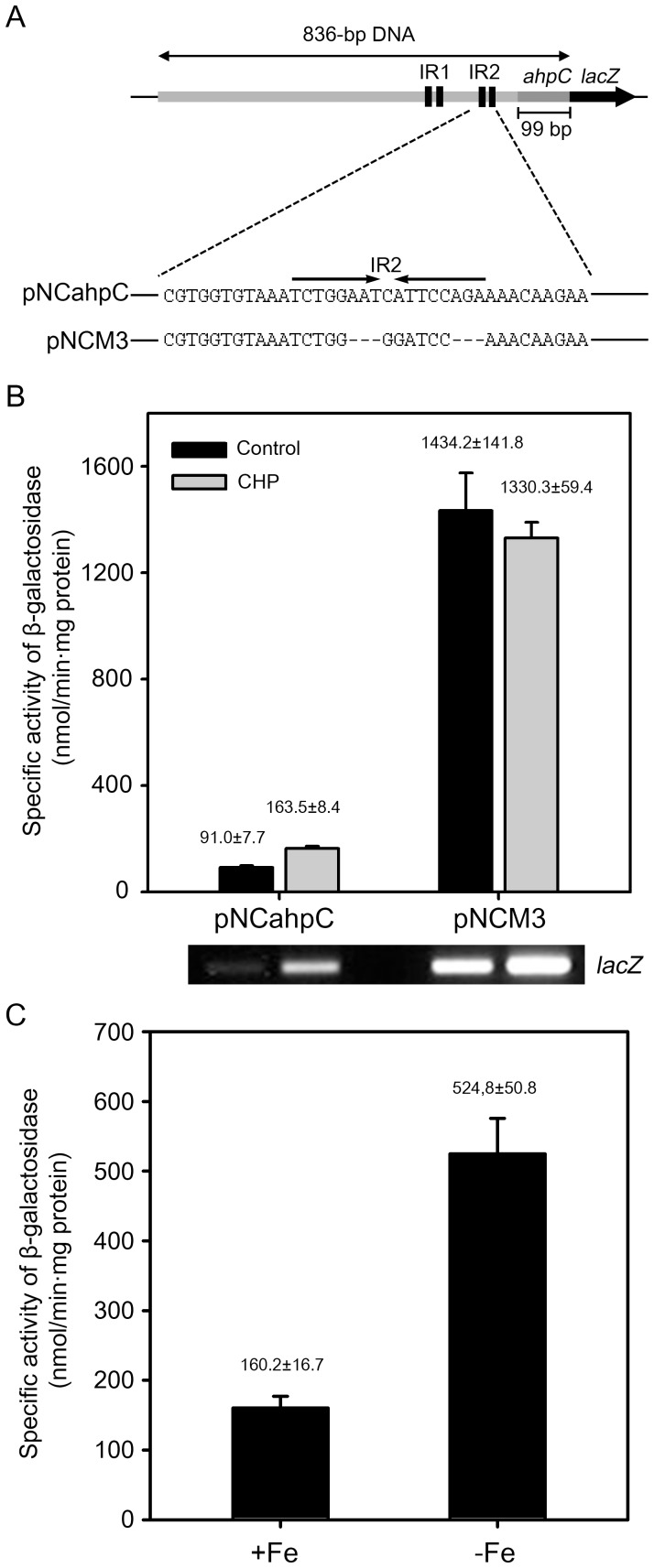
pNCM3 is a pNCahpC derivative carrying the same DNA fragment as pNCahpC except for the replacement of a 12-bp portion of IR2 with the BamHI recognition sequence (Figure 7A). Using pNCM3, the role of IR2 in the regulation of ahpC expression was examined. The wild-type strains harboring pNCahpC and pNCM3 were grown either with or without CHP treatment and promoter activities of ahpC in the strains were determined by means of β-galactosidase assay. Expression of ahpC was induced by CHP treatment in the wild-type strain with pNCahpC (Figure 7B). Expression of ahpC was strongly derepressed in the strain with pNCM3 in the presence and absence of CHP treatment. A slight decrease in ahpC expression by CHP was observed in the strain with pNCM3. When ahpC expression was measured by RT-PCR using primers for the detection of lacZ, transcription of lacZ on pNCM3 was shown to be slightly induced in the CHP-treated wild-type strain carrying pNCM3 relative to the untreated strain with pNCM3, indicating that the expression level of ahpC determined by β-galactosidase assay was underestimated in the presence of CHP, when compared with that determined by RT-PCR. The result indicates that IR2 serves as a repressor-binding site (operator) for the regulation of ahpC expression.
Figure 7. Effect of deletion of the IR2 sequence on ahpC expression and derepression of ahpC expression under iron-depleting conditions.
(A) Schematic diagram of pNCM3. The lacZ transcriptional fusion plasmid pNCM3 carries the same DNA fragment as pNCahpC except for the substitution of a part of IR2 with the BamHI recognition sequence. (B) M. smegmatis wild-type strains harboring pNCahpC and pNCM3 were grown to an OD600 of 0.45 to 0.5, and treated with CHP or DMSO (control). The cultures were further grown for 1 h. Cell-free crude extracts were used to measure β-galactosidase activity. Expression levels of lacZ in the wild-type strains carrying pNCahpC and pNCM3 were also determined by RT-PCR and the result is presented below the graph. All values are the means of two independent experiments. The error bars indicate the deviations from the means. (C) The wild-type strain of M. smegmatis harboring pNCahpC was grown in 7H9 medium to an OD600 of 1.5 to 2.0. Pre-cultured cells were washed twice with the original volume of MOPS medium supplemented with 0.02% Tween 80 and resuspended to the same volume of MOPS medium. 1 ml of the preculture was inoculated to 100 ml of MOPS medium supplemented with either 50 µM FeCl3 (+Fe) or 100 µM 2,2′-Dipyridyl (iron chelator) (−Fe). The strain was grown to an OD600 of 0.45 to 0.5 and harvested. Expression levels of ahpC were determined by performing β-galactosidase assay. The error bars indicate the deviations from the means of the two independent experiments.
The high similarity of the IR2 sequence to the known FurA-binding consensus sequence led us to speculate that FurA is involved in repression of ahpC. We identified three genes encoding FurA homologs (MSMEG_6383, MSMEG_3460, and MSMEG_6253) from M. smegmatis genome (Figure 8A). The genes encoding MSMEG_6383 (FurA1) and MSMEG_3460 (FurA2) are located immediately upstream of the duplicated katG genes coding for peroxidase-catalases, MSMEG_6384 (KatG1) and MSMEG_3461 (KatG2), respectively. All the amino acid residues involved in the coordination of the structural Zn2+ and regulatory Fe2+ ions are conserved in FurA1, FurA2, and FurA3, and their DNA-binding helix-turn-helix domains (amino acids 36 to 68 for FurA1) are relatively well conserved (Figure 8B). Phylogenetic analysis using their entire amino acid sequences revealed that FurA1 is more closely related with FurA2 than with FurA3 (data not shown). On account of difficulties in the construction of a furA triple mutant, we did not directly examine the involvement of FurA in ahpC expression by determining ahpC expression in the furA triple mutant. Instead, we determined the effect of iron depletion on ahpC expression on the basis of the fact that FurA is a Fe2+-dependent regulatory protein [46]. As shown in Figure 7C, the expression level of ahpC was increased 3.3-fold in M. smegmatis grown under iron-depleting conditions, compared with the control M. smegmatis strain grown in the medium replete with iron. This result indicates the involvement of an iron-dependent regulator in ahpC repression, possibly the FurA protein.
Figure 8. Genetic organization of the furA1, furA2, and furA3 loci in M. smegmatis mc2155 (A) and multiple alignment of the FurA homologs (B).
Multiple alignment was generated by using Clustal W. The position of the helix-turn-helix motif was extrapolated from the determined three-dimensional structure of FurA [58] and indicated with two cylinders. Identical and conservatively substituted residues are indicated by asterisks and colons, respectively. The amino acid residues, which comprise the helix-turn-helix motif and are conserved in two and three FurA homologs, are highlighted in black.
Discussion
Crp is an activator for ahpC expression in M. smegmatis
Two genes encoding Crp homologs (MSMEG_6189 and MSMEG_0539) occur in M. smegmatis genome. The primary structure of MSMEG_6189 is almost identical to CrpMtb (98% sequence identity), while MSMEG_0539 possesses 78% identity to CrpMtb. Expression of ahpC in the crp (MSMEG_6189) mutant strain of M. smegmatis was shown to be almost abolished or significantly decreased under induction conditions of ahpC, compared with the wild-type strain subject to the same conditions. Furthermore, purified Crp (MSMEG_6189) was shown to specifically bind to the IR1 sequence which has a high similarity to the Crp-binding consensus sequence. Both results indicate that Crp bound to IR1 serves as an activator for ahpC expression and that MSMEG_6189 is a major functional Crp protein in M. smegmatis. In many cases the role of Crp as an activator or a repressor is dependent on the position of its binding site relative to the transcription start point of its target gene. The Crp-binding sites located upstream of the promoters usually serve as activation sites and the binding sites located adjacent to the transcription start point serve as repression sites [47], [48]. In good agreement with this, the IR1 sequence is centered at −81.5 relative to the transcriptional start point of ahpC and serves as an activator-binding site. Since AhpC can remove organic peroxides, H2O2, and peroxynitrite (ONOO−) by using its peroxidase activity catalyzing the reduction of the peroxide linkage (-O-O-), increased susceptibility of the crp mutant strain to CHP and NO relative to the wild-type strain is attributable to a defect in ahpC expression in the crp mutant. AhpC was suggested to protect mycobacteria from deleterious effects of organic peroxides and peroxynitrite [5], [16], [49]. The production of reactive oxygen intermediates (ROIs) and RNIs by macrophages is considered to be the major mechanism restraining M. tuberculosis proliferation in vivo [50], [51]. The fact that a crp mutant strain of M. tuberculosis showed a reduced survival rate within macrophages and attenuated virulence in a murine infection model implies that Crp is also involved in the induction of genes related to adaptation to and defense mechanisms against ROIs and RNIs in M. tuberculosis [18]. There is a sequence (GGTGT-N6-TCACC) with a partial similarity to the Crp-binding consensus sequence (TGTGA-N6-TCACA), which is located 77 bp upstream of the M. tuberculosis ahpC gene [18]. Microarray analysis revealed that the ahpC gene is downregulated in a crp mutant of M. tuberculosis [18], indicating that ahpC of M. tuberculosis is also positively regulated by Crp.
The IR2 sequence is a cis-acting regulatory element involved in the negative regulation of ahpC expression
The location of a cis-regulatory DNA sequence determines its function in many cases. Transcriptional repressors bind predominantly to positions either overlapping with or downstream of the promoters of their target genes to prevent RNA polymerase from closed or open complex formation. The IR2 site is located between the promoter and the start codon of ahpC. Deletion of IR2 made ahpC strongly derepressed regardless of the presence and absence of CHP and its sequence (TCTGGAAT-N-ATTCCAGA) is very similar to the FurA-binding sites (TCTTGACT-N-ATTCCAGA: the nucleotides that are different from those of IR2 are underlined) located upstream of the autoregulated M. tuberculosis furA and M. smegmatis furA1 genes [43], [52]. Although we did not put forward direct evidence to support the involvement of FurA in ahpC expression due to the presence of three FurA homologs in M. smegmatis, derepression of ahpC expression under iron-depleting conditions together with the resemblance of the IR2 sequence to FurA-binding sequences strongly indicate that ahpC is under the negative regulation of FurA. Further study using a furA triple mutant is required to prove this suggestion and the construction of the mutant is under way. It was previously demonstrated by means of immunoblot analysis that the steady-state level of AhpC was not affected by inactivation of the furA1 gene in M. smegmatis [53], which can be explained by the presence of multiple FurA homologs. The functionality of FurA was suggested to be controlled by NO and ROIs [43], [54], [55]. NO and ROIs can inactivate the Fe2+-containing FurA protein, thereby inducing the genes that are under the negative regulation of FurA. This property of FurA enables it to serve as an RNI- and ROI-responsive regulator in addition to an iron-responsive regulator.
Cellular levels of cAMP correlate with the expression level of ahpC
The finding that more than 40% of genes in the Crp regulon overlap with hypoxia- and starvation-stimulated genes in M. tuberculosis gives a clue that Crp might activate expression of Crp regulon in response to increased levels of cAMP under hypoxic and starvation conditions [45], [56], [57]. Recently it was demonstrated in M. tuberculosis that increased cellular levels of cAMP by heat stress or exogenous dibutyryl cAMP treatment led to upregulation of some heat stress-induced genes that have the Crp-binding sequences in their control regions, strongly indicating that the cellular cAMP level correlates with expression levels of the Crp regulon [66]. In this study, we observed both reduced expression of ahpC in a PDE-overexpressed strain of M. smegmatis relative to the control strain and an increase in cellular levels of cAMP under oxidative stress conditions. lacZ expression from pNCM3 without IR2 was shown to be slightly induced by CHP treatment (see Figure 7B RT-PCR), which appears to be the consequence of elevated cAMP levels by CHP treatment. Taken together, these results strongly suggest that induction of ahpC expression under oxidative stress conditions probably results from a combinatory effect of both inactivation of FurA by oxidative stress and activation of Crp in response to an increase in cellular levels of cAMP.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This research was supported by Basic Science Research Program to JIO through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A2004243). The authors hereby declare that the funder, which is supporting the research related to this manuscript, had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wood ZA, Schroder E, Robin Harris J, Poole LB (2003) Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40. [DOI] [PubMed] [Google Scholar]
- 2. Hillas PJ, del Alba FS, Oyarzabal J, Wilks A, Ortiz De Montellano PR (2000) The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis . J Biol Chem 275: 18801–18809. [DOI] [PubMed] [Google Scholar]
- 3. Bryk R, Griffin P, Nathan C (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407: 211–215. [DOI] [PubMed] [Google Scholar]
- 4. Chen L, Xie QW, Nathan C (1998) Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol Cell 1: 795–805. [DOI] [PubMed] [Google Scholar]
- 5. Master SS, Springer B, Sander P, Boettger EC, Deretic V, et al. (2002) Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology 148: 3139–3144. [DOI] [PubMed] [Google Scholar]
- 6. Koshkin A, Knudsen GM, Ortiz De Montellano PR (2004) Intermolecular interactions in the AhpC/AhpD antioxidant defense system of Mycobacterium tuberculosis . Arch Biochem Biophys 427: 41–47. [DOI] [PubMed] [Google Scholar]
- 7. Guimaraes BG, Souchon H, Honore N, Saint-Joanis B, Brosch R, et al. (2005) Structure and mechanism of the alkyl hydroperoxidase AhpC, a key element of the Mycobacterium tuberculosis defense system against oxidative stress. J Biol Chem 280: 25735–25742. [DOI] [PubMed] [Google Scholar]
- 8. Chauhan R, Mande SC (2002) Site-directed mutagenesis reveals a novel catalytic mechanism of Mycobacterium tuberculosis alkylhydroperoxidase C. Biochem J. 367: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C (2002) Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 10. Jaeger T, Budde H, Flohe L, Menge U, Singh M, et al. (2004) Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis . Archives of Biochemistry and Biophysics 423: 182–191. [DOI] [PubMed] [Google Scholar]
- 11. Daugherty A, Powers KM, Standley MS, Kim CS, Purdy GE (2011) Mycobacterium smegmatis RoxY is a repressor of oxyS and contributes to resistance to oxidative stress and bactericidal ubiquitin-derived peptides. J Bacteriol 193: 6824–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pagan-Ramos E, Song J, McFalone M, Mudd MH, Deretic V (1998) Oxidative stress response and characterization of the oxyR-ahpC and furA-katG loci in Mycobacterium marinum . J Bacteriol 180: 4856–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deretic V, Philipp W, Dhandayuthapani S, Mudd MH, Curcic R, et al. (1995) Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol 17: 889–900. [DOI] [PubMed] [Google Scholar]
- 14. Dosanjh NS, Rawat M, Chung JH, Av-Gay Y (2005) Thiol specific oxidative stress response in Mycobacteria. FEMS Microbiol Lett 249: 87–94. [DOI] [PubMed] [Google Scholar]
- 15. Dhandayuthapani S, Zhang Y, Mudd MH, Deretic V (1996) Oxidative stress response and its role in sensitivity to isoniazid in mycobacteria: characterization and inducibility of ahpC by peroxides in Mycobacterium smegmatis and lack of expression in M. aurum and M. tuberculosis . J Bacteriol 178: 3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Springer B, Master S, Sander P, Zahrt T, McFalone M, et al. (2001) Silencing of oxidative stress response in Mycobacterium tuberculosis: expression patterns of ahpC in virulent and avirulent strains and effect of ahpC inactivation. Infect Immun 69: 5967–5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parish T, Smith DA, Roberts G, Betts J, Stoker NG (2003) The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149: 1423–1435. [DOI] [PubMed] [Google Scholar]
- 18. Rickman L, Scott C, Hunt DM, Hutchinson T, Menendez MC, et al. (2005) A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol 56: 1274–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domenech P, Honore N, Heym B, Cole ST (2001) Role of OxyS of Mycobacterium tuberculosis in oxidative stress: overexpression confers increased sensitivity to organic hydroperoxides. Microbes Infect 3: 713–721. [DOI] [PubMed] [Google Scholar]
- 20. Chawla M, Parikh P, Saxena A, Munshi M, Mehta M, et al. (2012) Mycobacterium tuberculosis WhiB4 regulates oxidative stress response to modulate survival and dissemination in vivo . Mol Microbiol 85: 1148–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Humpel A, Gebhard S, Cook GM, Berney M (2010) The SigF regulon in Mycobacterium smegmatis reveals roles in adaptation to stationary phase, heat, and oxidative stress. J Bacteriol 192: 2491–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gosset G, Zhang Z, Nayyar S, Cuevas WA, Saier MH Jr (2004) Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli . J Bacteriol 186: 3516–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reddy MC, Palaninathan SK, Bruning JB, Thurman C, Smith D, et al. (2009) Structural insights into the mechanism of the allosteric transitions of Mycobacterium tuberculosis cAMP receptor protein. J Biol Chem 284: 36581–36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallagher DT, Smith N, Kim SK, Robinson H, Reddy PT (2009) Profound asymmetry in the structure of the cAMP-free cAMP Receptor Protein (CRP) from Mycobacterium tuberculosis . J Biol Chem 284: 8228–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar P, Joshi DC, Akif M, Akhter Y, Hasnain SE, et al. (2010) Mapping conformational transitions in cyclic AMP receptor protein: crystal structure and normal-mode analysis of Mycobacterium tuberculosis apo-cAMP receptor protein. Biophys J 98: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stapleton M, Haq I, Hunt DM, Arnvig KB, Artymiuk PJ, et al. (2010) Mycobacterium tuberculosis cAMP receptor protein (Rv3676) differs from the Escherichia coli paradigm in its cAMP binding and DNA binding properties and transcription activation properties. J Biol Chem 285: 7016–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green J, Stapleton MR, Smith LJ, Artymiuk PJ, Kahramanoglou C, et al. (2014) Cyclic-AMP and bacterial cyclic-AMP receptor proteins revisited: adaptation for different ecological niches. Curr Opin Microbiol 18: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bai G, Schaak DD, McDonough KA (2009) cAMP levels within Mycobacterium tuberculosis and Mycobacterium bovis BCG increase upon infection of macrophages. FEMS Immunol Med Microbiol 55: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yadav M, Roach SK, Schorey JS (2004) Increased mitogen-activated protein kinase activity and TNF-α production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol 172: 5588–5597. [DOI] [PubMed] [Google Scholar]
- 30. Escolar L, Perez-Martin J, de Lorenzo V (1999) Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol 181: 6223–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pym AS, Domenech P, Honore N, Song J, Deretic V, et al. (2001) Regulation of catalase-peroxidase (KatG) expression, isoniazid sensitivity and virulence by furA of Mycobacterium tuberculosis . Mol Microbiol 40: 879–889. [DOI] [PubMed] [Google Scholar]
- 32. Maciag A, Dainese E, Rodriguez GM, Milano A, Provvedi R, et al. (2007) Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J Bacteriol 189: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press.
- 34. Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR Jr (1990) Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis . Mol Microbiol 4: 1911–1919. [DOI] [PubMed] [Google Scholar]
- 35. Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bardarov S, Bardarov S Jr, Pavelka MS Jr, Sambandamurthy V, Larsen M, et al. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis . Microbiology 148: 3007–3017. [DOI] [PubMed] [Google Scholar]
- 37. Kim MJ, Park KJ, Ko IJ, Kim YM, Oh JI (2010) Different roles of DosS and DosT in the hypoxic adaptation of Mycobacteria. J Bacteriol 192: 4868–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mouncey NJ, Kaplan S (1998) Redox-dependent gene regulation in Rhodobacter sphaeroides 2.4.1T: effects on dimethyl sulfoxide reductase (dor) gene expression. J Bacteriol 180: 5612–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oh JI, Kaplan S (1999) The cbb 3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38: 2688–2696. [DOI] [PubMed] [Google Scholar]
- 40. Rodrigue S, Provvedi R, Jacques PE, Gaudreau L, Manganelli R (2006) The sigma factors of Mycobacterium tuberculosis . FEMS Microbiol Rev 30: 926–941. [DOI] [PubMed] [Google Scholar]
- 41. Unniraman S, Chatterji M, Nagaraja V (2002) DNA gyrase genes in Mycobacterium tuberculosis: a single operon driven by multiple promoters. J Bacteriol 184: 5449–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berg OG, von Hippel PH (1988) Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J Mol Biol 200: 709–723. [DOI] [PubMed] [Google Scholar]
- 43. Sala C, Forti F, Di Florio E, Canneva F, Milano A, et al. (2003) Mycobacterium tuberculosis FurA autoregulates its own expression. J Bacteriol 185: 5357–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271: C1424–1437. [DOI] [PubMed] [Google Scholar]
- 45. Bai G, McCue LA, McDonough KA (2005) Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a cyclic AMP receptor protein-like DNA binding protein. J Bacteriol 187: 7795–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bagg A, Neilands JB (1987) Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli . Biochemistry 26: 5471–5477. [DOI] [PubMed] [Google Scholar]
- 47. Busby S, Ebright RH (1999) Transcription activation by catabolite activator protein (CAP). J Mol Biol 293: 199–213. [DOI] [PubMed] [Google Scholar]
- 48. Kahramanoglou C, Cortes T, Matange N, Hunt DM, Visweswariah SS, et al. (2014) Genomic mapping of cAMP receptor protein (CRPMt) in Mycobacterium tuberculosis: relation to transcriptional start sites and the role of CRPMt as a transcription factor. Nucleic Acids Res 42: 8320–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sherman DR, Mdluli K, Hickey MJ, Arain TM, Morris SL, et al. (1996) Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis . Science 272: 1641–1643. [DOI] [PubMed] [Google Scholar]
- 50. MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, et al. (1997) Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 94: 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD (2004) Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol 52: 1291–1302. [DOI] [PubMed] [Google Scholar]
- 52. Milano A, Forti F, Sala C, Riccardi G, Ghisotti D (2001) Transcriptional regulation of furA and katG upon oxidative stress in Mycobacterium smegmatis . J Bacteriol 183: 6801–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zahrt TC, Song J, Siple J, Deretic V (2001) Mycobacterial FurA is a negative regulator of catalase-peroxidase gene katG . Mol Microbiol 39: 1174–1185. [DOI] [PubMed] [Google Scholar]
- 54. D'Autreaux B, Touati D, Bersch B, Latour JM, Michaud-Soret I (2002) Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc Natl Acad Sci U S A 99: 16619–16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Varghese S, Wu A, Park S, Imlay KR, Imlay JA (2007) Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli . Mol Microbiol 64: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, et al. (2001) Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proc Natl Acad Sci U S A 98: 7534–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aung HL, Berney M, Cook GM (2014) Hypoxia-activated cytochrome bd expression in Mycobacterium smegmatis is cyclic AMP receptor protein dependent. J Bacteriol 196: 3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pohl E, Haller JC, Mijovilovich A, Meyer-Klaucke W, Garman E, et al. (2003) Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Mol Microbiol 47: 903–915. [DOI] [PubMed] [Google Scholar]
- 59. Jessee J (1986) New subcloning efficiency. Competent cells:>1×106 transformants/µg. Focus 8: 9. [Google Scholar]
- 60. Kriakov J, Lee S, Jacobs WR Jr (2003) Identification of a regulated alkaline phosphatase, a cell surface-associated lipoprotein, in Mycobacterium smegmatis . J Bacteriol 185: 4983–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jeong JA, Lee HN, Ko IJ, Oh JI (2013) Development of new vector systems as genetic tools applicable to mycobacteria. Journal of Life Science 23: 290–298. [Google Scholar]
- 62. Brown AK, Bhatt A, Singh A, Saparia E, Evans AF, et al. (2007) Identification of the dehydratase component of the mycobacterial mycolic acid-synthesizing fatty acid synthase-II complex. Microbiology 153: 4166–4173. [DOI] [PubMed] [Google Scholar]
- 63. Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, et al. (1991) New use of BCG for recombinant vaccines. Nature 351: 456–460. [DOI] [PubMed] [Google Scholar]
- 64. Oh JI, Park SJ, Shin SJ, Ko IJ, Han SJ, et al. (2010) Identification of trans- and cis-control elements involved in regulation of the carbon monoxide dehydrogenase genes in Mycobacterium sp. strain JC1 DSM 3803. J Bacteriol 192: 3925–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kang CM, Abbott DW, Park ST, Dascher CC, Cantley LC, et al. (2005) The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev 19: 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choudhary E, Bishai W, Agarwal N (2014) Expression of a subset of heat stress induced genes of Mycobacterium tuberculosis is regulated by 3′,5′-cyclic AMP. PLoS ONE 9: e89759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.