Abstract
Objectives:
With an increasing number of disease-modifying treatments (DMTs) for multiple sclerosis (MS), patient preferences will gain importance in the decision-making process. We assessed patients’ implicit preferences for oral versus parenteral DMTs and identified factors influencing patients’ treatment preference.
Methods:
Patients with relapsing–remitting MS (n = 156) completed a questionnaire assessing treatment preferences, whereby they had to decide between pairs of hypothetical treatment scenarios. Based on this questionnaire a choice-based conjoint analysis was conducted.
Results:
Treatment frequency and route of administration showed a stronger influence on patient preference compared with frequency of mild side effects. The latter attribute was more important for treatment-naïve patients compared with DMT-experienced patients. The higher the Extended Disability Status Scale score, the more likely pills, and the less likely fewer side effects were preferred. Pills were preferred over injections by 93% of patients, when treatment frequency and frequency of side effects were held constant. However, preference switched to injections when pills had to be taken three times daily and injections only once per week. Injections were also preferred when pills were associated with frequent side effects.
Conclusions:
Our results suggest that route of administration and treatment frequency play an important role in the patients’ preference for a given DMT.
Keywords: choice-based conjoint analysis, multiple sclerosis, oral, parenteral, patient preference, treatment frequency
Introduction
The treatment options for patients with multiple sclerosis (MS) have expanded dramatically during the past 20 years [Linker et al. 2008]. The aim of these disease-modifying treatments (DMTs) is the prevention of further relapses and disability. In the European Union, neurologists and patients can currently choose from seven different licensed first-line DMTs and this number is likely to increase within the next few years [Killestein et al. 2011], making it increasingly difficult for patients and their physicians to choose between treatments. It can be expected that convenience of treatment will therefore play a significant role in shaping the decision of patients and their physicians [Prosser et al. 2003; Patti, 2010; Killestein et al. 2011], which in turn might increase patient adherence, a critical factor for successful therapy [Patti, 2010]. Patient preferences are also relevant for those who are involved in the development or funding of new drugs as this factor is considered during the approval process in some health care systems [Zimmermann et al. 2013].
Until recently, all DMTs required either injection or intravenous infusion. In 2010 the first new oral DMT, fingolimod, became available in the US (2011 in the EU), followed by teriflunomide and dimethyl fumarate. Oral drugs offer a more convenient route of administration and we may therefore expect that patients will typically prefer them to other drugs that require parenteral administration [Wilke, 2009]. While this assumption seems plausible, it has not yet been tested in MS patients. The situation becomes more complex if we also take into consideration the fact that oral drugs typically have to be taken more frequently than parenteral drugs. How do patients decide in those circumstances? How do they trade off convenience of administration against treatment frequency or side effects?
Our study addresses these questions by applying a conjoint analysis (CA). CA is a technique for assessing consumer preferences, which is frequently used in research aimed at assessing and quantifying preferences for different products or service options [Green et al. 2001] with growing application in the health sector [Ryan and Farrar, 2000; Ryan et al. 2001; De Bekker-Grob et al. 2012]. In a choice-based conjoint (CBC) analysis, the variant used here, different levels of two or more attributes are combined in different ways to find out how these attributes are traded off against each other. The advantage of a CA over a direct customer survey is that it allows us to quantify the extent to which a certain option is preferred and to determine the relative importance of the different product attributes for the consumers’ decision [Ryan et al. 1998]. Furthermore, it avoids problems that handicap other direct measures such as the problem of social desirability (i.e. the tendency of respondents to express the preference which they think is expected from them by society or the examiner).
The aim of our study was to assess patients’ implicit preferences for oral versus parenteral DMTs and to determine how those preferences are influenced by treatment frequency, frequency of mild side effects, experience with different treatment modalities and patient characteristics. For this purpose a CBC analysis was performed in patients with relapsing–remitting MS.
Materials and methods
Target sample
Patients with relapsing–remitting MS according to the 2010 McDonald criteria [Polman et al. 2011] who had been in contact with the Department of Neurology of the Friedrich-Alexander University Erlangen-Nuremberg, Germany, between March and October 2013 were asked to participate. Patients below the age of 18 were not included. All participants gave their written informed consent prior to the investigation, which was conducted in accordance with the Declaration of Helsinki II and was approved by the local ethics committee. Patients were required to fill in two paper-based questionnaires either on site or at home and in the latter case to send back the filled-in questionnaires. The completion of the two questionnaires took approximately 20 minutes.
Preference questionnaire for CBC analysis
The data for the CBC analysis were collected via a paper-based preference questionnaire designed by us for the current study. As we aimed to assess the preference for oral versus parenteral DMTs, the attribute ‘route of administration’ with the levels ‘pill’ and ‘injection’ was predefined. The other attributes were chosen by literature research and discussion with neurologists being experienced in MS patient treatment with DMTs. To reduce complexity the number of attributes were limited to three. In addition to route of administration, the attributes ‘treatment frequency’ with 4 levels (1 monthly, 1 weekly, 1 daily, 3 daily) and ‘frequency of flu-like or gastrointestinal symptoms’ with 2 levels (2 days monthly, 7 days monthly) were included. The reason for our definition of side effects as frequency of flu-like or gastrointestinal symptoms was that we wanted to include side effects common to most of the available DMTs and which many of the patients likely had experiences with. Each choice contained the comparison of ‘pill’ versus ‘injection’. The levels of the other attributes were combined such that each combination was presented once, resulting in 64 choice scenarios (see Figure 1 for an example and Appendix A for further examples).
Figure 1.
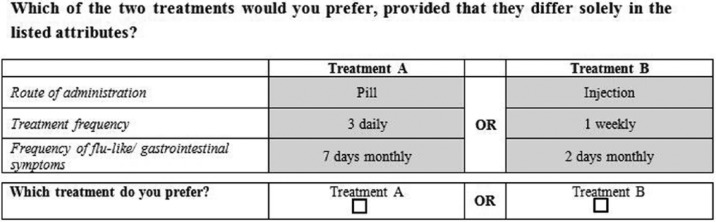
Example of a choice scenario.
Patients were instructed to decide between treatment A and B for each scenario and to assume that the therapeutic effect of both treatments is the same, as well as all other features not listed in the respective choice scenario. Patients were also informed that injection means a self-administered injection into the muscular or subcutaneous tissue.
Additional questions
In a second paper-based questionnaire, patients were asked explicitly for their preferred route of administration. Furthermore, they had to answer questions about their personal characteristics (e.g. age, profession, education), depressive symptoms, medical history of MS, their experiences with DMTs, their experience with switching DMTs and side effects (see Appendix B).
Data analysis
The data from the preference questionnaires were analysed with CBC/HB v5 (Sawtooth Software, Orem, UT, USA) using Hierarchical Bayes analysis. This technique allows the computation of individual part-worth utilities for each attribute level. Individual relative importance values of the attributes were calculated by division of the difference between the highest and lowest utility estimate (range) of an attribute by the sum of differences of all attributes, multiplied by 100. The relative importance value quantifies the influence of each attribute on the patient’s choice.
Differences of the importance values between attributes were analysed with Friedman test and subsequent Wilcoxon signed rank tests, and group differences were analysed via Kruskal–Wallis test and follow-up Mann–Whitney U-tests (IBM SPSS Statistics 19; alpha level: p = 0.05). Other group differences were computed using parametric or nonparametric tests depending on the scale of measurement of the variable and the differences in group sizes.
To determine the preference for one route of administration in relation to treatment frequency, the frequencies of choices for choice scenarios with different treatment frequencies of ‘pill’ and ‘injection’, but the same frequency of side effects (‘2 days weekly’) were compared for ‘pills’ versus ‘injections’. Likewise, the frequencies of choices for choice scenarios with different treatment frequencies of side effects for ‘pill’ and ‘injection’, but the same treatment frequency was compared for ‘pills’ versus ‘injections’.
To investigate how the patients’ characteristics influence their choice for pills or reduced side effects, we computed the utility scores for those choices and used them as dependent measures in stepwise multiple regressions with backward entry.
Results
Patient sample
Complete questionnaires were returned by 156 patients, of whom 24 patients were therapy-naïve, 92 had only experience with parenteral DMTs and 40 patients had experience with both parenteral and oral DMTs. Table 1 shows the characteristics of the different patient groups.
Table 1.
Patient characteristics.
| Characteristic | Whole sample (n = 156) | Therapy-naïve patients (n = 24) | Patients with experiences with parental DMTs only (n = 92) | Patients with experiences with parenteral and oral DMTs (n = 40) | p |
|---|---|---|---|---|---|
| Age in years, median (range) | 37 (18–72) | 38.5 (22–56) | 36 (20–72) | 38.5 (18–61) | 0.835 |
| Time since first diagnosis in months, median (range) | 52.5 (1–336) | 1 (1–228) | 60 (1–336) | 68.5 (7–312) | <0.001* |
| EDSS score, median (range) | 2 (0–7.5) | 2 (0–5) | 2 (0–7.5) | 2 (1–6) | 0.24 |
| Sex | 0.21 | ||||
| Male | 49 (31.4%) | 11 (45.8%) | 28 (30.4%) | 10 (25%) | |
| Female | 107 (68.6%) | 13 (54.2%) | 64 (69.6%) | 30 (75%) | |
| Marital status | 0.455 | ||||
| Single | 45 (28.8%) | 9 (37.5%) | 26 (28.3%) | 10 (25%) | |
| Married/partnership | 94 (60.3%) | 14 (58.3%) | 57 (61.9%) | 23 (57.5%) | |
| Divorced/separated/widowed | 17 (10.9%) | 1 (4.2%) | 9 (9.8%) | 7 (17.5%) | |
| Years of education | 0.38 | ||||
| 9 | 47 (30.1%) | 9 (37.5%) | 27 (29.3%) | 11 (27.5%) | |
| 10 | 49 (31.4%) | 9 (37.5%) | 30 (32.6%) | 10 (25%) | |
| 13 | 31 (19.9%) | 3 (12.5%) | 16 (17.4%) | 12 (30%) | |
| 18 | 29 (18.6%) | 3 (12.5%) | 19 (20.7%) | 7 (17.5%) | |
| Employment$ | 0.259 | ||||
| Fulltime job | 74 (47.4%) | 16 (66.7%) | 27 (29.3%) | 13 (32.5%) | |
| Part-time job | 39 (25%) | 6 (25%) | 21 (22.8%) | 12 (30%) | |
| Not working/retired | 42 (26.9%) | 2 (8.3%) | 43 (46.7%) | 15 (37.5%) | |
| Number of switches in DMT, median (range) | 1 (0–6) | 0 | 1 (0–5) | 2 (0–6) | <0.001‡ |
| Depression,§ median (range) | 2 (0–4) | 1 (0–3) | 2 (1–4) | 2 (1–4) | 0.051 |
| Sum score of side effects,|| median (range) | 5 (0–19) | 2 (0–3) | 4 (0–19) | 9 (0–19) | <0.05‡ |
Note: p refers to differences between the patient subgroups.
Therapy- naïve patients differ from other groups. $ Two patients did not respond. The values refer to n =155 for the whole sample and n = 91 for patients experienced with parental DMTs. ‡ All groups differ. § Patients had to indicate how often they felt depressive during the preceding three months (1 = seldom, 2 = sometimes, 3 = often, 4 = most of the time). || For further details, see the section on the influence of patient characteristics on preferences.
DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale.
Preferences
The Hierarchical Bayes analysis revealed a percent certainty of 0.835, meaning that the found solution is 83.5% better than chance. In comparison, a ‘perfect’ solution would correspond to 100%. Table 2 shows the mean utility scores for the different attribute levels. Within a given attribute, levels with higher utility scores are those that will be preferred by the patients. ‘Pill’ was the preferred route of administration to ‘injection’ and the lesser the treatment frequency the higher the utility score. Furthermore, less frequent flu-like or gastrointestinal symptoms were preferred to more frequent symptoms.
Table 2.
Utility scores of the choice-based conjoint analysis.
| Attribute | Level | Utility (SD) |
|---|---|---|
| Route of administration | Pill | 3.61 (2.22) |
| Injection | −3.61 (2.22) | |
| Treatment frequency | 1 monthly | 3.74 (2.48) |
| 1 weekly | 2.35 (1.35) | |
| 1 daily | −0.49 (0.88) | |
| 3 daily | −5.61 (3.31) | |
| Frequency of flu-like or gastrointestinal symptoms | 2 days monthly | 2.25 (1.78) |
| 7 days monthly | −2.25 (1.78) |
SD = mean within-patient standard deviation for each patient’s utility score. Utilities sum to zero within each attribute [Zimmermann et al. 2013].
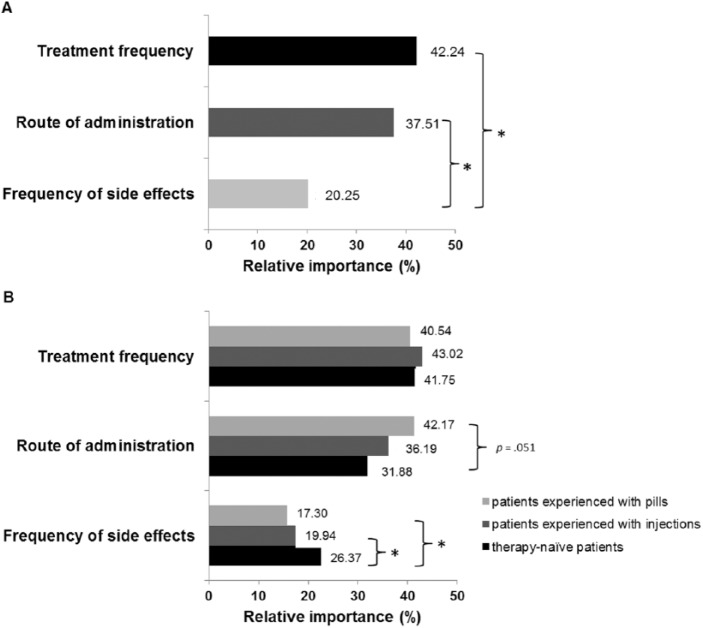
Friedman test showed a significant difference between the relative importance of the attributes [χ2(2) = 86.36; p < 0.001]. The relative importance of treatment frequency (z = −9.19; p < 0.001) as well as of route of administration (z = −1.47; p < 0.001) was significantly higher than the relative importance of frequency of side effects (Figure 2A). No significant difference between the relative importance of treatment frequency and route of administration was observed (z = −4.79; p = 0.141).
Figure 2.
Preference judgements: relative importance values of the different attributes. (A) Relative importance values of attributes for the whole sample. (B) Relative importance of attributes for different patient groups.
*p < 0.05.
Furthermore, Kruskal–Wallis tests revealed a significant group difference in the relative importance regarding frequency of side effects [H(2) = 7.36; p = 0.025], but not for route of administration or treatment frequency (all p > 0.05). The relative importance of frequency of side effects was significantly higher for therapy-naïve patients compared with patients experienced with injections (U = 790; z = −2.14; p = 0.032) and patients experienced with pills (U = 284; z = −2.718; p = 0.007). There was a trend, though not significant, towards a higher relative importance of route of administration for patients experienced with pills compared with treatment-naïve patients (U = 339; z = −1.955; p = 0.051). Further group differences were not found (all p > 0.05; Figure 2B).
Preference for one route of administration in relation to treatment frequency and frequency of side effects
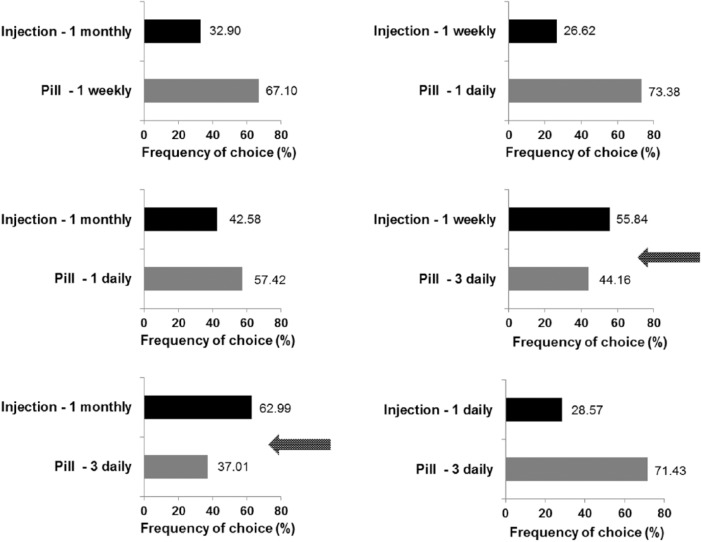
To illustrate what the findings on utility scores and relative importance actually mean, we directly compared different types of compounds (fictional drugs) and calculated how many patients prefer either of the two substances. Assuming everything to be equal (i.e. averaged across all treatment frequencies and with only mild side effects), pills are preferred by 93% of patients yielding a similar result to the explicitly stated preference for oral DMTs by 83% of patients. This preference for pills is, however, balanced against the treatment frequency. If pills have to be taken three times daily (as compared with an injection once per week or once per month), injections are preferred (for more details, see Figure3).
Figure 3.
Preference judgements: frequency of choices for one route of administration in relation of treatment frequency with frequency of side effects held constant at 2 days weekly.
Arrows indicate treatment scenarios where more patients preferred injections compared with pills.
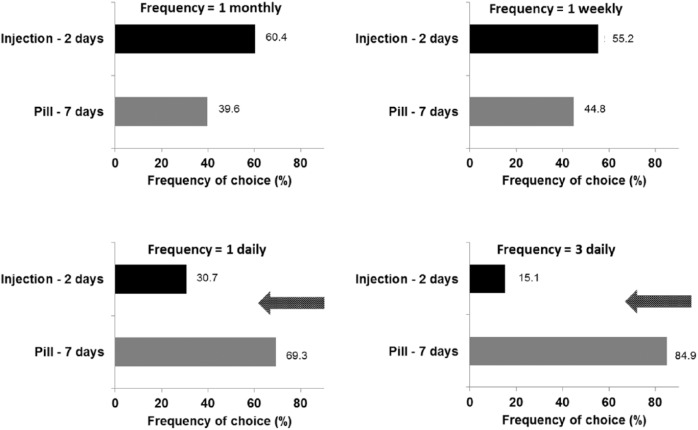
When pills were combined with side effects 2 days monthly and injection with side effects 7 days monthly and treatment frequency was held constant, 97.9% (range: 96.8–99.4%) of patients preferred pills and 2.1% (0.7–3.3%) preferred injections (averaged over all treatment frequencies). When pills were combined with the less preferred frequency of side effects (7 days monthly) and injections with side effects 2 days monthly, injections were preferred over pills, when treatment frequency was held constant at one per month or one per week for both treatment options. However, more frequent side effects associated with pills were tolerated when the treatment frequency for both routes of administration was one or three per day (Figure 4).
Figure 4.
Preference judgements: frequency of choices for one route of administration in relation of frequency of side effects with treatment frequency held constant.
Treatment scenarios are displayed where pills are combined with side effects 7 days monthly and injections with side effects 2 days monthly.
Treatment frequency for both routes of administration was held constant at the value indicated at the top of each graph.
Arrows indicate treatment scenarios where more patients preferred pills compared with injections.
Influence of patient characteristics on preferences
The stepwise multiple regression with the utility score of ‘pill’ as dependent variable and age, sex, Expanded Disability Status Scale (EDSS) score, time since diagnosis, number of switches in DMTs and depression as independent variables yielded the following results. The full model was not significant, but a model with sex, EDSS, time since diagnosis and number of switches in DMTs as independent variables was significant and accounted for 7.9% of variance in the utility score for ‘pill’ (R2 = 0.079; F4,134= 2.847; p = 0.026). EDSS score [squared semipartial correlation (sr2) = 0.049; standardized β = 0.27; p = 0.009] was the only significant predictor, meaning that patients with higher EDSS score were more likely to prefer pills over injections. Sex, time since diagnosis and number of switches in DMTs were not predictive for the utility score of ‘pill’.
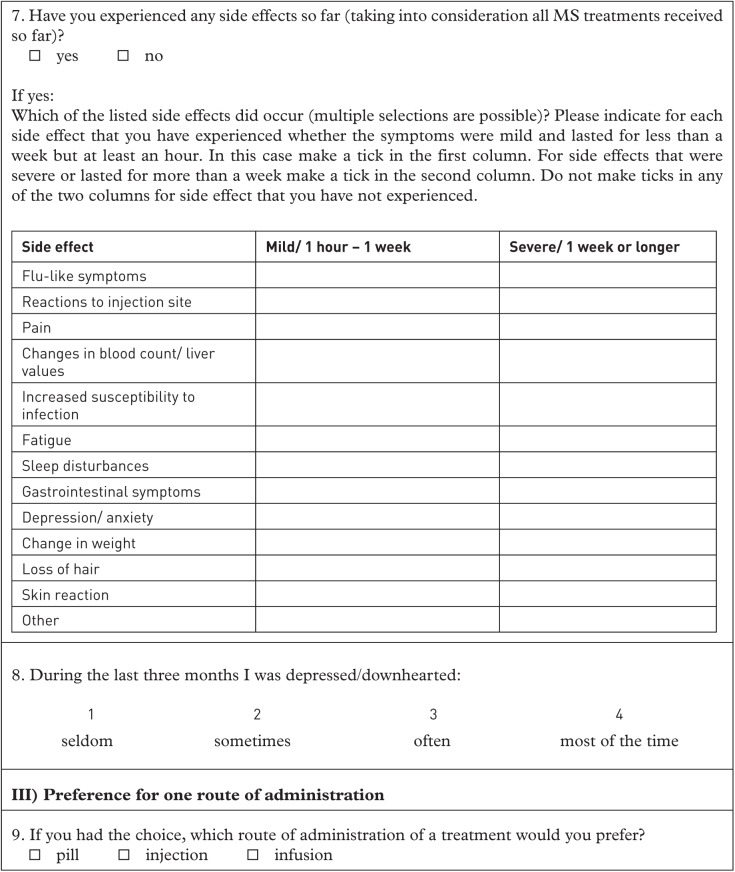
A sum score of side effects was computed based on patients’ reports. A list of 13 side effects (see Table 3) was provided and patients were required to indicate whether and how severe they had experienced one or several side effects when taking any of the DMTs. For every patient the sum of the ratings of each of the listed side effects was calculated (no = 0; mild = 1; severe = 2), ranging between 0 and 19. Then, a stepwise multiple regression with the utility score of side effects ‘2 days weekly’ as dependent variable and age, sex, EDSS score, time since diagnosis, number of switches in DMTs, depression and sum score of side effects as independent variables was computed.
Table 3.
Frequency and severity of DMT-related side effects as percentages.
| Side effect | No (%) | Mild (1 h to 1 week; %) | Severe (1 week or longer; %) |
|---|---|---|---|
| Flu-like symptoms | 23.6 | 55.5 | 20.9 |
| Reactions to injection site | 31.8 | 34.6 | 33.6 |
| Pain | 32.7 | 41.8 | 25.5 |
| Changes in blood count/ liver values | 67.3 | 19.1 | 13.6 |
| Increased susceptibility to infection | 71.8 | 17.3 | 10.9 |
| Fatigue | 37.3 | 25.4 | 37.3 |
| Sleep disturbances | 60.0 | 21.8 | 18.2 |
| Gastrointestinal symptoms | 75.4 | 18.2 | 6.4 |
| Depression/ anxiety | 66.4 | 20.0 | 13.6 |
| Change in weight | 69.1 | 14.5 | 16.4 |
| Loss of hair | 72.7 | 16.4 | 10.9 |
| Skin reaction | 62.7 | 19.1 | 18.2 |
| Other | 87.3 | 11.8 | 0.9 |
DMT, disease-modifying treatment.
In order to include only judgements of patients having experiences with side effects, the data of therapy-naïve patients and patients having experienced no side effects during their treatment were excluded from this analysis, resulting in a sample of n = 105. The full model was not significant, but a model with EDSS score, time since diagnosis and sum score of side effects as independent variables was significant and accounted for 7.5% of variance in the utility score for side effects ‘2 days weekly’ (R2 = 0.075; F3,104= 2.738; p = 0.047). Again, EDSS score (sr2 = 0.057; standardized β = −0.285; p = 0.014) was the only significant predictor, meaning that patients with higher EDSS score were less likely to prefer treatment with side effects ‘2 days weekly’. Time since diagnosis and sum score of side effects was not predictive.
Discussion
The majority of patients (93%) preferred pills over injections when treatment frequency and frequency of side effects were held constant. When comparing the different utilities for the levels of attributes, pills, low treatment frequency and infrequent side effects were preferred. Of particular interest is the comparative importance of attributes. Our patients’ choices were more strongly influenced by treatment frequency and route of administration than frequency of side effects. This relation between the importance values of attributes was modulated by the patients’ treatment experiences. Treatment-naïve patients’ preferences were more influenced by frequency of side effects compared with patients experienced with pills. In contrast, the latter group tended to be more influenced by route of administration compared with treatment-naïve patients, according to the motto ‘anyone who has ever enjoyed the benefits of oral medication, appreciates them’.
As the results of the explicit (83%) and implicit (93%) preference for oral over parenteral DMTs are similar, one could ask, why one should expend effort with a CA. One answer is that a CA allows showing how route of administration is traded off against treatment frequency and frequency of side effects. The general preference for oral DMTs switched to preference for injections when a pill had to be taken three times daily compared with an injection applied once monthly or weekly or when associated with less frequent side effects (treatment frequency held constant at one per month or one per week). This knowledge can be used to predict which combination of DMT attributes will be especially attractive for MS patients. Although the chosen attribute levels of frequency here do not correspond one to one to all currently available DMTs, our results imply that higher frequency can change patient preference.
In our regression models, the only significant predictor for the preference for pill and the preference for reduced side effects was the EDSS score. The higher the EDSS score the higher the preference value for pills and the lower the preference value for mild side effects. One reason for this observation could be that patients with higher EDSS scores have suffered longer from the disease, had more experience with DMTs, and therefore possess a better appreciation of the convenience associated with oral treatment. However, number of switches in DMTs was not a significant predictor for the preference for pill and the preference for few side effects.
It is important to note that the results of a CA depend very much on the definition of attributes and attribute levels. There are several other attributes such as severe side effects or efficacy which are known to influence MS patients’ preferences for DMTs [Johnson et al. 2009]. Those attributes may have a higher importance for patient preference than route of administration or treatment frequency. However, the more attributes the more possible combinations of scenarios and the more complex their evaluation for the patients. Furthermore, severe side effects such as progressive multifocal leukoencephalopathy are bound to one specific DMT (natalizumab) and thus cannot be generalized to other DMTs. In contrast, the efficacy of many DMTs is in a similar range and thus only few attribute levels can be differentiated. Therefore, we decided to limit the number of included attributes to three and to define the different levels for the attribute side effect by varying the assumed frequency of common side effects such as flu-like or gastrointestinal symptoms. In future studies, however, it would be good to include efficacy and frequency of severe side effects as attributes. The same is true for intravenous infusion as another route of administration. In the current study we only distinguished between oral administration and injections. However, since several intravenously administered antibodies are currently tested and one of them, alemtuzumab, was recently approved in Europe, it seems to be of particular interest to assess patients’ preference values for intravenously administered drugs.
We acknowledge the following limitations to our study. Firstly, we only recruited patients from the outpatient service and hospital wards of a neurological university hospital. It is possible that patients treated by resident neurologists may have different preferences. Future studies should therefore also include patients treated outside of a university hospital, for example, patients treated in local clinics and by resident neurologists. Secondly, it should be acknowledged that our patient sample is somewhat heterogeneous. Patients without any experience with DMT and those with experience with DMTs were included. Typically patients with experience had suffered longer from MS than those without experience and this difference should be taken into account when interpreting the different preference values obtained for these two groups of patients. However, it is noteworthy that the two groups did not differ with respect to their EDSS scores. Thirdly, the questionnaire used to assess clinical and demographical data of the patients had not been previously validated in another patient sample. The statements reflect subjective judgements of the patients which we did not objectively verify. While we think this issue is not relevant for the questions about demographical information, it might be more critical for questions about depressive feelings, explicit preference for a route of administration, number of switches in DMTs or experienced side effects. This should be kept in mind when interpreting our results.
In conclusion, this study shows that a majority of MS patients prefer oral DMTs. Their preference changes when the frequency of the oral treatment is substantially higher than that of the injectable treatment or when oral treatment is associated with more side effects (treatment frequency held constant at one/three daily). This knowledge may aid in helping patients to choose among the growing number of available DMTs [Stacey et al. 2011, Palace, 2013] and may be used to guide the development of new DMTs.
Acknowledgments
We thank all patients for their participation in the study. Furthermore, we thank Julia Kratzer and Tanja Stirnweiss for assistance in the recruitment of patients.
Appendix A
The following pages present the English translation of the instruction and the first eight choice scenarios of the questionnaire used for the conjoint analysis.
Dear patient,
On the following pages you will be presented repeatedly with a choice between two types of treatments for multiple sclerosis. The presented medications are not real but hypothetical treatments invented for research purposes. We would like to ask you to compare the two treatments and to indicate in each case which treatment you would choose. You should make your choice based on the assumption that both treatments have the same efficacy and are identical in all attributes except those stated explicitly in the description. ‘Injection’ refers to an injection that is administered by the patients themselves into their muscle or subcutaneous tissue. Each and every one of your answers is important to us. Please make sure that you do not pass over any of the questions, not even those questions where the choice might be difficult.
Which of the two treatments would you prefer, provided that they differ solely in the listed attributes?
-
1)
Treatment A Treatment B Route of administration Pill Injection Treatment frequency 3 daily OR 1 weekly Frequency of flu-like/ gastrointestinal symptoms 7 days monthly 2 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
2)
Treatment A Treatment B Route of administration Injection Pill Treatment frequency 1 weekly OR 1 monthly Frequency of flu-like/ gastrointestinal symptoms 7 days monthly 2 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
3)
Treatment A Treatment B Route of administration Pill Injection Treatment frequency 1 weekly OR 1 daily Frequency of flu-like/ gastrointestinal symptoms 2 days monthly 2 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
4)
Treatment A Treatment B Route of administration Injection Pill Treatment frequency 1 monthly OR 3 daily Frequency of flu-like/ gastrointestinal symptoms 7 days monthly 7 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
5)
Treatment A Treatment B Route of administration Injection Pill Treatment frequency 1 weekly OR 1 weekly Frequency of flu-like/ gastrointestinal symptoms 7 days monthly 7 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
6)
Treatment A Treatment B Route of administration Injection Pill Treatment frequency 1 daily OR 1 weekly Frequency of flu-like/ gastrointestinal symptoms 2 days monthly 7 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
7)
Treatment A Treatment B Route of administration Pill Injection Treatment frequency 1 daily OR 1 weekly Frequency of flu-like/ gastrointestinal symptoms 7 days monthly 2 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □ -
8)
Treatment A Treatment B Route of administration Pill Injection Treatment frequency 1 monthly OR 1 weekly Frequency of flu-like/ gastrointestinal symptoms 7 days monthly 2 days monthly Which treatment do you prefer? Treatment A OR Treatment B □ □
Appendix B
The following pages represent the English translation of the questions used to assess demographical and clinical patient data.
Please answer the following questions by choosing the most appropriate response option for you.
Footnotes
Conflict of interest statement: R.A.L. received travel grants or personal fees from Bayer, Biogen Idec, Genzyme, Merck Serono, Novartis and TEVA as well as research support from Biogen Idec, Merck Serono and Novartis. K.S.U. and T.S. received research support from Biogen Idec and Novartis. A. Waschbisch received grants and personal fees from Biogen Idec, Merck Serono and Bayer Schering, as well as personal fees from Teva/Sanofi and Genzyme. D.H.L. received personal fees from Genzyme and Biogen Idec. S.B. received personal fees from Biogen Idec and Novartis. A.L., B.J., J.A. and A. Wentrup declare no conflict of interest.
Funding: This work was supported by Biogen Idec.
Contributor Information
Kathrin S. Utz, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Schwabachanlage 6, 91054 Erlangen, Germany
Jana Hoog, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
Andreas Wentrup, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
Sebastian Berg, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
Alexandra Lämmer, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
Britta Jainsch, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
Anne Waschbisch, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
De-Hyung Lee, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
Ralf A. Linker, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany
Thomas Schenk, Department of Neurology, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany.
References
- De Bekker-Grob E., Ryan M., Gerard K. (2012) Discrete choice experiments in health economics: a review of the literature. Health Econ 21: 145–172 [DOI] [PubMed] [Google Scholar]
- Green P., Krieger A., Wind Y. (2001) Thirty years of conjoint analysis: reflections and prospects. Interfaces 31: S56–S73 [Google Scholar]
- Johnson F., Van Houtven G., Ozdemir S., Hass S., White J., Francis G., et al. (2009) Multiple sclerosis patients’ benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol 256: 554–562 [DOI] [PubMed] [Google Scholar]
- Killestein J., Rudick R., Polman C. (2011) Oral treatment for multiple sclerosis. Lancet Neurol 10: 1026–1034 [DOI] [PubMed] [Google Scholar]
- Linker R., Kieseier B., Gold R. (2008) Identification and development of new therapeutics for multiple sclerosis. Trends Pharmacol Sci 29: 558–565 [DOI] [PubMed] [Google Scholar]
- Palace J. (2013) Partnership and consent in MS treatment choice. J Neurol Sci 335: 5–8 [DOI] [PubMed] [Google Scholar]
- Patti F. (2010) Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adher 4: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C. H., Reingold S. C., Banwell B., Clanet M., Cohen J. A., Filippi M., et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. [Research Support, Non-U.S. Gov't]. Ann Neurol 69(2): 292–302. DOI: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser L., Kuntz K., Bar-Or A., Weinstein M. (2003) Patient and community preferences for treatments and health states in multiple sclerosis. Mult. Scler. 9: 311–319 [DOI] [PubMed] [Google Scholar]
- Ryan M., Farrar S. (2000) Using conjoint analysis to elicit preferences for health care. Br Med J 320: 1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M., Mcintosh E., Shackley P. (1998) Methodological issues in the application of conjoint analysis in health care. Health Econ 7: 373–378 [DOI] [PubMed] [Google Scholar]
- Ryan M., Scott D., Reeves C., Bate A., Van Teijlingen E., Russell E., et al. (2001) Eliciting public preferences for healthcare: a systematic review of techniques. Health Technol Assess 5: 1–186 [DOI] [PubMed] [Google Scholar]
- Stacey D., Bennett C., Barry M., Col N., Eden K., Holmes-Rovner M., et al. (2011) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev DOI: 10.1002/14651858.CD001431.pub3 [DOI] [PubMed] [Google Scholar]
- Wilke T. (2009) Patient preferences for an oral anticoagulant after major orthopedic surgery: results of a German survey. Patient 2: 39–49 [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Clouth J., Elosge M., Heurich M., Schneider E., Wilhelm S., et al. (2013) Patient preferences for outcomes of depression treatment in Germany: a choice-based conjoint analysis study. J Affect Disord 148: 210–219 [DOI] [PubMed] [Google Scholar]