Abstract
Objective
Dysregulation of c-MET signaling pathway results from various molecular mechanisms including mutations, amplification, and overexpression. Overexpression and amplification of c-MET have been correlated with poor clinical outcome in gastric cancer, whereas the associations between c-MET polymorphisms and prognosis have not been well defined. We examined the prognostic impact of functional polymorphisms of MET gene on clinical outcome in gastric cancer.
Methods
Candidate polymorphisms of MET gene were analyzed by PCR-based direct sequencing for the associations with clinical outcome across three independent cohorts, including 161 Japanese, 101 U.S. and 63 Austrian patients, with loco-regional gastric cancer treated with surgery.
Results
The univariable analysis showed patients with any G (A/G or G/G genotype) allele of MET rs40239 had significantly longer disease-free survival and overall survival compared to those with the AA genotype in the Japanese cohort (HR: 0.43; P=0.001, HR: 0.47; P=0.006, respectively); this remained significant upon multivariable analysis adjusted for age, sex, stage, and type of adjuvant therapy (HR: 0.48; P=0.009, HR: 0.50; P=0.017, respectively). However, there was no significant association of the polymorphism with clinical outcome in the U.S. and Austrian cohort. When stratified by gender in the Japanese cohort, males, but not females, with the G allele maintained a clinical outcome benefit in both univariable and multivariable analysis.
Conclusions
The MET rs40239 may serve as a prognostic biomarker in loco-regional gastric cancer. These data also suggest that genetic variants of the c-MET may have a gender-related difference in the impact on clinical outcome.
Keywords: c-MET, polymorphism, prognostic biomarker, gastric cancer, Japanese
Introduction
The development strategy of molecularly targeted drugs is now strongly focused on genetic alterations found in specific malignancies, with clinical trial enrollment limited to patients with tumors exhibiting oncogenic alterations affecting target kinases, including gene amplifications, point mutations, and chromosomal translocations. Mutations and amplifications of certain kinases are associated with human gastric carcinogenesis [1]. A role of human epidermal growth factor receptor 2 (HER2) as a prognostic factor in gastric cancer remains controversial, although overexpression of the HER2 served as a predictive marker for response to anti-HER2 antibody. Some studies demonstrated the HER2 overexpression had no impact on survival, whereas other studies showed associations with poor outcomes in patients with gastric cancer [2]. Besides, the HER2 overexpression is associated with intestinal-type or gastroesophageal junctuion (GEJ) cancers [3], whereas loss-of-function of E-cadherin and c-MET overexpression are more likely to be found in diffuse-type [4,5]. Several studies using immunohistochemistry (IHC) have reported that frequency of the c-MET overexpression was higher than that of the HER2 [6,7], suggesting that the c-MET may be a clinically useful biomarker in gastric cancer. Molecular diversities of genetic alternations which reflect clinicopathologic features based on regional differences may impact the outcome of molecular biomarkers. Understanding of the molecular biologic and pathologic characteristics of gastric cancer is critical to identify predictive or prognostic biomarkers.
The N-methyl-N0-nitroso-guanidine human osteosarcoma transforming gene (MET) tyrosine kinase is a cell-surface receptor for hepatocyte growth factor (HGF) that plays a pivotal role in tumor cell proliferation, apoptosis, and migration as well as in the process of tissue repair [8,9]. c-MET pathway is activated not only in cancer but also in degenerative diseases, such as renal and lung fibrosis [10,11]. Cellular deregulation of the c-MET can occur through mechanisms including the c-MET overexpression, genomic amplification, mutation, or alternative splicing [9]. Activation of the c-MET results in activation of downstream signaling intermediates such as mitogen-activated protein kinase, mammalian target of rapamycin pathway, and signal transducer and activator of transcription pathway. The c-MET has also been shown to interact with other cell-surface receptors to enhance downstream signaling and tumorigenesis and to promote drug resistance [12]. There are many cross-talks between the c-MET, epidermal growth factor receptor (EGFR), and Wnt/b-catenin signaling [13–16].
The overexpression and amplification of the c-MET have been found frequently in gastric cancer [17,18]. c-MET gene amplification causes protein overexpression and constitutive activation of the kinase domain and has also been reported in different human cancers [19–21]. Approximately 10–20% of gastric cancer tissues and up to 40% of the scirrhous histological subtype was shown to harbor increased c-MET gene copy numbers [22,23]. Thus, targeting the c-MET pathway would be a promising therapeutic approach that may potentiate the standard treatments for patients with gastric cancer. More recently, it was reported that c-MET polymorphism rs11762213 might increase risk of recurrence after nephrectomy in patients with localized renal-cell carcinoma [24]. However, to the best of our knowledge, there are still no studies indicating associations of the c-MET polymorphisms with prognosis in gastrointestinal cancer including gastric cancer. We hypothesized that polymorphisms of c-MET will be associated with clinical outcome in patients with loco-regional gastric cancer treated with surgery. We therefore evaluated the prognostic impact of the polymorphisms on clinical outcome across three independent cohorts harboring each different ethnicity.
Methods
Patients
This study enrolled the three independent cohorts, Japanese, U.S. and Austrian cohort. The Japanese cohort consisted of 161 Japanese patients with histologically confirmed (stage IB to stage IV; AJCC 6th) loco-regional gastric adenocarcinoma treated with surgery alone or surgery plus S-1 or fluoropyrimidine-based adjuvant chemotherapy in Japan [Fukushima Red Cross Hospital (Fukushima) and Kitasato University East Hospital (Sagamihara)] between 1991 and 2011. The U.S. cohort consisted of 101 patients with histologically confirmed (stage IA to stage IV; AJCC 6th) loco-regional gastric adenocarcinoma treated with surgery alone or surgery plus fluoropyrimidine-based adjuvant (radio)-chemotherapy in United States [University of Southern California (USC)/Norris Comprehensive Cancer Center (Los Angeles, CA), Los Angeles County Hospital (Los Angeles, CA)] between 1992 and 2007. The Austrian cohort consisted of 63 patients with histologically confirmed (stage IA to stage IIIB; AJCC 6th) loco-regional gastric adenocarcinoma treated with surgery alone or surgery plus fluoropyrimidine-based adjuvant chemotherapy in Austria [Comprehensive Cancer Center Graz, Medical University of Graz (Graz)] between 2001 and 2010. Patients were followed clinically every 3 months for the first 2 years and then every 6 months. Patient data were collected retrospectively through chart review. Pathologic stage was decided according to tumor–node–metastasis (TNM) classification sixth edition. The tissue analysis presented in this study was conducted at the USC/Norris Comprehensive Cancer Center following approval by the USC Institutional Review Board (IRB) of Medical Sciences. Patients signed an informed consent for the analysis of molecular correlates. The Medical University of Graz IRB approved the retrospective analysis in a few patients without an informed consent.
DNA extraction, single-nucleotide polymorphism (SNP) selection and genotyping
Genomic DNA was extracted from peripheral blood or formalin-fixed paraffin-embedded (FFPE) tissue derived from tumor samples to obtain germline DNA using the QIAmp Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol (www.qiagen.com). The blood samples were used in 24 of all Japanese patients and all patients of the U.S. cohort, the FFPE samples were used in the other Japanese patients and all patients of the Austrian cohort. The reference SNP identification numbers, location, base exchange, predictive function, forward and reverse primer are summarized in Table 1. Stringent predefined criteria were used to select candidates for SNPs and included (a) SNPs, which shown to be of biological significance according to the literature review [either published data or predicted function using Functional-SNP (F-SNP) database, http://compbio.cs.queensu.ca/F-SNP] [25,26] and (b) a minor allele frequency ≥ 10% in Asians and Caucasians (with relative allelic frequencies of polymorphisms in different ethnicities obtained from the genetics section in the Ensembl Genome Browser: http://uswest.ensembl.org/index.html). The candidates for SNPs were tested by using PCR-based direct DNA sequence analysis. Briefly, forward and reverse primers were used for PCR amplification. PCR fragments were sequenced on an ABI 3100A Capillary Genetic Analyzer (Applied Biosystems) and analyzed in either sense or antisense directions for the presence of the polymorphism. DNA sequence analyses were performed by using the ABI Sequencing Scanner v1.0 (Applied Biosystems). For quality control purposes, a random selection of 10% of the samples was examined for each polymorphism and genotype concordance rate was 100%.
Table 1.
Analyzed polymorphisms in MET gene and the functional significance
| rs number | Location of polymorphism |
Base exchange |
Predictive function of polymorphism |
Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|---|---|---|
| rs41736 | Exon | C>T | Exonic Splicing Enhancer/Exonic Splicing Silencer | GGGAGCTGATGACAAGAGGA | AAGGGGTCTGGGCAGTATTC |
| rs41739 | 3'-UTR | A>G | MicroRNA binding | TTGGGGAGTTTTATTTTGCATT | TCAGGGTGGCTATTCCATCT |
| rs40239 | Intron | A>G | Tag SNP | GTTCCTATTGGCACGTGGTT | GCCATGATGGATTCAGGTTT |
| rs1621 | 3'-UTR | A>G | MicroRNA binding | AGGCAATTGAAAATCCCAGCT | GAGTAACCTACACCACATGCAC |
| rs10234854 | Intron | G>A | Tag SNP | GAGGCACATTCTGGTTCTATTGT | CCCCTCAAACTACCACCACT |
| rs184953 | 5’-UTR | C>A | Transcription Factor Binding | GCGGTGCCCAAATCTCTCTA | CTTGGGAGGCGAGTGTCA |
Statistical analysis
The primary endpoints of current study were overall survival (OS) and disease-free survival (DFS) or time-to-tumor recurrence (TTR). The OS was defined as the period from the date of surgery to death. The DFS and TTR were defined as the period from the date of surgery to the date of the first documented relapse or death, and the first observation of tumor recurrence, respectively. The OS was censored at the date when patients were alive, the DFS was censored at the date of last follow-up if patients were still relapse-free and alive, and the TTR was censored at the date of death or last follow-up if patients remained tumor recurrence-free at that time. Chi-square tests were performed to examine differences in baseline patient characteristics between three cohorts. Kaplan–Meier curves and log-rank tests were conducted for univariable analysis of the association between the c-MET polymorphism and DFS or TTR and OS. A forward stepwise Cox regression model was conducted to select baseline patient demographic and tumor characteristics in the Japanese cohort, to be included in the multivariable analyses of the c-MET polymorphisms and clinical outcome. Tumor stage, gender, age, and type of adjuvant chemotherapy which were significantly associated with DFS or OS at 0.10 level were adjusted in multivariable Cox model to evaluate the independent effects of the c-MET polymorphisms on DFS and OS in the Japanese cohort. Additionally, interactions between the c-MET polymorphisms and gender on DFS and OS were tested by comparing likelihood ratio statistics between the baseline and nested Cox regression models that include the multiplicative product term. With the sample size of 161 patients, we would have 80% power to identify the polymorphisms with hazard ratio of 1.92 to 2.16 and minor allele frequency of >10% using a 2-sided log-rank test. To simplify the scenarios of power calculation, we only considered the dominant model of inheritance. All tests were two-sided at a 0.05 significance level and performed by using the SAS statistical package version 9.3. (SAS Institute, Cary, NC, USA).
Results
The baseline characteristics in the three cohorts were summarized in Table 2. In the Japanese cohort, median follow-up time was 4.0 years. The median DFS and OS were 4.8 and 5.8 years, respectively. All patients were Eastern Cooperative Oncology Group (ECOG) PS 0 or 1. In the U.S. cohort, median follow-up time was 3.3 years. The median TTR and OS were 2.2 and 4.1 years, respectively. Ninety-three (92 %) of all patients were ECOG PS 0 or 1. In the Austrian cohort, median follow-up time was 6.5 years. The median TTR and OS were 4.9 and 9.4 years, respectively. The U.S. cohort was more likely to have young patients compared with the other cohorts. The Austrian cohort was more likely to have early stage, diffuse-type pathology and less frequent adjuvant chemotherapy compared with the other cohorts. With respect to primary tumor site, a higher incidence of GEJ cancer was significantly found in both the U.S. and Austrian cohort compared with the Japanese cohort.
Table 2.
Baseline clinical characteristics of Japanese, U.S. and Austrian cohort in loco-regional gastric cancer patients
| Japan (n=161) |
U.S. (n=101) |
Austria (n=63) |
p-value* | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender | |||||||
| Male | 103 | 64.0 | 61 | 60.4 | 32 | 50.8 | |
| Female | 58 | 36.0 | 40 | 39.6 | 31 | 49.2 | 0.19 |
| Age (year) | |||||||
| Median (range) | 68 (31–88) | 57 (26–85) | 66 (38–86) | <0.001 | |||
| < 65 | 60 | 37.3 | 81 | 80.2 | 28 | 44.4 | |
| 65 – 74 | 57 | 35.4 | 12 | 11.9 | 21 | 33.3 | <0.001 |
| ≥ 75 | 44 | 27.3 | 8 | 7.9 | 14 | 22.2 | |
| Stage | |||||||
| I – II | 76 | 47.2 | 36 | 35.6 | 37 | 59.7 | 0.01 |
| III – IV | 85 | 52.8 | 65 | 64.4 | 25 | 40.3 | |
| Tumor stage | |||||||
| T1 | 12 | 7.5 | 1 | 1.0 | 1 | 1.6 | |
| T2 | 61 | 37.9 | 36 | 36.0 | 37 | 58.7 | <0.001 |
| T3 | 85 | 52.8 | 54 | 54.0 | 24 | 38.1 | |
| T4 | 3 | 1.8 | 9 | 9.0 | 1 | 1.6 | |
| N stage | |||||||
| N0 | 36 | 22.4 | 21 | 20.8 | 19 | 30.2 | |
| N1 | 79 | 49.0 | 49 | 48.5 | 19 | 30.2 | 0.14 |
| N2 | 32 | 19.9 | 19 | 18.8 | 13 | 20.6 | |
| N3 | 14 | 8.7 | 12 | 11.9 | 12 | 19.0 | |
| Tumor site | |||||||
| Stomach | 159 | 98.7 | 60 | 59.4 | 49 | 77.8 | |
| GEJ | 2 | 1.3 | 30 | 29.7 | 14 | 22.2 | <0.001 |
| unknown | 0 | 11 | 10.9 | 0 | |||
| Lauren classification | |||||||
| Intestinal | 64 | 39.8 | 36 | 35.6 | 13 | 20.6 | |
| Diffuse | 97 | 60.2 | 29 | 28.7 | 27 | 42.9 | <0.001 |
| Mixed | 0 | 18 | 17.8 | 7 | 11.1 | ||
| Unknown | 0 | 18 | 17.8 | 16 | 25.4 | ||
| Adjuvant chemotherapy | |||||||
| Yes | 103 | 64.0 | 78 | 77.2 | 2 | 3.3 | |
| No | 58 | 36.0 | 23 | 22.8 | 58 | 96.7 | <0.001 |
| Ethnicity | |||||||
| Asian | 161 | 100 | 24 | 23.8 | 0 | ||
| Caucasian | 0 | 35 | 34.7 | 63 | 100 | NA | |
| Hispanic | 0 | 41 | 40.6 | 0 | |||
| Other | 0 | 1 | 1.0 | 0 | |||
Abbreviations: GEJ, Gastroesophageal Junction
Based on Chi-square test or Wilcoxon test whenever appropriate.
Univariable and multivariable analysis in the Japanese cohort
In the Japanese cohort, we found a significant association between MET rs40239 and both DFS and OS in univariable analysis among 6 candidates for SNPs. The patients with any G (A/G or G/G genotype) allele had significantly longer DFS and OS compared to those with the A/A genotype although both median DFS and OS had not been reached yet [hazard ratio (HR): 0.43; 95% confidence interval (CI): 0.25–0.74; P=0.001, HR: 0.47; 95% CI: 0.27–0.81; P=0.006, respectively] (Figure 1). Multivariable analysis for the MET rs40239 was stratified by stage, gender, age and type of adjuvant chemotherapy, it remained significantly associated with both DFS and OS (HR: 0.48; 95% CI: 0.27–0.83; P=0.009, HR: 0.50; 95% CI: 0.28–0.88; P=0.017, respectively) (Table 3). In this cohort, age, stage, T-category, N-category, and type of adjuvant chemotherapy were significantly associated with DFS, whereas age, stage, T-category, and N-category were significantly associated with OS (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/FPC/A776).
Figure 1.
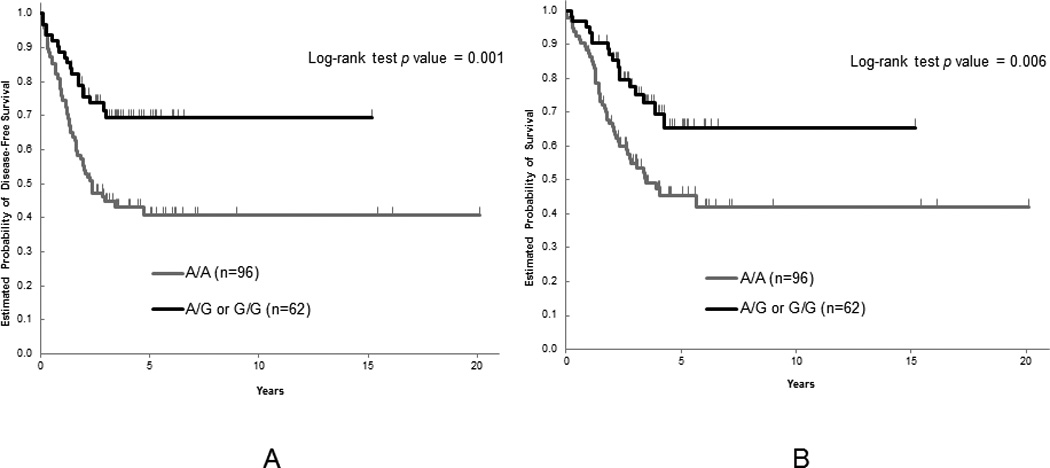
(A) Disease-free survival and (B) overall survival by MET rs40239 in the Japanese cohort
Table 3.
Associations between c-MET SNPs and clinical outcome among loco-regional gastric cancer patients (stage IB to stage IV; AJCC 6th) in the Japanese cohort
| Disease-free Survival |
Overall Survival |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | N | Median, yrs (95%CI) |
3-year of recurrence rate ± SE |
HR (95%CI) | HR (95%CI) ‡ | Median, yrs (95%CI) |
5-year of survival rate ± SE |
HR (95%CI) | HR (95%CI) ‡ |
| rs41736 | |||||||||
| T/T | 42 | 2.9 (1.5, 16.1+) | 0.51 ± 0.08 | 1 (Reference) | 1 (Reference) | 4.3 (2.2, 16.1+) | 0.45 ± 0.09 | 1 (Reference) | 1 (Reference) |
| T/C | 82 | 20.1+ (2.9, 20.1+) | 0.40 ± 0.06 | 0.71 (0.42, 1.22) | 0.58 (0.32, 1.05) | 20.1+ (3.4, 20.1+) | 0.60 ± 0.06 | 0.70 (0.39, 1.23) | 0.60 (0.33, 1.11) |
| C/C | 30 | 2.5 (1.3, 7.1+) | 0.52 ± 0.10 | 1.04 (0.54, 2.01) | 0.76 (0.37, 1.55) | 2.8 (1.7, 7.1+) | 0.44 ± 0.10 | 1.20 (0.61, 2.35) | 0.86 (0.42, 1.78) |
| P value† | 0.32 | 0.20 | 0.18 | 0.25 | |||||
| rs41739 | |||||||||
| G/G | 49 | 16.1+ (2.0, 16.1+) | 0.46 ± 0.07 | 1 (Reference) | 1 (Reference) | 16.1+ (2.8, 16.1+) | 0.54 ± 0.08 | 1 (Reference) | 1 (Reference) |
| G/A | 72 | 20.1+ (2.2, 20.1+) | 0.44 ± 0.06 | 0.92 (0.54, 1.58) | 0.74 (0.43, 1.28) | 20.1+ (3.1, 20.1+) | 0.54 ± 0.07 | 0.97 (0.54, 1.71) | 0.77 (0.43, 1.38) |
| A/A | 33 | 2.9 (1.3, 7.1+) | 0.51 ± 0.09 | 1.25 (0.67, 2.33) | 0.93 (0.47, 1.82) | 4.1 (1.7, 7.1+) | 0.46 ± 0.10 | 1.48 (0.77, 2.82) | 1.01 (0.50, 2.04) |
| P value† | 0.60 | 0.53 | 0.34 | 0.60 | |||||
| rs40239 | |||||||||
| A/A | 96 | 2.3 (1.6, 20.1+) | 0.55 ± 0.05 | 1 (Reference) | 1 (Reference) | 3.4 (2.3, 20.1+) | 0.45 ± 0.06 | 1 (Reference) | 1 (Reference) |
| A/G* | 53 | 15.2+ | 0.28 ± 0.06 | 0.43 (0.25, 0.74) | 0.48 (0.27, 0.83) | 15.2+ (4.3, 15.2+) | 0.65 ± 0.07 | 0.47 (0.27, 0.81) | 0.50 (0.28, 0.88) |
| G/G* | 9 | ||||||||
| P value† | 0.001 | 0.009 | 0.006 | 0.017 | |||||
| rs1621 | |||||||||
| A/A | 109 | 20.1+ (2.3, 20.1+) | 0.45 ± 0.05 | 1 (Reference) | 1 (Reference) | 20.1+ (3.4, 20.1+) | 0.55 ± 0.05 | 1 (Reference) | 1 (Reference) |
| A/G* | 44 | 3.4 (2.0, 9.0+) | 0.47 ± 0.07 | 1.04 (0.63, 1.70) | 0.89 (0.54, 1.48) | 4.1 (2.7, 9.0+) | 0.48 ± 0.08 | 1.11 (0.66, 1.84) | 0.95 (0.56, 1.60) |
| G/G* | 4 | ||||||||
| P value† | 0.88 | 0.65 | 0.70 | 0.85 | |||||
| rs10234854 | |||||||||
| G/G | 80 | 16.1+ (2.9, 16.1+) | 0.40 ± 0.06 | 1 (Reference) | 1 (Reference) | 16.1+ (4.3, 16.1+) | 0.60 ± 0.06 | 1 (Reference) | 1 (Reference) |
| G/A | 54 | 2.2 (1.3, 20.1+) | 0.54 ± 0.07 | 1.49 (0.90, 2.45) | 1.14 (0.67, 1.95) | 3.9 (2.3, 20.1+) | 0.41 ± 0.08 | 1.60 (0.94, 2.70) | 1.15 (0.66, 2.01) |
| A/A | 20 | 6.5+ (1.4, 6.5+) | 0.37 ± 0.11 | 0.82 (0.37, 1.86) | 0.73 (0.31, 1.72) | 6.5+ (1.9, 6.5+) | 0.60 ± 0.12 | 1.01 (0.44, 2.30) | 0.89 (0.37, 2.12) |
| P value† | 0.19 | 0.58 | 0.18 | 0.80 | |||||
| rs184953 | |||||||||
| C/C | 114 | 3.4 (2.2, 20.1+) | 0.49 ± 0.05 | 1 (Reference) | 1 (Reference) | 5.7 (3.0, 20.1+) | 0.52 ± 0.05 | 1 (Reference) | 1 (Reference) |
| C/A* | 36 | 6.1+ (3.0, 6.1+) | 0.32 ± 0.07 | 0.66 (0.37, 1.19) | 0.69 (0.37, 1.27) | 6.1+ (3.9, 6.1+) | 0.54 ± 0.11 | 0.77 (0.42, 1.39) | 0.75 (0.40, 1.39) |
| A/A* | 6 | ||||||||
| P value† | 0.16 | 0.23 | 0.38 | 0.36 | |||||
Combined in the analysis in the dominant genetic model.
Based on the log-rank test in the univariable analysis and Wald test in the multivariable analysis within Cox regression model.
Estimates were not reached.
Adjusted for stage (I, II, III, and IV), gender, age (<65, 65–74, ≥75 as continuous), and type of adjuvant therapy (no vs yes)
Analysis of MET rs40239 polymorphism in the U.S. and Austrian cohort
We tested the association between clinical outcome and the MET rs40239, which was significant in the Japanese cohort, in two other cohorts with mainly Caucasian and Hispanic patients. However, there was no significant association between them in both univariable and multivariable analysis using a dominant genetic model (A/A vs. A/G and G/G genotype) (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/FPC/A777 ). The Austrian cohort consisted of all Caucasian patients, whereas the U.S. cohort of 41% Hispanic, 35% Caucasian and 24% Asian. To exclude an influence of ethnic differences in the U.S. cohort, we also evaluated the association in the Caucasian patients among the U.S. cohort and among patients combined with the U.S. and Austrian cohort. However, there was no significant association between them. When stratified by gender in the U.S. cohort and the Caucasian among patients combined with the U.S. and Austrian cohort, the subgroup analyses showed no significance (Supplementary Table 3, Supplemental Digital Content 3, http://links.lww.com/FPC/A778).
Subgroup analysis of c-MET SNPs by gender
When stratified by gender in the Japanese cohort, males, but not females, with any G allele of the MET rs40239 maintained a clinical outcome benefit in both univariable and multivariable analysis. The males with the G allele had significantly longer DFS and OS compared to those with the A/A genotype (Figure 2). Interestingly, when we correlated the MET SNPs with clinical outcome by gender, in 4 of 6 candidates for SNPs, rs41736, rs41739, rs1621, and rs10234854, we found that males with minor allele of the SNPs had favorite DFS and OS but females with those had a worse DFS and OS. Additionally, these differences reached statistical significance in both DFS and OS of rs41739 and in DFS of rs10234854 (Table 4).
Figure 2.
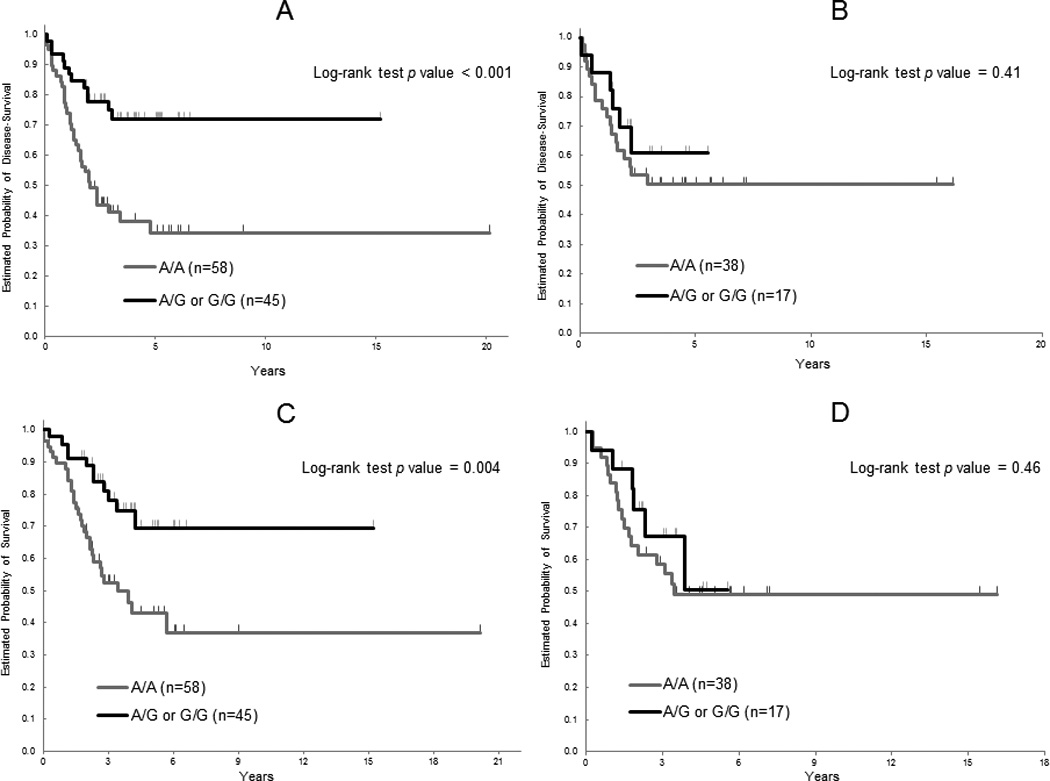
Disease-free survival (DFS) and overall survival (OS) by MET rs40239 in the Japanese cohort according to gender. A) DFS in males, B) DFS in females, C) OS in males, D) OS in females
Table 4.
Associations between c-MET SNPs and clinical outcome in the Japanese cohort by gender
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | N | DFS HR (95%CI) |
DFS HR (95%CI) ‡ |
OS HR (95%CI) |
OS HR (95%CI) ‡ |
N | DFS HR (95%CI) |
DFS HR (95%CI) ‡ |
OS HR (95%CI) |
OS HR (95%CI) ‡ |
| rs41736 | ||||||||||
| T/T | 29 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 13 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| any C | 71 | 0.50 (0.27, 0.90) | 0.44 (0.23, 0.88) | 0.49 (0.26, 0.93) | 0.47 (0.22, 0.97) | 41 | 2.25 (0.77, 6.57) | 1.92 (0.51, 7.28) | 2.21 (0.76, 6.46) | 1.81 (0.47, 6.99) |
| P value† | 0.019 | 0.020 | 0.026 | 0.042 | 0.12 | 0.34 | 0.13 | 0.39 | ||
| Pfor interaction§ | 0.089 | 0.092 | ||||||||
| rs41739 | ||||||||||
| G/G | 35 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 14 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| any A | 63 | 0.62 (0.34, 1.11) | 0.54 (0.29, 1.01) | 0.65 (0.35, 1.23) | 0.59 (0.30, 1.18) | 42 | 3.55 (1.07, 11.82) | 3.83 (0.91, 16.13) | 3.57 (1.07, 11.86) | 4.09 (0.91, 18.32) |
| P value† | 0.10 | 0.053 | 0.18 | 0.14 | 0.026 | 0.067 | 0.026 | 0.066 | ||
| Pfor interaction§ | 0.027 | 0.043 | ||||||||
| rs40239 | ||||||||||
| A/A | 58 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 38 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| any G | 45 | 0.34 (0.18, 0.66) | 0.40 (0.20, 0.79) | 0.37 (0.19, 0.75) | 0.39 (0.19, 0.78) | 17 | 0.68 (0.27, 1.72) | 0.72 (0.26, 2.00) | 0.71 (0.28, 1.78) | 0.89 (0.32, 2.49) |
| P value† | 0.001 | 0.008 | 0.004 | 0.008 | 0.41 | 0.53 | 0.46 | 0.82 | ||
| Pfor interaction§ | 0.33 | 0.18 | ||||||||
| rs1621 | ||||||||||
| A/A | 71 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 38 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| any G | 30 | 0.83 (0.44, 1.58) | 0.63 (0.32, 1.24) | 0.92 (0.47, 1.80) | 0.75 (0.37, 1.53) | 18 | 1.53 (0.70, 3.38) | 1.69 (0.73, 3.95) | 1.50 (0.68, 3.31) | 1.35 (0.57, 3.19) |
| P value† | 0.57 | 0.18 | 0.80 | 0.43 | 0.29 | 0.22 | 0.31 | 0.49 | ||
| Pfor interaction§ | 0.076 | 0.30 | ||||||||
| rs10234854 | ||||||||||
| G/G | 54 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 26 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| any A | 46 | 0.80 (0.44, 1.45) | 0.72 (0.37, 1.39) | 0.90 (0.48, 1.69) | 0.81 (0.41, 1.63) | 28 | 3.30 (1.36, 7.98) | 3.06 (1.06, 8.87) | 3.19 (1.32, 7.72) | 2.97 (1.04, 8.50) |
| P value† | 0.46 | 0.33 | 0.73 | 0.56 | 0.004 | 0.039 | 0.006 | 0.043 | ||
| Pfor interaction§ | 0.044 | 0.082 | ||||||||
| rs184953 | ||||||||||
| C/C | 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 44 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| any A | 31 | 0.59 (0.29, 1.20) | 0.59 (0.29, 1.23) | 0.74 (0.36, 1.52) | 0.60 (0.29, 1.26) | 11 | 0.87 (0.30, 2.54) | 1.02 (0.32, 3.28) | 0.86 (0.30, 2.53) | 1.25 (0.37, 4.20) |
| P value† | 0.14 | 0.16 | 0.41 | 0.18 | 0.79 | 0.97 | 0.79 | 0.72 | ||
| Pfor interaction§ | 0.38 | 0.37 | ||||||||
Based on the log-rank test in the univariable analysis and the Wald test in the multivariable analysis within Cox regression model.
Adjusted for stage (I, II, III, and IV), age (<65, 65–74, ≥75 as continuous), and type of adjuvant therapy (no vs yes.)
Based on the likelihood ratio test of the Cox regression model including the interaction term of sex and SNP.
Discussion
Our results showed that the c-MET polymorphism, rs40239, may serve as a prognostic biomarker in loco-regional gastric cancer treated with surgery alone or surgery plus adjuvant therapy in Japanese patients. To the best of our knowledge, this is the first report demonstrating that the c-MET polymorphism was associated with clinical outcome in gastric cancer among Japanese patients. Moreover, our results provide preliminary evidence suggesting that there may be a gender-related difference in the impact on clinical outcome by genetic variants of the c-MET.
The c-MET pathway is becoming an important target for development of novel anti-cancer drugs. Currently, multiple agents are under investigation as monotherapy or combined chemotherapy with other targeted agents for the treatment of a wide variety of tumors, and some of them are in phase III trials. Rilotumumab (AMG-102), antibody against HGF which binds to the HGF light chain, is in a phase III trial testing whether the combination with chemotherapy in advanced gastric cancer is superior to chemotherapy alone based on promising data of a phase II trial in patients with high c-MET expression disease measured by IHC [27]. In a phase II trial of foretinib in advanced gastric cancer, tumors with c-MET amplification were more likely to respond to therapy [28]. The detection of c-MET signaling aberration may identify clinically important subgroup of gastric cancer patients who are likely to respond to c-MET inhibitors.
In this study, we tested whether a germline polymorphism of the c-MET is a prognostic biomarker in loco-regional gastric cancer. We found a significant association between the G allele of the MET rs40239 and both longer DFS and OS in univariable and multivariable analysis among the Japanese cohort. Liu et al have reported that a germline variant N375S in MET was associated with gastric cancer susceptibility [29]. However, the variant may not play a clinically important role because the frequency of the minor allele of the polymorphism is 5 % or less in all ethnic groups. The frequency of the minor allele of the MET rs40239 is much higher, hence this polymorphism may become clinically useful as a biomarker. The overexpression and amplification of the c-MET have been found to be associated with advanced disease stage, worse clinical outcome and liver metastases in gastric cancer [17,18]. The F-SNP database predicted tagging SNP as a role of the MET rs40239 [25]. Given the location of the MET rs40239 within intron of the gene, it is biologically plausible that this polymorphism may have an impact on the splicing of the gene transcript. However, the functions of the polymorphism remain unclear. Further mechanistic studies confirming the functional role of this polymorphism are warranted.
We failed to find the association of the MET rs40239 with clinical outcome in the U.S. and Austrian cohort. Certainly, the small number of patient population along with variety of patients’ characteristics, different surgical technique of lymphadenectomy, and different adjuvant treatment across the cohorts limits the statistical power. There was no significant difference in the frequency of the minor allele in the polymorphism between the three cohorts. One of the other reasons for the failure to find the association in the U.S. and Austrian cohort may be an ethnic difference which might be due to different biology and etiology of gastric cancer carcinogenesis. There has been shown to be several biological regional ethnic differences in gastric cancer. The GEJ cancers or proximal gastric cancers are more prevalent in Europe and the U.S. than in Asia [30]. In this study, it appeared that the U.S. and Austrian patients had a significantly higher incidence of GEJ cancer than the Japanese patients. Recent meta-analysis including Asian and Caucasian patients with gastric cancer showed that higher c-MET gene amplification and expression were indicators of poor prognosis, they remained significance in the subgroup analysis based on race [31]. However, a Western study showed a lack of the c-MET amplification or pathway activation in patients with loco-regional gastric cancer, suggesting that c-MET-driven gastric cancer was relatively rare in Western population [7]. Germline studies including an investigation about relationships between pathology type and the c-MET polymorphism may provide evidences that support our results and elucidate which factor causes the difference, ethnicity or pathology.
Our results also indicated that there may be a gender-related difference in the impact of the c-MET polymorphism on clinical outcome. When stratified by gender in the Japanese cohort, males with minor allele of the c-MET SNPs had favorite prognosis but females with those had a worse prognosis in 4 of 6 candidates for SNPs. Recently, a retrospective study of a phase III trial in adjuvant gastric cancer showed that a frequency of c-MET amplification had a significant difference between males and females. The c-MET amplification was more frequent in males than in females [32]. Accumulated epidemiologic data has indicated that young females have had not only a lower risk but also a longer survival particular in colorectal cancer and esophageal cancer [33–35]. These data suggest that the gender-related difference may be critical to characterize predictive and prognostic values of the c-MET pathway in gastric cancer. This might be highly important since the c-MET pathway has significant cross-talks to EGFR and Wnt pathway in tumor progression. The c-MET interacts with the EGFR and acts as a compensatory pathway for EGFR signaling, suggesting that they have substantially overlapped downstream mediators [13]. The c-MET expression can be regulated by the Wnt pathway which is another important pathway for tumor growth and invasion, whereas an activation of the c-MET enhances activity of the Wnt pathway [16]. Activated EGFR signaling can activate Ras/Raf/MAPK pathway, which in turn will phosphorylate estrogen receptors (ERs), resulting in dimerization and ligand-independent activation of gene expression [36,37] and several studies support the cross-talk between the Wnt pathway and ERs activation [38]. Estrogen has been shown to play a protective role to reduce an incidence of gastric cancer [39,40]. An indirect influence of estrogen on the EGFR or Wnt pathway may explain in part the gender-related difference we observed in current study. In this study, there was no gender dependent association between the MET rs40239 and clinical outcome in the U.S. and the Caucasian patients. One of potential reasons could be small sample size which is even smaller in the stratified analysis. The other reasons may involve that higher estradiol (E2) levels in U.S. people were reported in each gender possible related to the higher BMI compared with those in Japan [41,42]. Moreover, expression rates of ER-α and -β in gastric cancer by IHC in Asian population were 0% to 22.7 % and 43.6 % to 100 %, respectively [43,44], whereas those in Western population were 36 % and 11 %, respectively [45]. These physiologic and biologic differences among gender and region may impact the protective estrogen effect in gastric cancer, and also affect the impact of the c-MET pathway on clinical outcome through the cross-talk such as the EGFR or Wnt pathway.
Our data are hypothesis-generating and a selection bias cannot be fully excluded because of the retrospective design of our study. Moreover, this study has a number of limitations that need to be considered, in particular the U.S. and Austrian cohort. A larger sample size is necessary to determine if our results are attributable to a real biological phenomenon or a limitation of the small sample size. In addition, retrospective studies involving independent Asian cohorts should be considered due to consideration of possible ethnic differences.
In conclusion, our results provide the initial evidence that the MET rs40239 may serve as a prognostic biomarker in loco-regional gastric cancer among Japanese patients. This may help to select patients who benefit from more intense observation or aggressive treatment. These data also suggest that genetic variants of the c-MET may have a gender-related difference in the impact on clinical outcome. Further functional correlative preclinical analyses and external clinical validation studies are needed to confirm these results.
Supplementary Material
Acknowledgments
Source of Funding
This study was partly funded by the NIH grant 5 P30CA14089-27S1 and Yvonne Bogdanovich.
It was performed in the Sharon A. Carpenter Laboratory at USC/Norris Comprehensive Cancer Center and in memory of David Donaldson.
Footnotes
Conflict of Interest
The authors disclose no potential conflicts of interest.
References
- 1.Lin W, Kao HW, Robinson D, Kung HJ, Wu CW, Chen HC. Tyrosine kinases and gastric cancer. Oncogene. 2000;19:5680–5689. doi: 10.1038/sj.onc.1203924. [DOI] [PubMed] [Google Scholar]
- 2.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. International Journal of Cancer. 2012;130:2845–2856. doi: 10.1002/ijc.26292. [DOI] [PubMed] [Google Scholar]
- 3.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Annals of Oncology. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Annals of Oncology. 2003;14:1705–1713. doi: 10.1093/annonc/mdg486. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi K, Yonemura Y, Nojima N, Hirono Y, Fushida S, Fujimura T, et al. The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c-met), autocrine motility factor receptor, and urokinase-type plasminogen activator receptor. Cancer. 1998;82:2112–2122. [PubMed] [Google Scholar]
- 7.Janjigian YY, Tang LH, Coit DG, Kelsen DP, Francone TD, Weiser MR, et al. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1021–1027. doi: 10.1158/1055-9965.EPI-10-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 9.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Yang J. Hepatocyte growth factor: new arsenal in the fights against renal fibrosis? Kidney Int. 2006;70:238–240. doi: 10.1038/sj.ki.5001661. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Ebina M, Orson FM, Nakamura A, Kubota K, Koinuma D, et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol Ther. 2005;12:58–67. doi: 10.1016/j.ymthe.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Corso S, Comoglio PM, Giordano S. Cancer therapy: can the challenge be MET? Trends Mol Med. 2005;11:284–292. doi: 10.1016/j.molmed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, et al. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36:547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boon EM, van der Neut R, van de Wetering M, Clevers H, Pals ST. Wnt signaling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res. 2002;62:5126–5128. [PubMed] [Google Scholar]
- 17.Amemiya H, Kono K, Itakura J, Tang RF, Takahashi A, An FQ, et al. c-Met expression in gastric cancer with liver metastasis. Oncology. 2002;63:286–296. doi: 10.1159/000065477. [DOI] [PubMed] [Google Scholar]
- 18.Graziano F, Galluccio N, Lorenzini P, Ruzzo A, Canestrari E, D'Emidio S, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29:4789–4795. doi: 10.1200/JCO.2011.36.7706. [DOI] [PubMed] [Google Scholar]
- 19.Houldsworth J, Cordon-Cardo C, Ladanyi M, Kelsen DP, Chaganti RS. Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res. 1990;50:6417–6422. [PubMed] [Google Scholar]
- 20.Umeki K, Shiota G, Kawasaki H. Clinical significance of c-met oncogene alterations in human colorectal cancer. Oncology. 1999;56:314–321. doi: 10.1159/000011985. [DOI] [PubMed] [Google Scholar]
- 21.Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nessling M, Solinas-Toldo S, Wilgenbus KK, Borchard F, Lichter P. Mapping of chromosomal imbalances in gastric adenocarcinoma revealed amplified protooncogenes MYCN, MET, WNT2, and ERBB2. Genes Chromosomes Cancer. 1998;23:307–316. doi: 10.1002/(sici)1098-2264(199812)23:4<307::aid-gcc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Sakakura C, Mori T, Sakabe T, Ariyama Y, Shinomiya T, Date K, et al. Gains, losses, and amplifications of genomic materials in primary gastric cancers analyzed by comparative genomic hybridization. Genes Chromosomes Cancer. 1999;24:299–305. doi: 10.1002/(sici)1098-2264(199904)24:4<299::aid-gcc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Schutz FA, Pomerantz MM, Gray KP, Atkins MB, Rosenberg JE, Hirsch MS, et al. Single nucleotide polymorphisms and risk of recurrence of renal-cell carcinoma: a cohort study. Lancet Oncol. 2013;14:81–87. doi: 10.1016/S1470-2045(12)70517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:D820–D824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee PH, Shatkay H. An integrative scoring system for ranking SNPs by their potential deleterious effects. Bioinformatics. 2009;25:1048–1055. doi: 10.1093/bioinformatics/btp103. [DOI] [PubMed] [Google Scholar]
- 27.Oliner KS, Tang R, Anderson A, Lan Y, Iveson T, Donehower RC, et al. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer. ASCO Meeting Abstracts. 2012;30:4005. [Google Scholar]
- 28.Jhawer MP, Kindler HL, Wainberg ZA, Hecht JR, Kerr RO, Ford JM, et al. Preliminary activity of XL880, a dual MET/VEGFR2 inhibitor, in MET amplified poorly differentiated gastric cancer (PDGC): Interim results of a multicenter phase II study. ASCO Meeting Abstracts. 2008;26:4572. [Google Scholar]
- 29.Liu Y, Zhang Q, Ren C, Ding Y, Jin G, Hu Z, et al. A germline variant N375S in MET and gastric cancer susceptibility in a Chinese population. J Biomed Res. 2012;26:315–318. doi: 10.7555/JBR.26.20110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 31.Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Ge S, et al. Prognostic Significance of MET Amplification and Expression in Gastric Cancer: A Systematic Review with Meta-Analysis. PLoS One. 2014;9:e84502. doi: 10.1371/journal.pone.0084502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochiai A, Kitada K, Ichikawa W, Terashima M, Kurahashi I, Katai H, et al. Expression analysis of MET, EGFR, and HER2, and K-ras mutation status in patients with stage II/III gastric cancer enrolled in the ACTS-GC study. ASCO Meeting Abstracts. 2013;31:40. [Google Scholar]
- 33.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 34.Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–6397. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohanes P, Yang D, Chhibar RS, Labonte MJ, Winder T, Ning Y, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30:2265–2272. doi: 10.1200/JCO.2011.38.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin LA, Farmer I, Johnston SR, Ali S, Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocr Relat Cancer. 2005;12(Suppl 1):S75–S84. doi: 10.1677/erc.1.01023. [DOI] [PubMed] [Google Scholar]
- 38.Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, et al. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem. 2004;279:40255–40258. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:20–38. doi: 10.1158/1055-9965.EPI-11-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasui T, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R, et al. Associations of endogenous sex hormones and sex hormone-binding globulin with lipid profiles in aged Japanese men and women. Clin Chim Acta. 2008;398:43–47. doi: 10.1016/j.cca.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Vaidya D, Dobs A, Gapstur SM, Golden SH, Cushman M, Liu K, et al. Association of baseline sex hormone levels with baseline and longitudinal changes in waist-to-hip ratio: Multi-Ethnic Study of Atherosclerosis. Int J Obes (Lond) 2012;36:1578–1584. doi: 10.1038/ijo.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195–201. doi: 10.1016/j.ejso.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, Shen JY, et al. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol. 2010;17:2503–2509. doi: 10.1245/s10434-010-1031-2. [DOI] [PubMed] [Google Scholar]
- 45.Chandanos E, Rubio CA, Lindblad M, Jia C, Tsolakis AV, Warner M, et al. Endogenous estrogen exposure in relation to distribution of histological type and estrogen receptors in gastric adenocarcinoma. Gastric Cancer. 2008;11:168–174. doi: 10.1007/s10120-008-0475-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


