Abstract
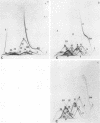
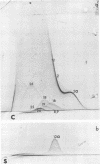
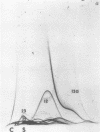
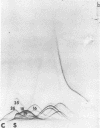
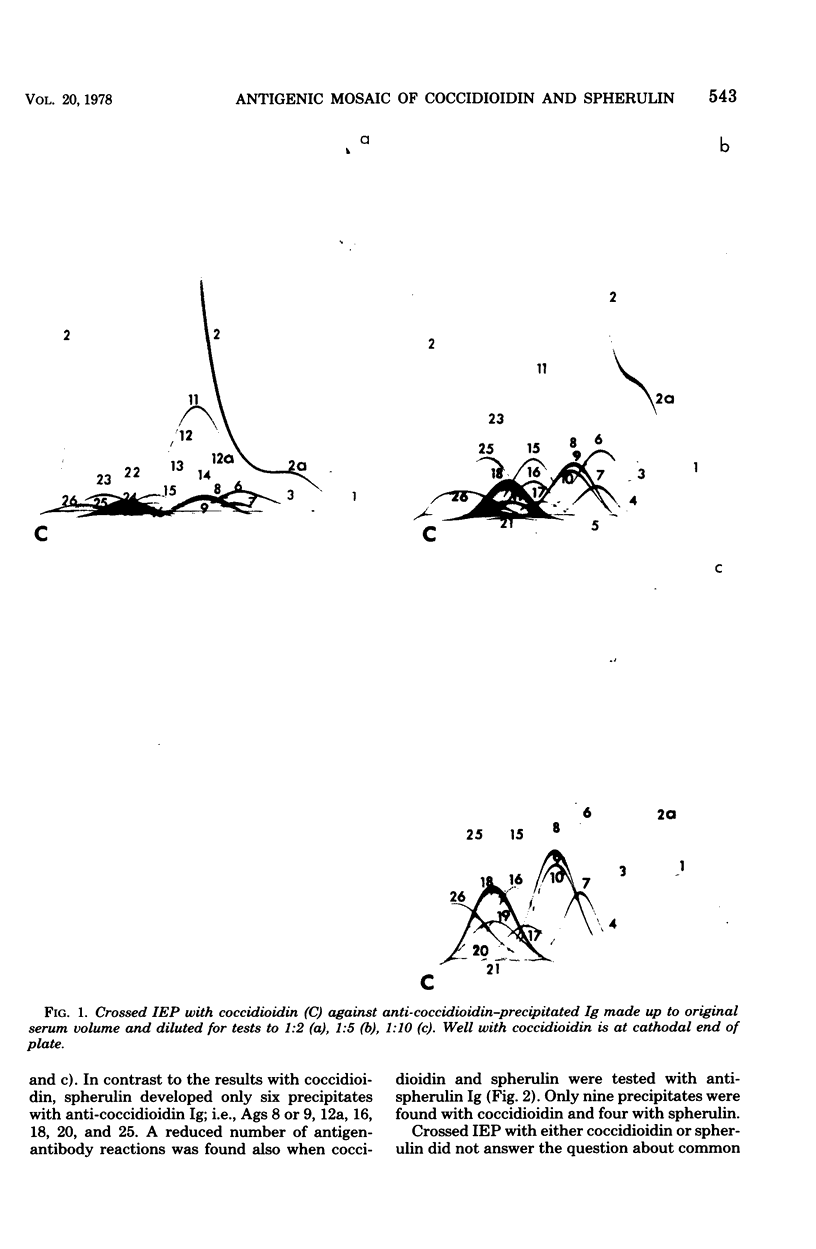
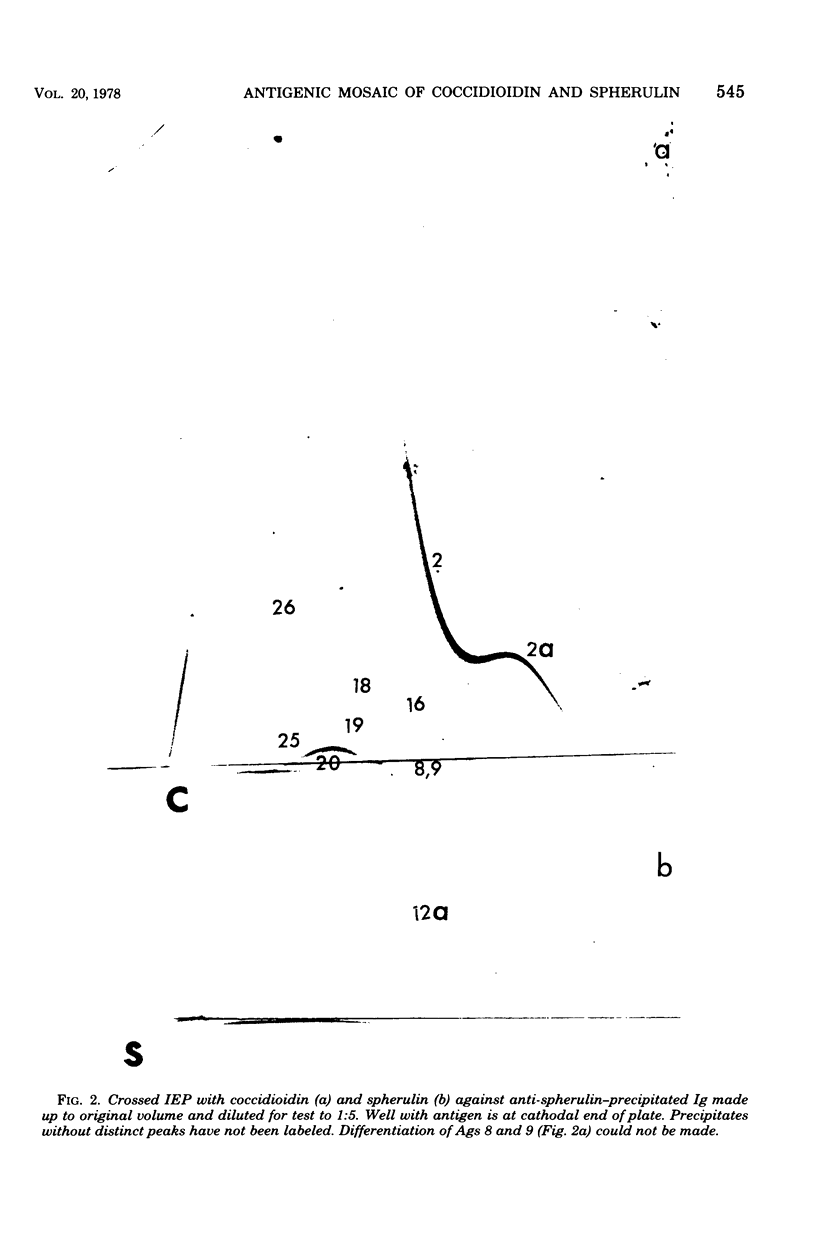
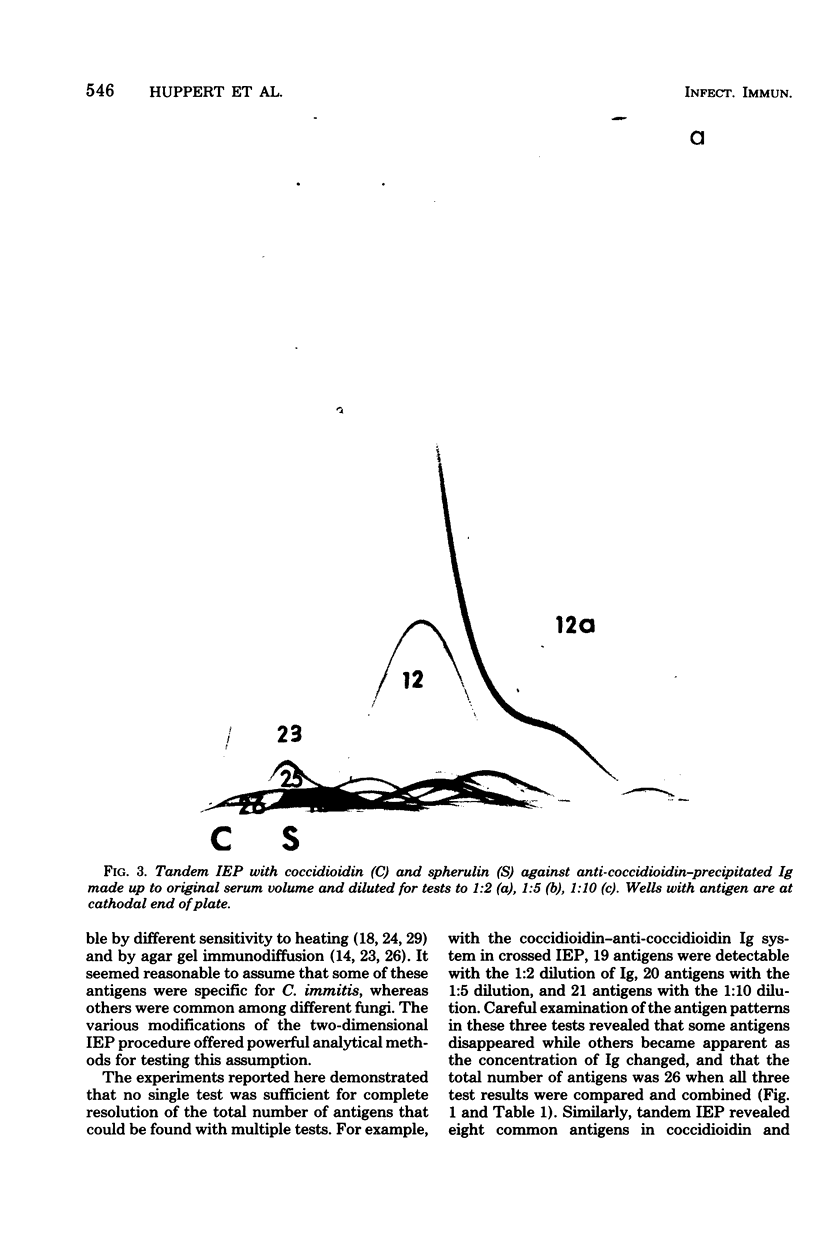
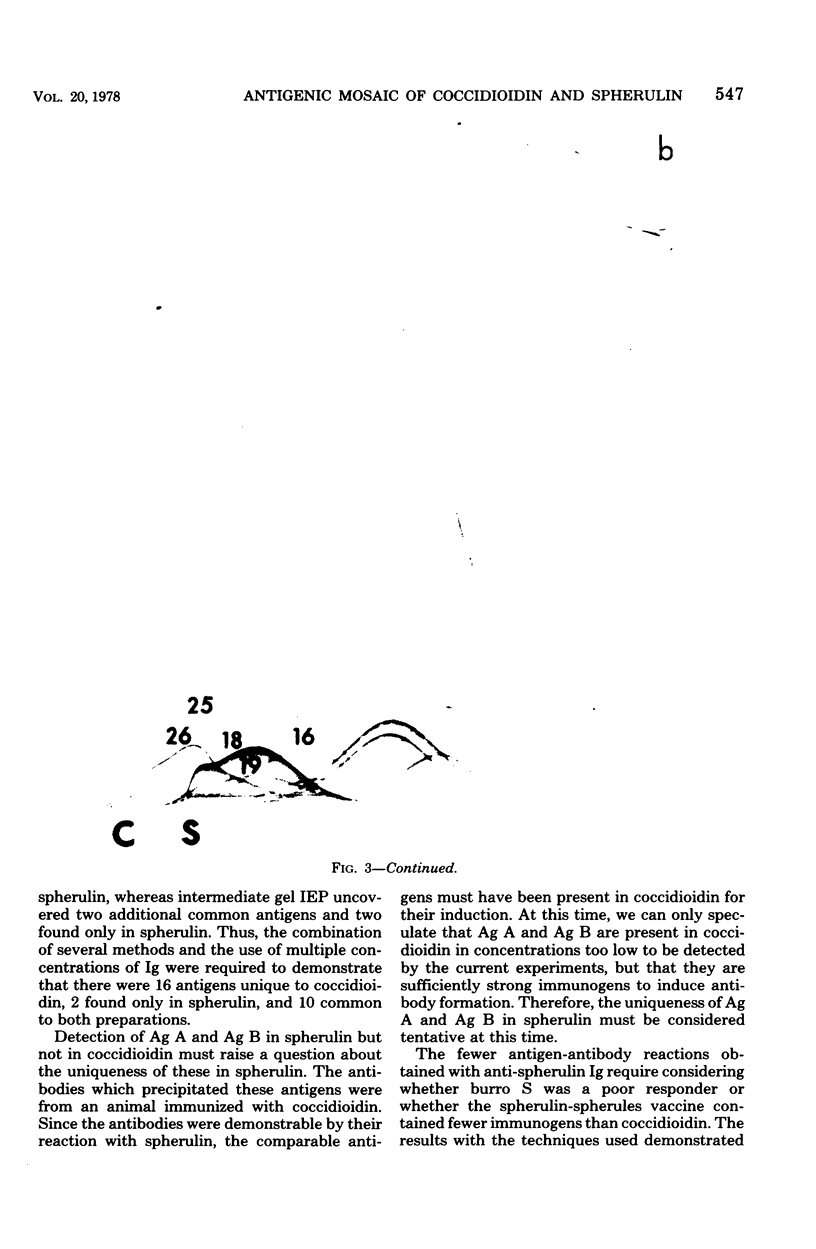
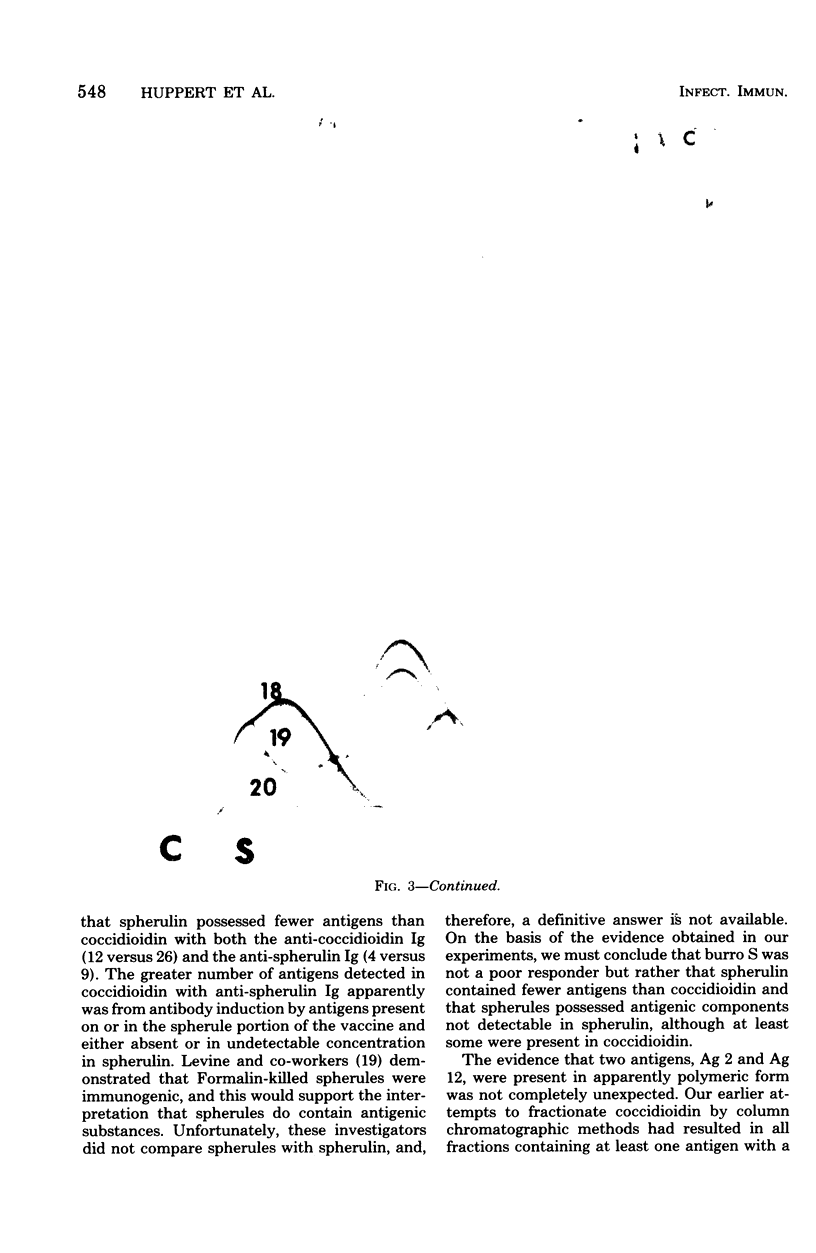
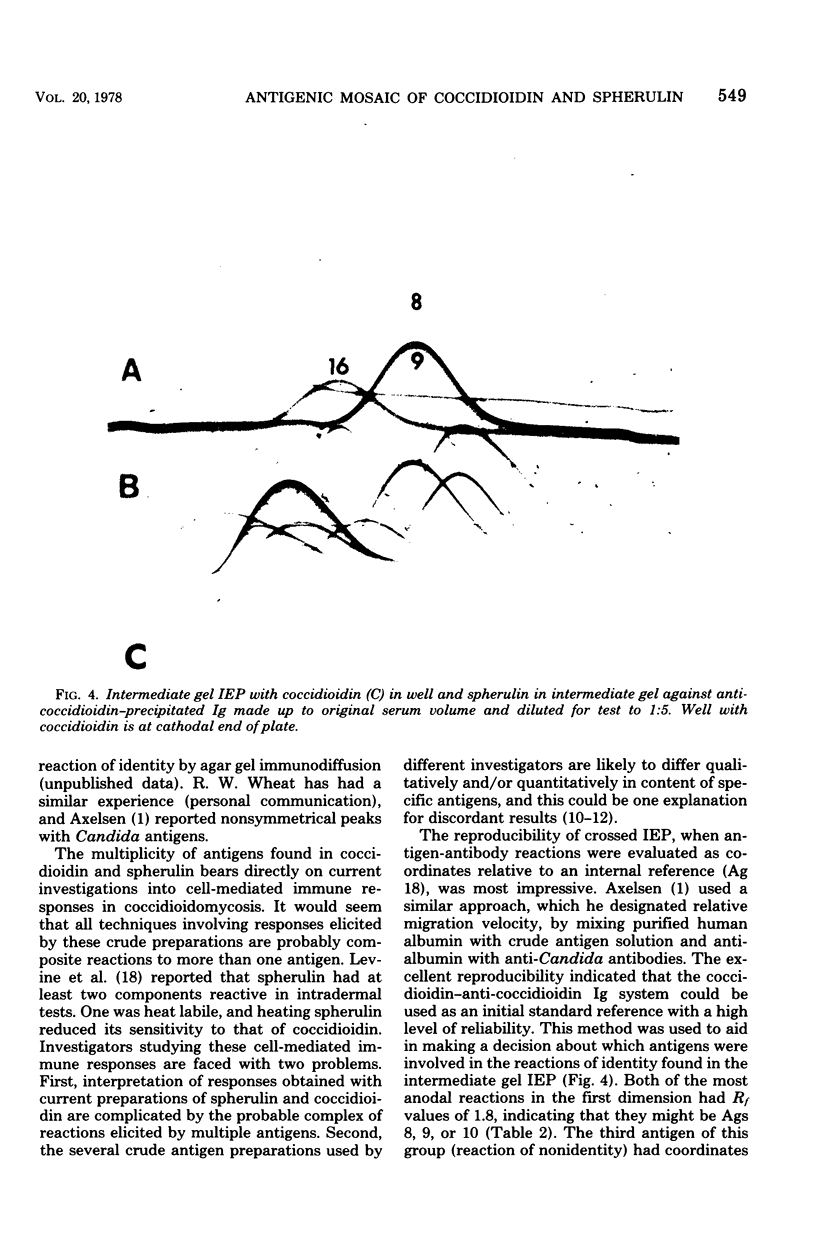
Immunological tests are valuable aids for diagnosis of mycotic infections and, in some cases, as objective guides for clinical management and prognosis. The usefulness of these procedures is limited to the extents that crude antigen preparations are employed, that these are difficult to standardize uniformly, and that they contain antigens common to several species of pathogenic fungi. Analysis by two-dimensional immunoelectrophoresis methods of the two crude preparations used for coccidioidomycosis demonstrated that coccidioidin contained at least 26 antigens, with 10 of these found also in spherulin. In addition, spherulin contained two antigens not demonstrated in coccidioidin. No single test detected all antigens present, and multiple procedures were required to display the complete array of antigens. A reference system was established for coccidioidin and precipitated immunoglobulins from a burro hyperimmunized with coccidioidin. Evaluation of the reference system demonstrated that it was highly reproducible with respect to the reagents used, to repeated tests by the same person, and to comparative tests by two individuals using the same reagents. Applications of this reference system for standardization of reagents, for detecting common antigens, for monitoring successive steps during fractionation of crude preparations, and for fingerprinting strains for ecological and epidemiological studies are presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H., Bock E. Identification and quantitation of antigens and antibodies by means of quantitative immunoelectrophoresis. A survey of methods. J Immunol Methods. 1972 Jan;1(2):109–121. doi: 10.1016/0022-1759(72)90038-5. [DOI] [PubMed] [Google Scholar]
- Axelsen N. H. Quantitative immunoelectrophoretic methods as tools for a polyvalent approach to standardization in the immunochemistry of Candida albicans. Infect Immun. 1973 Jun;7(6):949–960. doi: 10.1128/iai.7.6.949-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL C. C., BINKLEY G. E. Serologic diagnosis with respect to histoplasmosis, coccidioidomycosis, and blastomycosis and the problem of cross reactions. J Lab Clin Med. 1953 Dec;42(6):896–906. [PubMed] [Google Scholar]
- CAMPBELL C. C. The accuracy of serologic methods in diagnosis. Ann N Y Acad Sci. 1960 Aug 27;89:163–177. doi: 10.1111/j.1749-6632.1960.tb20139.x. [DOI] [PubMed] [Google Scholar]
- CONVERSE J. L. Effect of physico-chemical environment of spherulation of Coccidioides immitis in a chemically defined medium. J Bacteriol. 1956 Dec;72(6):784–792. doi: 10.1128/jb.72.6.784-792.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONVERSE J. L. Effect of surface active agents on endosporulation of Coccidioides immitis in a chemically defined medium. J Bacteriol. 1957 Jul;74(1):106–107. doi: 10.1128/jb.74.1.106-107.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse J. L., Besemer A. R. NUTRITION OF THE PARASITIC PHASE OF COCCIDIOIDES IMMITIS IN A CHEMICALLY DEFINED LIQUID MEDIUM. J Bacteriol. 1959 Aug;78(2):231–239. doi: 10.1128/jb.78.2.231-239.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Brummer E., Lecara G. In vitro lymphocyte responses of coccidioidin skin test-positive and -negative persons to coccidioidin, spherulin, and a coccidioides cell wall antigen. Infect Immun. 1977 Mar;15(3):751–755. doi: 10.1128/iai.15.3.751-755.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell Immunol. 1977 Jun 1;31(1):130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Huppert M., Krasnow I., Vukovich K. R., Sun S. H., Rice E. H., Kutner L. J. Comparison of coccidioidin and spherulin in complement fixation tests for coccidioidomycosis. J Clin Microbiol. 1977 Jul;6(1):33–41. doi: 10.1128/jcm.6.1.33-41.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. Tandem-crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:57–59. [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Levine H. B., Cobb J. M., Scalarone G. M. Spherule coccidioidin in delayed dermal sensitivity reactions of experimental animals. Sabouraudia. 1969 Feb;7(1):20–32. doi: 10.1080/00362177085190051. [DOI] [PubMed] [Google Scholar]
- Levine H. B., Gonzalez-Ochoa A., Ten Eyck D. R. Dermal sensitivity to Coccidioides immitis. A comparison of responses elicited in man by spherulin and coccidioidin. Am Rev Respir Dis. 1973 Mar;107(3):379–386. doi: 10.1164/arrd.1973.107.3.379. [DOI] [PubMed] [Google Scholar]
- Levine H. B., Pappagianis D., Cobb J. M. Development of vaccines for coccidioidomycosis. Mycopathol Mycol Appl. 1970;41(1):177–185. doi: 10.1007/BF02051493. [DOI] [PubMed] [Google Scholar]
- Levine H. B., Restrepon A., Eyck D. R., Stevens D. A. Spherulin and coccidioidin: cross-reactions in dermal sensitivity to histoplasmin and paracoccidioidin. Am J Epidemiol. 1975 Jun;101(6):512–516. doi: 10.1093/oxfordjournals.aje.a112122. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., PUTMAN E. W., KOBAYASHI G. S. Polysaccharide of Coccidioides immitis. J Bacteriol. 1961 Nov;82:714–723. doi: 10.1128/jb.82.5.714-723.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- ROWE J. R., NEWCOMER V. D., WRIGHT E. T. STUDIES OF THE SOLUBLE ANTIGENS OF COCCIDIOIDES IMMITIS BY IMMUNODIFFUSION. J Invest Dermatol. 1963 Oct;41:225–233. doi: 10.1038/jid.1963.100. [DOI] [PubMed] [Google Scholar]
- SMITH C. E. Coccidioidomycosis. Pediatr Clin North Am. 1955 Feb;:109–125. [PubMed] [Google Scholar]
- SMITH C. E. Diagnosis of pulmonary coccidioidal infections. Calif Med. 1951 Dec;75(6):385–394. [PMC free article] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., BEARD R. R., KEPP R. M., CLARK R. W., EDDIE B. U. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am J Hyg. 1950 Jul;52(1):1–21. doi: 10.1093/oxfordjournals.aje.a119404. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T. Histoplasmin sensitivity and coccidioidal infection; occurrence of cross-reaction. Am J Public Health Nations Health. 1949 Jun;39(6):722–736. doi: 10.2105/ajph.39.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation and effect of different adjuvants on the immunogenic activity of mycobacterial ribosomal fraction. J Bacteriol. 1967 Oct;94(4):836–843. doi: 10.1128/jb.94.4.836-843.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]