Abstract
Background
Strong theoretical models suggest implicit learning deficits may exist among children with Attention Deficit Hyperactivity Disorder (ADHD).
Method
We examine implicit contextual cueing (CC) effects among children with ADHD (n=72) and non-ADHD Controls (n=36).
Results
Using Ratcliff’s drift diffusion model, we found that among Controls, the CC effect is due to improvements in attentional guidance and to reductions in response threshold. Children with ADHD did not show a CC effect; although they were able to use implicitly acquired information to deploy attentional focus, they had more difficulty adjusting their response thresholds.
Conclusions
Improvements in attentional guidance and reductions in response threshold together underlie the CC effect. Results are consistent with neurocognitive models of ADHD that posit sub-cortical dysfunction but intact spatial attention, and encourage the use of alternative data analytic methods when dealing with reaction time data.
Keywords: Contextual Cueing, Diffusion Model, ADHD, Implicit Learning, Developmental Psychopathology, Neuropsychology
Introduction
ADHD is a behavioral syndrome marked by age-inappropriate levels of inattention, hyperactivity, and impulsivity that is present across multiple environments and leads to significant impairment in adaptive functions (APA, 1994). Executive dysfunction is considered a core cognitive mechanism of the disorder (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), but it is by no means ubiquitous (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005), and recent theories have begun to consider non-executive domains as well (Halperin & Schulz, 2006). In particular, implicit learning, or the ability to learn complex associations without conscious awareness or effort, is one intriguing candidate because the process is dependent upon the functioning of the basal ganglia and frontostriatal neural loops (Seger, 1994), structures which are implicated in ADHD (Dickstein, Bannon, Castellanos, & Milham, 2006; Nigg & Casey, 2005; Sagvolden, Johansen, Aase, & Russell, 2005).
Although behavioral control is most commonly conceptualized as an effortful and conscious process, the acquisition and utilization of implicit knowledge is also critical to self-regulation. Implicit links between cues or objects help guide attentional focus (King, Korb, & Egner, 2012; Moores, Laiti, & Chelazzi, 2003) as well as social behavior and non-social cognition (Bargh, 2007; Dijksterhuis, Bos, Nordgren, & van Baaren, 2006; Frith & Frith, 2006). This has led some (e.g. Nigg & Casey, 2005) to argue that if children with ADHD are less able to implicitly learn critical covariations within their environment, then their ability to utilize these structures in the service of self-regulation would be impaired. Despite this, the possible presence of an implicit learning deficit in ADHD has not been well evaluated in part because of the predominant focus on documenting impairments of conscious, effortful control in the extant literature.
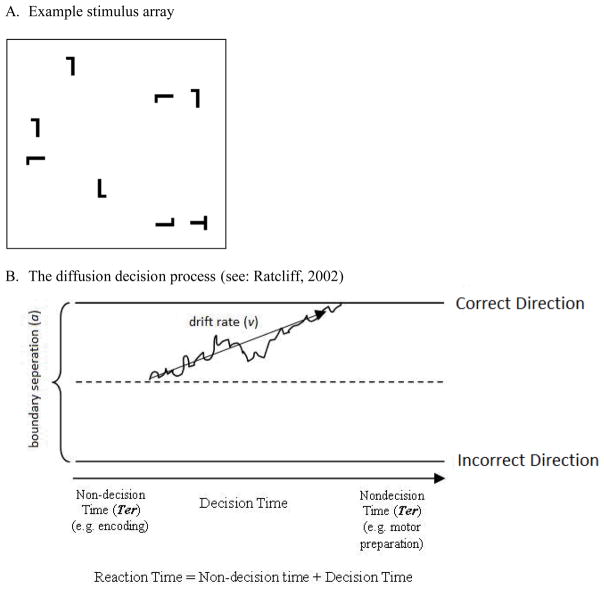
To examine implicit learning processes in ADHD, we focus on the spatial contextual cueing (CC) effect (Chun & Jian, 1998). In the most common form of the paradigm, participants indicate whether the target, the letter “T” which is placed among distractor letter “L”s, is rotated to the left or right (Figure 1A). CC refers to an effect in which the reaction times (RT) for target detection are faster when the target position repeatedly covaries with the spatial configurations of distractors, than when the target’s position is independent of distractor placement. These RT benefits occur despite the fact that participants display no conscious knowledge that some configurations are repeated.
Figure 1.
The existing literature on the CC effect suggests that it occurs for two reasons. First, mediated by the medial temporal lobe (MTL) (Chun, 2000; Chun & Jian, 1998; 1999), the memory of repeated displays efficiently guides attention to the target location. Second, once attention is focused, recognition of the repeated context allows individuals to reduce their response thresholds, or the amount of information needed to confirm the target has been identified (Kunar, Flusberg, Horowitz, & Wolfe, 2007; Kunar & Wolfe, 2011; Schankin & Schubo, 2009, 2010; Zhao et al., 2012). This reduces unnecessary time spent double checking when a stimulus appears in an expected context. For example, a person would be less inclined to seek confirmatory information and would be faster to identify the television remote if it were near the television. But, that same person would require more information and be more cautious if it were in an unusual location, say in the kitchen or bathroom. The striatum is believed to be the neural substrate responsible for mediating changes in response threshold (Forstmann et al., 2008; Ivanoff, Branning, & Marois, 2008; Kuhn et al., 2011) so populations that are hypothesized to have striatal disturbances, including ADHD (Nigg & Casey, 2005; Sagvolden et al., 2005), may demonstrate reduced CC effects due to difficulty in modulating their response thresholds but not in attentional guidance.
No group differences were found in the only other study of CC effects in ADHD (Barnes, Howard, Howard, Kenealy, & Vaidya, 2010). However, the sample size was modest (20 per group) and the average IQ of the children with ADHD was high average (FSIQ = 113), which is atypical. Thus, their analyses may have been underpowered and/or the normative CC effect may not generalize to a more representative population of children with ADHD.
Beyond issues of sampling, Barnes et. al. (2010) focused their analyses on median RTs, a data analytic strategy that is frequently used to diminish the impact that highly variable RTs have on findings. However, slow and/or variable RTs among children with ADHD are well documented (e.g. Epstein et al., 2011; C. L. Huang-Pollock, Karalunas, Tam, & Moore, 2012; Nikolas & Nigg, 2013), and recent work has suggested that RT variability may not only predict real world behavior (Antonini, Narad, Langberg, & Epstein, 2013) but may also be an important endophenotype associated with white matter abnormality (Castellanos, Kelly, & Milham, 2009; Valera, Faraone, Murray, & Seidman, 2007). Thus, the use of analytic techniques to reduce variance in performance may in fact remove true variance that is associated with the disorder.
Furthermore, when only median or mean RTs are used, an individual’s rate of information processing, speed-accuracy trade-off setting, and speed of encoding/motor preparation are all collapsed into a single estimate of performance (Ratcliff, 2002). Accurately modelling these processes and how they ultimately produce a RT is critical to the interpretability of RT data, as group differences in performance can be obscured when groups differ from one another on two subprocesses with opposing effects on the final RT (e.g. slower rate of information processing offset by faster motor preparation: Karalunas & Huang-Pollock, 2013). Thus, dependence on RT alone prevents us from determining why the CC effect occurs and may obscure the integrity of the effect in populations of interest.
The diffusion model (Ratcliff & Smith, 2004; Andreas Voss, Rothermund, & Voss, 2004) is one way to address this problem. This computational model assumes that simple forced choice decisions are made after a noisy information accumulation process (for a discussion of this process and its biological underpinnings, see: Smith & Ratcliff, 2004). As information accumulates, it gradually moves the diffusion process towards one of two boundaries (Figure 1B). When the process reaches one of the boundaries, the corresponding response is initiated.
The model has three primary parameters: drift rate (v), boundary separation (a), and non-decision time (Ter). Drift rate represents the efficiency with which an individual is able to gather evidence to make a forced choice decision (e.g., in which direction is the target pointing?). Boundary separation, an index of response threshold, represents how much evidence an individual requires to make a decision. Non-decision time represents the time it takes to complete all other processes not involved in the stimulus discrimination process (e.g., encoding or motor preparation). As any given set of parameter values predicts specific correct and error RT distributions, the model can be fit to empirical data by identifying a set of values that best corresponds to the observed distributions (for review of methods, see: van Ravenzwaaij & Oberauer, 2009). The model has been used to identify the source of individual differences in cognitive processing in college-aged adults (Ratcliff, Schmiedek, & McKoon, 2008), normal aging (Ratcliff, Thapar, & McKoon, 2004) and clinical populations (White, Ratcliff, Vasey, & McKoon, 2010) including childhood ADHD (C. L. Huang-Pollock et al., 2012; Karalunas & Huang-Pollock, 2013; Karalunas, Huang-Pollock, & Nigg, 2012).
Previous research on learning effects has found that, with practice, faster RTs to repeated vs. novel stimuli are due to preferential improvements in v (Dutilh, Krypotos, & Wagenmakers, 2011; Dutilh, Vandekerckhove, Tuerlinckx, & Wagenmakers, 2009). Correct orienting of spatial attention has also been suggested to speed evidence accumulation by improving the quality of the stimulus representation (Smith & Ratcliff, 2009). Similarly, we assume that improvements in attentional guidance on repeated arrays would speed target localization (detection) and thus facilitate target discrimination (left/right judgment), which would be reflected by faster v for repeated vs. novel configurations over time. Because changes in response threshold are also believed to contribute to the CC effect, a reduction in a (representing the amount of evidence required for response initiation) for repeated vs. novel configurations over time is also expected.
In summary, disturbances in frontostriatal neural loops may impair the ability of children with ADHD to acquire information implicitly. Or, it may be that the use of that knowledge to guide behavior is impaired by the disruption of a separate neurocognitive mechanism. If the CC effect is diminished among children with ADHD, the locus of that deficit could be due to problems (a) acquiring implicit knowledge, in which case no CC effect would be seen on any index of performance, (b) using that implicitly acquired information to guide their attention, in which case a CC effect would not be seen for v, or (c) using that information to adjust their response thresholds on repeated trials, in which case a CC effect would not be seen for a. In the following study, we ask whether children with ADHD have impairments in spatial contextual cueing, and why.
Method
Participants
Children ages 9–12, with (n=72;39 male) and without (n=36;19 male) ADHD were recruited from local schools, newspaper and radio ads, and distributed flyers in the Centre and Dauphin county areas of Pennsylvania. Sample ethnicity reflected regional demographics: 76.9% Caucasian, 10.2% African American, 1.8% Hispanic, 0.9% Asian, 4.6% mixed, and 5.6% unknown. Exclusion criteria included (a) current non-stimulant medication treatment, (b) pervasive developmental disorder, intellectual or sensorimotor disability, psychosis, or other parent-reported neurological disorder, and (c) estimated Full Scale IQ (FSIQ)<70 based on a 2-subtest short-form (vocabulary and matrix reasoning, test-retest reliability=0.93, predictive validity=0.87) of the Wechsler Intelligence Scale for Children (WISC-IV: Wechsler, 2003).
Children with ADHD
Children with ADHD met DSM-IV criteria (APA, 1994) for ADHD including age of onset, duration, cross situational severity, and impairment as determined by parental report on the Diagnostic Interview Schedule for Children-IV (DISC-IV) (Shaffer, Fisher, & Lucas, 1997). At least one parent and one teacher report of behavior on the Attention, Hyperactivity, or ADHD subscales of the Behavioral Assessment Scale for Children (BASC-2: Reynolds & Kamphaus, 2004) or the Conners’ Rating Scales (Conners’: Conners, 2001) was required to exceed the 85th percentile (T-score>61). Following DSM-IV field trials (Lahey et al., 1994), an “or” algorithm integrating parent report on the DISC and teacher report on the ADHD Rating-Scale (DuPaul, Power, Anastopoulos, & Reid, 1998) was used to determine symptom count and subtype (Table 1). Children prescribed psychostimulant medication (N= 24, 33%) were asked to discontinue medication use for 24–48 hours (mean=66 hours).
Table 1.
Description of groups. Means, with standard deviation in parentheses. All ratings scales reported in T-scores.
| Control | ADHD | |
|---|---|---|
| N(Males:Females) | 36(19:17) | 72(39:33) |
| #Subtypes (H,I,C) | 5,40,33 | |
| Age | 10.50(1.16) | 10.17(1.02) |
| Estimated FSIQ | 103.69(9.18) | 103.75(12.56) |
| Hyperactivity/Impulsivity | ||
| Total # of symptoms | 0.25(0.65) | 4.89(2.89)*** |
| Parent/Self BASC-2 | 43.72(5.48) | 63.93(14.00)*** |
| Parent Conners | 46.67(3.38) | 66.79(14.48)*** |
| Teacher BASC-2 | 43.58(4.04) | 58.15(12.36)*** |
| Teacher Conners | 45.39(2.43) | 58.68(12.59)*** |
| Inattention | ||
| Total # of symptoms | 0.83(1.34) | 7.89(1.42)*** |
| Parent/Self BASC-2 | 45.42(5.56) | 66.63(6.32)*** |
| Parent Conners | 46.00(3.47) | 71.24(11.91)*** |
| Teacher BASC-2 | 43.17(6.07) | 61.42(6.35)*** |
| Teacher Conners | 46.06(4.63) | 60.01(10.88)*** |
| Comorbidity | ||
| MDD | 0 | 6 |
| GAD | 1 | 10 |
| ODD/CD | 1/0 | 23/6 |
Controls
Controls did not meet criteria for ADHD on the DISC-IV, had T-scores below the 80th percentile (T-score≤58) on all listed rating scales, and had never been previously diagnosed or treated for ADHD. To equate IQ levels between groups, controls with IQs>115 were excluded. The presence of anxiety, depression, oppositional defiant disorder, and conduct disorder was not exclusionary.
Procedures
The CC task was completed as part of a larger task battery that took place over two three-hour sessions. All data were collected in compliance with human subjects’ approval from the Pennsylvania State University Institutional Review Board (IRB#32126). Informed written consent from parents and verbal assent from children were obtained prior to participation. Parents received monetary compensation and informal clinical feedback. Children were given a small prize.
Contextual Cueing Paradigm
In a paradigm modeled after that of Chun and Jiang (1998), children viewed an array of 1 target and 7 distractors randomly distributed within an invisible 6×6 grid (Figure 1A) subtending a visual angle of ~10°×10° in the center of the computer screen. The target was a “T” rotated 90° to the left or right. Target direction was randomly chosen and did not covary with the target’s spatial location or with the configuration of the distractors. Distractors were “L”s rotated 0, 90, 180 or 270 degrees at random. Each quadrant of the grid contained two items and the target appeared in one of 16 possible locations (4 to a quadrant). Eight target locations were associated with repeated configurations and 8 target locations were associated with the novel configurations.
Children were instructed to find the “T” and to indicate by pressing a key on the response box whether it pointed right or left. The array remained visible until the child made a response. Each trial was followed by a 500ms blank screen. If an error, a feedback tone was produced during this interval. Children completed 5 practice trials followed by 30 blocks of 16 trials (8 novel, 8 repeated). For analysis, trials were grouped into 3 epochs, containing 10 blocks each resulting in a total of 160 trials (80 novel, 80 repeated) per epoch.
Explicit Recognition Post-Tests
Two explicit recognition post-tests followed. Children first saw 16 configurations (8 novel, 8 repeated) in random order in which the target was replaced by a distractor. Children indicated with a keypress in which quadrant the target would most likely appear. For the second post-test, they were presented 16 configurations (8 novel, 8 repeated) in random order with the target present and asked to indicate whether they had previously seen the configuration.
Diffusion Model Variable Analysis
Drift rate (v), boundary separation (a), and nondecision time (Ter) were estimated using the Fast-dm modelling program (A. Voss & Voss, 2007), downloaded from the authors’ website: http://www.psychologie.uni-heidelberg.de/ae/meth/fast-dm. Fast-dm estimates parameters by creating an empirical cumulative distribution function (CDF) of correct and error trials (terminating at the upper and lower boundaries, respectively). It then begins a multidimensional search, in which combinations of model parameters produce predicted CDFs, which are then compared to the empirical CDF for each condition. Fast-dm uses a simplex-downhill method to fit the predicted and observed distributions in three successive attempts with increasingly strict fit criteria until the best possible model-fit is achieved. To index model fit, the maximal vertical distance between the predicted and empirical CDFs (Kolmogorov-Smirnov statistic) is transformed into a probability value (with higher p-values indicating better fit, and p<.05 values denoting poor fit). For the current analysis, v, a, and Ter, were allowed to vary by individual subject, epoch and configuration, while other parameters of the diffusion model were only allowed to vary between individual subjects.
As outlier RTs can impede accurate estimation of diffusion model parameters (Ratcliff & Tuerlinckx, 2002), trials with RTs that were <300ms and >3000ms were excluded on the basis of cutoffs used in prior research applying the diffusion model with the same age group (Ratcliff, Love, Thompson, & Opfer, 2012). Participants for whom this procedure removed >25% of trials (5 ADHD, 1 Control) were excluded from analysis to allow accurate estimations of the model parameters. One additional participant with ADHD was excluded from analysis for poor task compliance (<80% accuracy). Among the remaining participants, ~8% of trials were excluded from analysis (4% for Controls, 9% for ADHD). Outliers were equally distributed between configurations (i.e. 49.6% of excluded trials were repeated). The six ADHD participants who were ultimately excluded did not differ from the ADHD sample that was retained in age, t(76)=−1.17, p=.25, estimated FSIQ, t(76)=−1.74, p=.09, or total number of ADHD symptoms, t(76)=1.81, p=.07. Fast-dm was able to fit the data of all remaining participants to the diffusion model parameters, as indicated by non-significant p-values for all model fits (all p>.22). After these exclusions, the final sample contained 72 children with ADHD and 36 typically-developing controls as reported in the Participants section.
Data analysis Plan
A Configuration (2: Novel/Repeated) × Epoch (1–3) × Diagnosis (2: ADHD/Control) repeated measures ANOVA was conducted on mean RTs (to correct trials), accuracy, v, a, and Ter.
Results
Preliminary Analyses
Table 1 provides descriptive statistics. Children with ADHD were more inattentive, t(106)=−24.79, p<.001, and hyperactive/impulsive, t(106)=−9.51, p<.001, than Controls. There were no group differences in FSIQ, t(106)=−.02, p=.98, or age, t(106)=1.53, p=.13.
Primary Analyses
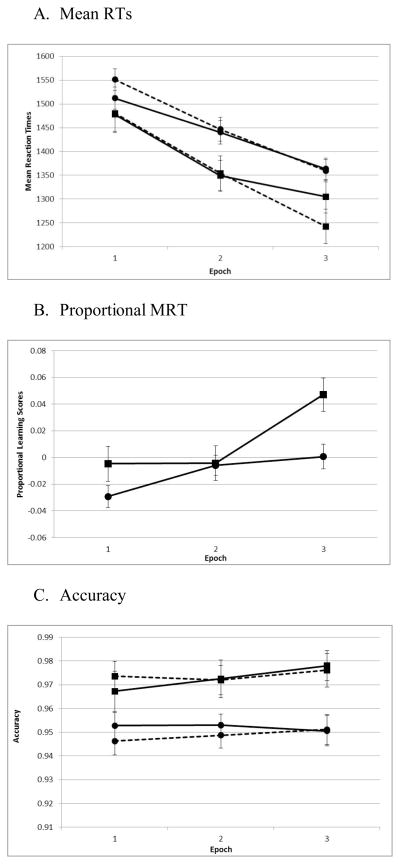
Children with ADHD had slower RTs, F(1,106)=4.11,η2=.04, p=.045, and RTs decreased with epoch, F(2,212)=116.81,η2=.52, p <.001. Although there was no effect of configuration, F(1,106)=.12,η2=.001, p=.73, children had faster RTs to repeated vs. novel configurations over time (EpochxConfiguration, F(2,212)=8.60,η2=.08, p<.001; Figure 2A), indicating the presence of a CC effect. Controls, but not children with ADHD, also had shorter RTs to repeated vs. novel configurations (Configuration×Diagnosis, F(1,106)=5.56,η2 =.05, p=.02).
Figure 2.
Solid line=Novel configuration; Dotted line=Repeated configuration; Squares=Controls; Circles=ADHD. Error bars represent standard error of the mean.
The three way interaction was not significant, F(2,212)=2.35,η2=.02, p=.10. However, planned post-hoc comparisons found a significant Epoch×Configuration interaction for Controls, F(2,35)=8.96,η2=.20, p<.001, in which RTs decreased over time for repeated vs. novel configurations, and particularly in the final Epoch, t(35)=3.93,η2=.18, p<.001. Comparatively, the Epoch×Configuration interaction for ADHD, F(2,71)=8.96,η2=.05, p=.03, was in the reverse direction; RTs for repeated vs. novel configurations were longer in the first epoch, t(71)=−3.24,η2=.07, p=.002.
To control for baseline differences in RT, proportional learning scores [(Novel MRT – Repeated MRT)/Novel MRT] were calculated (Barnes et al., 2010). Expected effects of epoch, F(2,212)=9.84,η2=.09, p<.001, and diagnosis, F(1,106)=6.27,η2=.06, p=.01, were found which were qualified by a marginally significant Epoch×Diagnosis interaction, F(2,212)=2.81,η2 =.03, p=.06. The RT difference between novel and repeated configurations, controlling for baseline speed, was much greater for Controls than for children with ADHD, and particularly in the last epoch (Figure 2B). Collectively, these results suggest that Controls, but not children with ADHD, displayed a CC effect.
Children with ADHD were less accurate than Controls, F(1,106)=9.49,η2=.08, p=.003. There were no other main effects or interactions (all p>0.24, all η2<0.01; Figure 2C), indicating that RT improvements on repeated trials did not come at a significant cost to response accuracy.
Diffusion Model Parameters
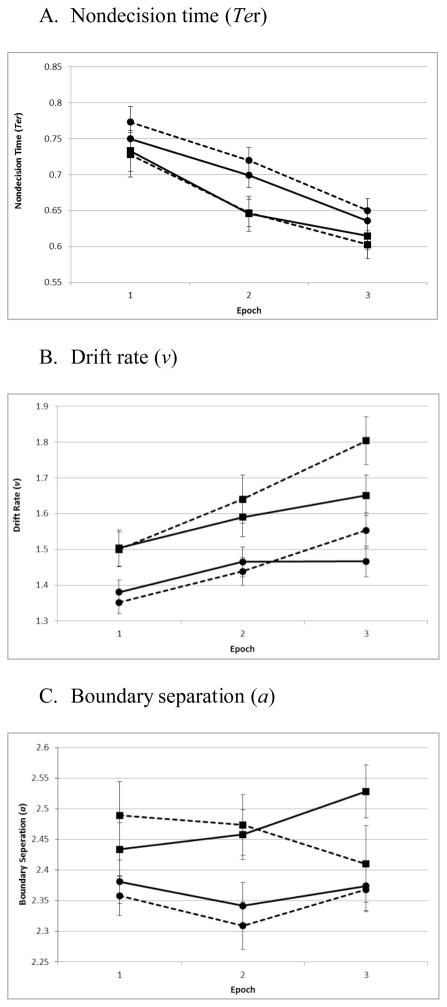
Non-decision time (Ter) became faster with practice, F(2,212)=48.46,η2=.31, p<.001, and was slightly longer for repeated vs. novel configurations among children with ADHD (Diagnosis: F(1,106)=2.96,η2=.03, p=.09; Configuration×Diagnosis: F(1,106)=2.88,η2=.03, p=.09; Figure 3A). There were no other main effects or interactions (all p>0.35, all η2<0.01).
Figure 3.
Solid line=Novel Configuration; Dotted line=Repeated Configuration; Squares=Controls; Circles=ADHD. Error bars represent standard error of the mean.
Drift rate (v) was slower for children with ADHD (Diagnosis:F(1,106)=10.19,η2=.09, p=.002), improved with practice (Epoch:F(2,212)=22.56,η2=.18, p<.001), and was faster for repeated vs. novel configurations (Configuration:F(1,106)=4.04,η2=.04, p=.047). v was also faster for repeated vs. novel configurations over time (Epoch×Configuration: F(2,212)=5.88,η2=.05, p=.003; Figure 3B), particularly in the final epoch, t(107)=-3.42, η2=.05, p=.001, confirming that part of the CC effect can be attributed to changes in attentional guidance. Both groups displayed similar improvements in v for repeated vs. novel configurations over time (remaining two and three-way interactions, all p>0.14; all η2<.02).
Boundary separation (a) was narrower for children with ADHD (Diagnosis:F(1,106)= 5.94,η2=.05, p=.02), but did not change with practice or by configuration (both p >0.40, both η2<.007). However, a marginally significant Epoch×Configuration×Diagnosis interaction was detected, F(2,212)=2.87,η2=.03, p= .06. Planned post-hoc comparisons found a significant Epoch×Configuration interaction for Controls, F(2,70)=3.39,η2=.09, p=.04, but not children with ADHD, F(2,142)=.15,η2<.01, p=.86, in which Controls narrowed their response threshold to repeated configurations, and increased them to novel configurations over time, particularly in the final epoch, t(35)=3.00,η2=.06, p=.04 (Figure 3C). Thus, changes in response threshold contribute to the CC effect for Controls, and the absence of such changes explains the absence of the CC effect in children with ADHD.
Results using diagnosis as an independent categorical variable were upheld when analyses were repeated using continuous indices of behavioral inattention and hyperactivity. Drift rate for both novel and repeated configurations was negatively correlated with parent and teacher-reported inattention and hyperactivity (all r’s >−0.17, all p’s <0.05). Boundary separation on novel configurations was also negatively correlated with parent and teacher reported inattention and hyperactivity (all r’s >0.19, all p’s <0.05). This was not the case for repeated configurations, reflecting the Diagnosis × Configuration interaction in which typically-developing children, but not children with ADHD, lowered their boundaries on repeated configurations.
Post-Tests
Participants performed no better than chance on either the first t(107)=.83, p=.41, or the second post-test, t(107)=1.80, p=.07, suggesting that the CC effect was implicit. Results did not differ by diagnosis.
Discussion
We found evidence of impaired CC effects among children with ADHD and used the drift diffusion model to determine that this was due to a relative inability to appropriately adjust their response thresholds.
In adults, the CC effect can be attributed to changes in attentional guidance (Chun & Jiang, 1999), operationalized in the current study as improvements in drift rate (v), and to changes in response threshold (Kunar et al., 2007; Kunar & Wolfe, 2011), operationalized as reductions in a. We also found this to be the case for non-ADHD Controls. A significant Configuration×Epoch interaction was observed in which v became faster and a became smaller with practice for repeated vs. novel configurations. CC effects have been found in typically-developing children ranging in age from 5–14 years (Barnes et al., 2008; Barnes et al., 2010; Dixon, Zelazo, & De Rosa, 2010), but see Vaidya et al. (2007). This is, however, the first study of which we are aware that has demonstrated that both mechanisms are responsible for this effect in children.
v was slower among children with ADHD, a finding that is consistent with performance on a variety of speeded RT tasks (C. L. Huang-Pollock et al., 2012; Karalunas & Huang-Pollock, 2013; Karalunas et al., 2012; Metin et al., 2013). Despite this, the rate at which v improved for repeated vs. novel configurations was similar between groups. Children with ADHD are therefore capable of implicitly acquiring associative knowledge, and are able to use that knowledge to guide their attentional focus. These findings are consistent with previous work documenting normative automatic and effortful deployment of spatial attention in ADHD (C. Huang-Pollock & Nigg, 2003; C. Huang-Pollock, Nigg, & Carr, 2005).
However, a was more narrow in children with ADHD, and did not show the same flexibility observed among Controls, who reduced a for repeated configurations, and increased a for novel configurations. Thus, children with ADHD are able to learn implicit associations and to use that information to guide their visual attention (as demonstrated by changes in v), but they have particular difficulty adjusting their response thresholds in response to this implicit base of knowledge. Mulder et al. (2010) similarly reported ADHD-related difficulties in the flexible adjustment of response threshold. In that study, children were instructed either to emphasize speed or accuracy on a perceptual discrimination task. Although there were no group differences in a in the speed condition, a was smaller for children with ADHD in the accuracy condition. Group differences in a are not always found (C. L. Huang-Pollock et al., 2012; Karalunas & Huang-Pollock, 2013; Karalunas et al., 2012), so together these data suggest that the most impairing aspect of performance among children with ADHD may best be conceptualized as a lack of flexibility in this parameter, as opposed to a stable reduction in threshold. Interestingly, this appears to be the case whether the required changes in threshold are consciously controlled through explicit changes in instruction (as in Mulder et al. 2010) or implicitly, as in the current study.
Recent theories (Nigg & Casey, 2005; Sagvolden et al., 2005) have proposed that children with ADHD might have difficulties acquiring implicit knowledge, or have difficulty utilizing that knowledge to adjust their behavior. Our results suggest the latter, and encourage the field to shift from an almost exclusive focus on top-down executive processes to the examination of other theoretically relevant processes that may also be impaired in ADHD. Our findings also support a functional dissociation for the role of the MTL and striatum in the execution of the CC task. Whereas attentional guidance in the CC effect is believed to depend upon the MTL (Chun, 2000; Chun & Phelps, 1999), changes in response threshold have been linked to activity in the striatum (Forstmann et al., 2008; Ivanoff et al., 2008; Kuhn et al., 2011). The striatum, but not MTL, is implicated in ADHD, and our findings of normative attentional guidance but impaired modulation of response threshold suggest that the processes that underlie the CC effect are dissociable at both a behavioral and a neural level.
Non-decision time (Ter) decreased with practice for both groups irrespective of stimulus configuration and so was responsible for RT speed up due to general task familiarity (Dutilh et al., 2011), but not the specific CC effect. Ter was marginally slower for children with ADHD consistent with a large body of literature identifying motor speed and coordination problems in ADHD (Pitcher, Piek, & Hay, 2003; Rommelse et al., 2009).
It bears mentioning that it is not clear whether there is strong evidence to support the existence of implicit or unintentional learning (Shanks, 2010). Our post-test analyses suggest that children were not explicitly aware of the target location-distractor configuration pairings, although the number of post-test trials may have been underpowered (Smyth & Shanks, 2008). In the end, as unlikely as it may be that a completely unconscious form of learning exists, it is similarly unlikely that all forms of learning are conscious or effortful (Shanks, 2010). Whether the CC effect is fully unconscious is not critical to the interpretation of our findings.
One of the limitations of this study is that eye-tracking data was not available. In a CC paradigm, RT can be affected by any process that extends between the eye and the hand. Thus, a more accurate estimate of the response threshold effect requires the removal of the amount of time that elapses from stimulus onset to pupilary fixation of the target, time which is attributed to attentional guidance effects (Zhao et al., 2012). However using eye tracking data, Zhao et al. (2012), also found evidence of response threshold effects in the production of the CC effect in young adults. It remains to be seen if the explanatory power of the response threshold effect to explain diminished CC among children with ADHD would be reduced if eye tracking data were available.
Additionally, though we have operationalized changes in response threshold as changes in boundary separation (a), whether response threshold as conceptualized by Kunar and others, can be conceptualized in this way remains an empirical question. However, both constructs refer to an individual’s response conservatism, so the comparison appears reasonable.
And finally, we proposed that the implicit learning of spatial context improves attentional guidance, which in turn allows participants to more efficiently decide whether a target points left or right. A stricter interpretation of the data may hold that the decision process only begins once the target is identified, and thus improvements in v only reflect improvements in the left/right judgment. However, only the location of the “T” covaried with distractors in repeated configurations; whether it pointed left or right was randomized. If v only reflected the decisional efficiency of the left/right judgment, the CC effect for v would not have been observed. Thus, we are reasonably confident in our interpretation, but future applications of decision-making models to this task may benefit from more explicit considerations of these issues.
Conclusion
Over the last 40 years, research in the cognitive neuroscience of ADHD has been almost exclusively dominated by the examination of effortful control processes. Thus, the potential explanatory power of other promising cognitive mechanisms has been largely overlooked. Our findings help broaden the scope of cognitive research pertinent to ADHD, and to better understanding the source of the CC effect more broadly. Using a diffusion model framework allowed us to separate the contributions of attentional guidance (operationalized as drift rate) and response threshold reduction (operationalized as boundary separation) to the CC effect, which would not have been possible using molar RT or accuracy values alone. We found that although children with ADHD were able to implicitly acquire associative pairings, and to use those pairings to deploy their attentional focus, they had difficulty flexibly altering their response thresholds to the same. This finer grained analysis of performance would not have been possible through the use of traditional analytic techniques that depend upon molar RT variables.
Key Points.
Neurocognitive models of ADHD suggest that children with ADHD may display either impaired acquisition of implicit information, or impaired ability to utilize that information to guide behavior.
We found that children with ADHD could acquire implicit associations of spatial context, but were unable to optimally utilize that information to adjust their response thresholds, or the amount of information required to make a perceptual decision.
Our results indicate that the relative inflexibility in the adjustment of response threshold is one mechanism through which striatal dysfunction impacts cognitive performance in ADHD.
These findings encourage further inquiry into other non-executive cognitive domains that might be affected in the disorder.
Acknowledgments
This work was supported in part by National Institute of Mental Health Grant R01 MH084947 to C.H-P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors thank the parents, teachers, and children who participated, and tireless research assistants who helped in the conduct of the study.
Footnotes
Conflicts of interest statement: No conflicts declared.
The authors have declared that they do not have any potential or competing conflicts of interest.
References
- Antonini TN, Narad ME, Langberg JM, Epstein JN. Behavioral correlates of reaction time variability in children with and without ADHD. Neuropsychology. 2013;27(2):201–209. doi: 10.1037/a0032071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: Author; 1994. [Google Scholar]
- Bargh J. Social psychology and the unconscious: The automaticity of higher mental processes. New York, NY: Psychology Press; 2007. [Google Scholar]
- Barnes KA, Howard DV, Howard JH, Gilotty L, Kenworthy L, Gaillard WD, Vaidya CJ. Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology. 2008;22(5):563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Howard JH, Howard DV, Kenealy L, Vaidya CJ. Two Forms of Implicit Learning in Childhood ADHD. Developmental Neuropsychology. 2010;35(5):494–505. doi: 10.1080/875656412010494750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C, Milham MP. The Restless Brain: Attention-Deficit Hyperactivity Disorder, Resting-State Functional Connectivity, and Intrasubject Variability. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 2009;54(10):665–672. doi: 10.1177/070674370905401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends in Cognitive Sciences. 2000;4(5):170–178. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jian YH. Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology. 1998;36(1):28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang YH. Top-down attentional guidance based on implicit learning of visual covariation. Psychological Science. 1999;10(4):360–365. [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience. 1999;2(9):844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales—Revised Technical Manual. NY: Multi-Health Systems Inc; 2001. [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Bos MW, Nordgren LF, van Baaren RB. On making the right choice: The deliberation-without-attention effect. Science. 2006;311(5763):1005–1007. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Zelazo PD, De Rosa E. Evidence for intact memory-guided attention in school-aged children. Developmental Science. 2010;13(1):161–169. doi: 10.1111/j.1467-7687.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- DuPaul G, Power T, Anastopoulos A, Reid R. ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York: Guildford Press; 1998. [Google Scholar]
- Dutilh G, Krypotos AM, Wagenmakers EJ. Task-Related Versus Stimulus-Specific Practice A Diffusion Model Account. Experimental Psychology. 2011;58(6):434–442. doi: 10.1027/1618-3169/a000111. [DOI] [PubMed] [Google Scholar]
- Dutilh G, Vandekerckhove J, Tuerlinckx F, Wagenmakers EJ. A diffusion model decomposition of the practice effect. Psychonomic Bulletin & Review. 2009;16(6):1026–1036. doi: 10.3758/16.6.1026. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, Altaye M. Evidence for Higher Reaction Time Variability for Children With ADHD on a Range of Cognitive Tasks Including Reward and Event Rate Manipulations. Neuropsychology. 2011;25(4):427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmaker EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17538–17542. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. How we predict what other people are going to do. Brain Research. 2006;1079:36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: The case of visuospatial orienting. Clinical Psychology Review. 2003;23(6):801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C, Nigg JT, Carr TH. Deficient attention is hard to find: applying the perceptual load model of selective attention to attention deficit hyperactivity disorder subtypes. Journal of Child Psychology and Psychiatry. 2005;46(11):1211–1218. doi: 10.1111/j.1469-7610.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating Vigilance Deficits in ADHD: A Meta-Analysis of CPT Performance. Journal of Abnormal Psychology. 2012;121(2):360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff J, Branning P, Marois R. fMRI Evidence for a Dual Process Account of the Speed-Accuracy Tradeoff in Decision-Making. Plos One. 2008;3(7) doi: 10.1371/journal.pone.0002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL. Integrating Impairments in Reaction Time and Executive Function Using a Diffusion Model Framework. Journal of Abnormal Child Psychology. 2013;41(5):837–850. doi: 10.1007/s10802-013-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL, Nigg JT. Decomposing Attention-Deficit/Hyperactivity Disorder (ADHD)-Related Effects in Response Speed and Variability. Neuropsychology. 2012;26(6):684–694. doi: 10.1037/a0029936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Korb FM, Egner T. Priming of Control: Implicit Contextual Cuing of Top-down Attentional Set. Journal of Neuroscience. 2012;32(24):8192–8200. doi: 10.1523/jneurosci.0934-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Schmiedek F, Schott B, Ratcliff R, Heinze HJ, Duzel E, Lovden M. Brain Areas Consistently Linked to Individual Differences in Perceptual Decision-making in Younger as well as Older Adults before and after Training. Journal of Cognitive Neuroscience. 2011;23(9):2147–2158. doi: 10.1162/jocn.2010.21564. [DOI] [PubMed] [Google Scholar]
- Kunar MA, Flusberg S, Horowitz TS, Wolfe JM. Does contextual cuing guide the deployment of attention? Journal of Experimental Psychology-Human Perception and Performance. 2007;33(4):816–828. doi: 10.1037/0096-1523.33.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunar MA, Wolfe JM. Target absent trials in configural contextual cuing. Attention Perception & Psychophysics. 2011;73(7):2077–2091. doi: 10.3758/s13414-011-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151(11):1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Metin B, Roeyers H, Wiersema JR, van der Meere JJ, Thompson M, Sonuga-Barke E. ADHD performance reflects inefficient but not impulsive information processing: A diffusion model analysis. Neuropsychology. 2013;27(2):193–200. doi: 10.1037/a0031533. [DOI] [PubMed] [Google Scholar]
- Moores E, Laiti L, Chelazzi L. Associative knowledge controls deployment of visual selective attention. Nature Neuroscience. 2003;6(2):182–189. doi: 10.1038/nn996. [DOI] [PubMed] [Google Scholar]
- Mulder MJ, Bos D, Weusten JMH, van Belle J, van Dijk SC, Simen P, Durston S. Basic Impairments in Regulating the Speed-Accuracy Tradeoff Predict Symptoms of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2010;68(12):1114–1119. doi: 10.1016/j.biopsych.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/s0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, Nigg JT. Neuropsychological Performance and Attention-Deficit Hyperactivity Disorder Subtypes and Symptom Dimensions. [Article] Neuropsychology. 2013;27(1):107–120. doi: 10.1037/a0030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher TM, Piek JP, Hay DA. Fine and gross motor ability in males with ADHD. [Article] Developmental Medicine and Child Neurology. 2003;45(8):525–535. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A diffusion model account of response time and accuracy in a brightness discrimination task: Fitting real data and failing to fit fake but plausible data. Psychonomic Bulletin & Review. 2002;9(2):278–291. doi: 10.3758/bf03196283. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Love J, Thompson CA, Opfer JE. Children Are Not Like Older Adults: A Diffusion Model Analysis of Developmental Changes in Speeded Responses. Child Development. 2012;83(1):367–381. doi: 10.1111/j.1467-8624.2011.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Schmiedek F, McKoon G. A diffusion model explanation of the worst performance rule for reaction tune and IQ. Intelligence. 2008;36(1):10–17. doi: 10.1016/j.intell.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL. A Comparison of Sequential Sampling Models for Two-Choice Reaction Time. Psychological Review. 2004;111(2):333–367. doi: 10.1037/0033-295x.111.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. A diffusion model analysis of the effects of aging on recognition memory. Journal of Memory and Language. 2004;50(4):408–424. doi: 10.1016/j.jml.2003.11.002. [DOI] [Google Scholar]
- Ratcliff R, Tuerlinckx F. Estimating parameters of the diffusion model: Approaches to dealing with contaminant reaction times and parameter variability. Psychonomic bulletin & review. 2002;9(3):438–481. doi: 10.3758/bf03196302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C, Kamphaus R. Behavior Assessment for Children, (BASC-2) Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Rommelse NN, Altink ME, Fliers EA, Martin NC, Buschgens CJ, Hartman CA, Oosterlaan J. Comorbid problems in ADHD: degree of association, shared endophenotypes, and formation of distinct subtypes. Implications for a future DSM. J Abnorm Child Psychol. 2009;37(6):793–804. doi: 10.1007/s10802-009-9312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Schankin A, Schubo A. Cognitive processes facilitated by contextual cueing: Evidence from event-related brain potentials. Psychophysiology. 2009;46(3):668–679. doi: 10.1111/j.1469-8986.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- Schankin A, Schubo A. Contextual cueing effects despite spatially cued target locations. [Article] Psychophysiology. 2010;47(4):717–727. doi: 10.1111/j.1469-8986.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- Seger CA. Implicit Learning. Psychological Bulletin. 1994;115(2):163–196. doi: 10.1037/0033-2909.115.2.163. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas R. NIMH Diagnostic INterview Schedule for Children-IV. New York: Ruane Center for Early Diagnosis, Division of Child Psychiatry, Columbia University; 1997. [Google Scholar]
- Shanks DR. Learning: From Association to Cognition. Annual Review of Psychology. 2010;61:273–301. doi: 10.1146/annurev.psych.093008.100519. [DOI] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. An Integrated Theory of Attention and Decision Making in Visual Signal Detection. Psychological Review. 2009;116(2):283–317. doi: 10.1037/a0015156. [DOI] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends in neurosciences. 2004;27(3):161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Smyth AC, Shanks DR. Awareness in contextual cuing with extended and concurrent explicit tests. Memory & Cognition. 2008;36(2):403–415. doi: 10.3758/mc.36.2.403. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Huger M, Howard DV, Howard JH. Developmental differences in implicit learning of spatial context. Neuropsychology. 2007;21(4):497–506. doi: 10.1037/0894-4105.21.4.497. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- van Ravenzwaaij D, Oberauer K. How to use the diffusion model: Parameter recovery of three methods: EZ, fast-dm, and DMAT. [Article] Journal of Mathematical Psychology. 2009;53(6):463–473. doi: 10.1016/j.jmp.2009.09.004. [DOI] [Google Scholar]
- Voss A, Rothermund K, Voss J. Interpreting the parameters of the diffusion model: An empirical validation. Memory & Cognition. 2004;32(7):1206–1220. doi: 10.3758/bf03196893. [DOI] [PubMed] [Google Scholar]
- Voss A, Voss J. Fast-dm: A free program for efficient diffusion model analysis. Behavior Research Methods. 2007;39(4):767–775. doi: 10.3758/bf03192967. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Weschler Intelligence Scale for Children - IV, Technical Manual. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- White CN, Ratcliff R, Vasey MW, McKoon G. Using diffusion models to understand clinical disorders. Journal of Mathematical Psychology. 2010;54(1):39–52. doi: 10.1016/j.jmp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E, Doyle A, Nigg J, Faraone S, Pennington B. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zhao G, Liu Q, Jiao J, Zhou PL, Li H, Sun HJ. Dual-state modulation of the contextual cueing effect: Evidence from eye movement recordings. Journal of Vision. 2012;12(6) doi: 10.1167/12.6.11. [DOI] [PubMed] [Google Scholar]