Abstract
Using a rodent model of ischemic stroke (permanent middle cerebral artery occlusion; pMCAO), our laboratory has previously demonstrated that sensory-evoked cortical activation via mechanical single whisker stimulation treatment delivered under an anesthetized condition within 2 hours of ischemic onset confers complete protection from impending infarct. There is a limited time window for this protection: rats that received the identical treatment 3 hours following ischemic onset lost neuronal function and sustained a substantial infarct. Rats in these studies, however, were anesthetized with sodium pentobarbital or isoflurane, whereas most human stroke patients are typically awake. To optimize our animal model, the present study examined, using functional imaging, histological, and behavioral analysis, whether self-induced sensory-motor stimulation is also protective in unrestrained, behaving, rats that actively explore an enriched environment. Rats were revived from anesthesia either immediately or three hours after pMCAO – at which point they were allowed to freely explore an enriched environment. Rats that explored immediately after ischemic onset maintained normal cortical function and did not sustain infarct, even when their whiskers were clipped. Rats that were revived 3 hours post-pMCAO exhibited eliminated cortical function and sustained cortical infarct. Further, the data suggest that the level of individual active exploration could influence the outcome. Thus, early activation of the ischemic cortical area via unrestrained exploration results in protection from ischemic infarct, whereas late activation results in infarct, irrespective of level of arousal or whisker-specific stimulation.
Keywords: plasticity, ischemia, neuroprotection, animal models, somatosensory cortex
Introduction
Stroke is a major cause of disability and mortality worldwide (Roger et al., 2012). Both clinical and preclinical research has clearly established that disruption of cerebral blood flow during ischemic stroke triggers large-scale reorganization of the brain at multiple levels, including cortical function and blood flow (for a recent review see Xerri, 2012). Growing evidence based on functional, structural and behavioral techniques in a rodent model of ischemia (permanent middle cerebral artery occlusion; pMCAO) demonstrated that, when administered soon after ischemic onset, sensory-induced evoked cortical activity initiates neurovascular plasticity resulting in a complete protection from impending ischemic stroke (Lay et al., 2010; Davis et al., 2011; Lay et al., 2011; Hancock et al., 2013; Lay et al., 2013) as reviewed in (Frostig et al., 2013). These studies have shown that multiple forms of whisker tactile stimulation treatment can completely protect the cortex from impending damage when initiated within two hours of pMCAO, but when initiated three hours post-pMCAO treatment resulted in irreversible damage.
The central hypothesis driving this line of research has been that cortical activation within the ischemic region of cortex plays the critical role in protecting the brain from stroke (Frostig et al., 2013). This hypothesis suggests that following pMCAO it is possible to completely protect the adult rat ischemic cortex not solely through passive mechanical stimulus delivered under anesthetized conditions, but also through active, natural self-stimulation in awake, behaving animals to create a more realistic animal model for ischemic stroke. Here we tested this hypothesis by allowing rats to freely explore an enriched environment. Rats were revived from anesthesia either immediately or three hours after pMCAO – at which point they were also allowed to freely explore an enriched environment.
Because the ischemic MCA territory can also be stimulated by other types (i.e., not only whiskers) of sensory-motor inputs present in an enriched environment during active exploration (e.g. auditory, tactile, and motor stimuli), these types of stimulations could, according to our hypothesis, also lead to protection. To address this issue, we tested whether clipping the large facial whisker (vibrissae) array of rats immediately post-pMCAO and prior to active exploration modified any potential protective effect.
While the majority of our lab’s previous work was conducted using sodium pentobarbital, this anesthesia can render animals unresponsive for many hours post-anesthesia cessation and therefore missing the 2 hour window of opportunity for protection. In order to circumvent this problem, we have recently incorporated the inhalational anesthetic isoflurane, from which animals regain consciousness within minutes of cessation. Our studies using isoflurane yielded identical results to those obtained previously with sodium pentobarbital (Lay et al., 2013) confirming that isoflurane-anesthetized rat pMCAO model can be used in for pMCAO stroke model, and therefore allowed us to investigate sensory-based protection from impending ischemic stroke using awake, behaving animals.
Methods
All procedures were in compliance with NIH guidelines and approved by UC Irvine Animal Care and Use Committee (protocol #: 1997-1608, assurance ID#: A3416.01).
Surgical Preparation & Anesthesia
Male Sprague-Dawley rats (295–400g; Charles River Laboratories) used in this study were individually housed in standard cages with food and water ad libitum on a 12-h light/12-h dark cycle prior to experimental procedures. All rats were anaesthetized using isoflurane, and maintained at 1.5% – 2.0% (E-Z Anesthesia Machine) isoflurane in 100% oxygen as described in (Lay et al., 2013). In order to ensure that all rats were exposed to the same duration of anesthesia, each subject was anesthetized for 7 hours prior to exposure an enriched environment, regardless of experimental group. Anesthetic depth was assessed by monitoring the animal’s breathing rate and regular testing of reflexes. Body temperature was maintained at 37°C. Five percent dextrose (3 mL, subcutaneous) was administered to prevent dehydration. In a subset of rats, arterial oxygen saturation, respiration, pulse distension (a proxy for blood pressure), and heart rate were measured using pulse oximetry (Starr Life Sciences, Allison Park, PA) to ensure that any observed changes following pMCAO were not due to variability in vital parameters. Previous studies in our lab have shown that pMCAO and stimulation treatment do no alter systemic vital parameters (Lay et al., 2011; Lay et al., 2013), and here again significant alterations in vital parameters were not observed.
Experimental Groups
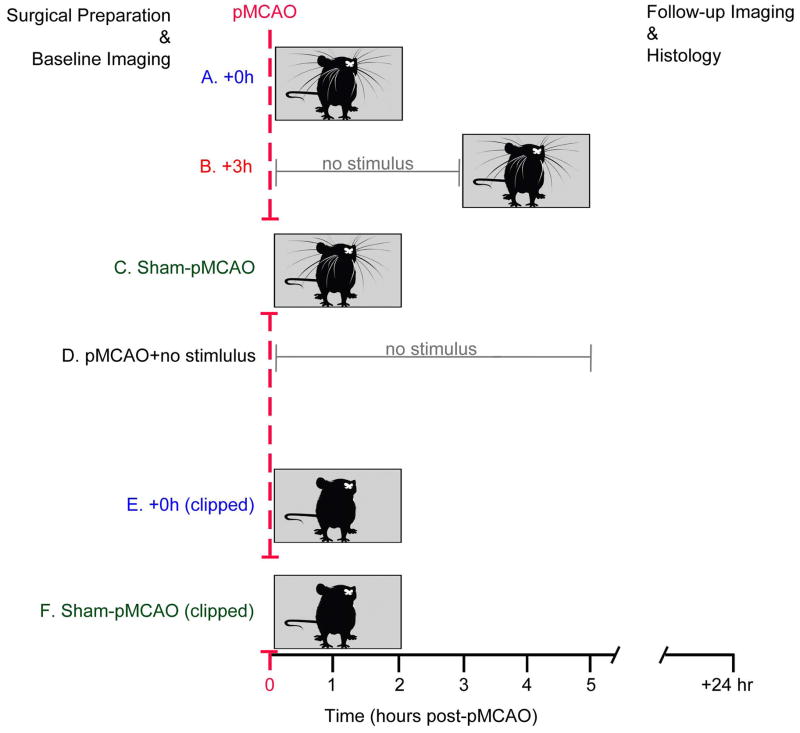
Rats were randomly assigned to one of the following experimental groups (n=8 per group; Figure 1):
Figure 1.
Experiment 1: The effects of early and late active exploration on protection
The aim of Experiment 1 was to determine whether sensory-induced neuroprotection is possible in awake, behaving subjects. Rats were revived from isoflurane anesthesia either immediately (+0h, n=8; Figure 1A) or three hours (+3h, n=8; Figure 1B) following pMCAO (ischemia onset) – at which point they were placed into an enriched environment (see below for details). Two control groups were also employed: a sham-operation group, and an untreated pMCAO group. In the sham-operated awake treatment group (sham controls, n=8; Figure 1C), rats underwent identical surgical procedures but MCA was never blocked. These sham control rats were revived immediately following surgery, and were thus included to rule out potential confounding effects that the experimental procedure and/or novel environment might have exerted on the rodent cortex. Finally in order to determine the degree of stroke damage sustained as a result of pMCAO in the absence of treatment, an untreated pMCAO control group (pMCAO controls, n=8; Figure 1D) was also included in experiment 1. These untreated pMCAO control rats underwent pMCAO and were maintained under isoflurane for 5 hours prior to being placed in the enriched environment in order to match experimental animals on all variables aside from exposure to enriched environment within the protective time window.
Experiment 2: Is sensory-induced protection whisker-specific?
The aim of Experiment 2 was to determine whether sensory-induced neuroprotection is whisker stimulus-specific. To address this question, an additional +0h awake group of rats had their whisker array clipped prior to pMCAO, and were then immediately placed into the enriched environment (+0h clipped, n=8; Figure 1E). To functionally assess these rats 24 hours post-pMCAO, whiskers were re-attached using superglue just prior to evoked functional imaging. To control for any changes that whisker clipping and re-attachment may have had on cortical function and plasticity, a whisker clipped sham-pMCAO control group, which is comprised of rats that underwent identical surgical procedure but the MCA was never blocked (clipped sham controls, n=8; Figure 1F) was also assessed at baseline, and 24 hours after whisker clipping and placement into the enriched environment.
Permanent Middle Cerebral Artery Occlusion (pMCAO)
Ischemic conditions were achieved via surgical occlusion and transection of the M1 segment (just distal to lenticulostriate branching) of the left middle cerebral artery (Davis et al., 2013). Only MCA cortical branches were affected and thus only cortical infarct (no subcortical damage) was expected (Coyle, 1982; Risedal et al., 1999). The skull and dura were carefully removed from a 2×2mm ‘surgical window’ just anterior and lateral to the imaging window (over the occlusion location; the M1 segment just distal to MCA’s lenticulostriate branching) and a half-curve reverse cutting suture needle and thread (4-0 silk) was passed through the pial layer of the meninges, below MCA and above the cortical surface. A double ligature was tied and tightened around MCA and the vessel was then transected (completely severed) between the two knots; for more details about the procedure see (Davis et al., 2013). Experiments were terminated if there was any sign of bleeding from MCA, or if there were obvious arterial abnormalities or malformations (Niiro et al., 1996).
Enriched Environment and behavioral analysis
Following pMCAO, rats were individually placed into an enriched environment including a tunnel connecting two large cages that included small objects and buried food treats to encourage active exploration. All rats were monitored with a digital video camera for 120 minutes following revival from anesthesia. Afterwards, observers blind to experimental group identity quantified how long each animal took to revive from anesthesia, and total time spent actively exploring the environment. Revival was defined as spontaneous movement from a prone to sitting position. Active exploration was defined as occurring when the animal sniffed, oriented towards, or made contact with the cage walls, tunnel, or toys with any part of its head, whiskers, or forelimbs (Sahakian et al., 1977). After each experiment, the enriched environment and all components were thoroughly washed prior to re-use.
Intrinsic Signal Optical Imaging and Analysis
We used the functional imaging technique intrinsic signal optical imaging to assess whisker functional representation prior to, and 24 hours after pMCAO to assess the functional integrity of the rat cortex. Intrinsic signal optical imaging has been used extensively to provide high spatial resolution maps of stimulus evoked hemodynamic-related signals as an indirect means to image the functional organization of the cortex, and examine how these contribute to brain function (Grinvald et al., 1986; Frostig et al., 1990; Ts’o et al., 1990). The initial dip phase of the whisker functional representation is generally associated with evoked neuronal activity and the overshoot phase with blood flow response (for a recent review of ISOI see Frostig and Chen-Bee, 2012). (Frostig & Chen-Bee, 2012)
A ~6mm × 5mm of skull overlying the left somatosensory cortex was exposed and thinned, and a vaseline wall was built around the periphery of the thinned skull region, filled with saline, and covered with a glass coverslip to enhance image quality. A charge-coupled device (CCD) camera was used for imaging with red light illumination (630 nm). Stimulus began 1.5 s into the start of an imaging trial. During stimulus delivery, a single whisker was deflected for 1 s in the rostral-caudal direction at 5 Hz frequency. A complete data set of 64 stimulation trials was collected, in which each 15-s trial consisted of 1.5 s of collected pre-stimulus data followed by 13.5 s of collected post-stimulus data with a 6±5 s random inter-trial interval. Imaging trials were then collapsed from 100-ms frames to 500-ms frames to increase the signal-to-noise ratio. Ratio images were created by dividing each 500-ms frame of post-stimulus signal activity by the 500-ms frame of pre-stimulus intrinsic signal activity. Under isoflurane anesthesia, the areal extent of the initial dip and overshoot were quantified at a threshold level of 5.0×10−4 away from zero and peak amplitude in fractional change units from the pixel with peak activity within the maximum areal extent of the evoked activity (Chen-Bee et al., 2007; Lay et al., 2010; Davis et al., 2011; Lay et al., 2011). While previous work using sodium pentobarbital has utilized 2.5 × 10−4 FC, the higher 5.0 × 10−4 FC threshold was chosen here to achieve areal extent values that were comparable to the previous studies. Peak amplitude was quantified in FC units from the pixel with peak activity within the maximum areal extent for each of the two phases. (for details see Lay et al., 2013).
Histology
Twenty-four hours after pMCAO, the brain was removed, sectioned into 2-mm coronal slices, then incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C for 20 minutes in the dark. Red stain intensity correlates to the functional activity of mitochondria and thus reflects viable neural tissues, whereas unstained (white) areas are indicative of cortical infarct. The TTC-stained sections were photographed, and the total infarct volume was determined by multiplying the infarct area of each slice by the slice thickness following the protocol described in (Lay et al., 2010; Davis et al., 2011; Lay et al., 2011).
Statistical Analysis
For statistical analysis, evoked area and amplitude were converted to difference score values (post-occlusion - baseline), with values away from 0 signifying a change from baseline. A constant was added to obtain only positive numbers in order to allow for ANOVA. Scores were transformed with a natural log function to better satisfy the assumptions of an ANOVA, and inferential statistics were performed on the transformed data. Alpha level was set to 0.05 and Bonferroni adjustments were applied to account for multiple contrasts. Separate ANOVAs followed by respective contrasts were performed for the two phases of the whisker functional representation. The alpha level was set to 0.05 and Bonferroni adjustment applied to account for multiple contrasts. In order to compare infarct volumes between experimental groups, Student’s t-tests (two-tailed) were used.
Results
Experiment 1: Early active exploration completely protects the cortex from ischemic stroke whereas late exploration leads to infarct
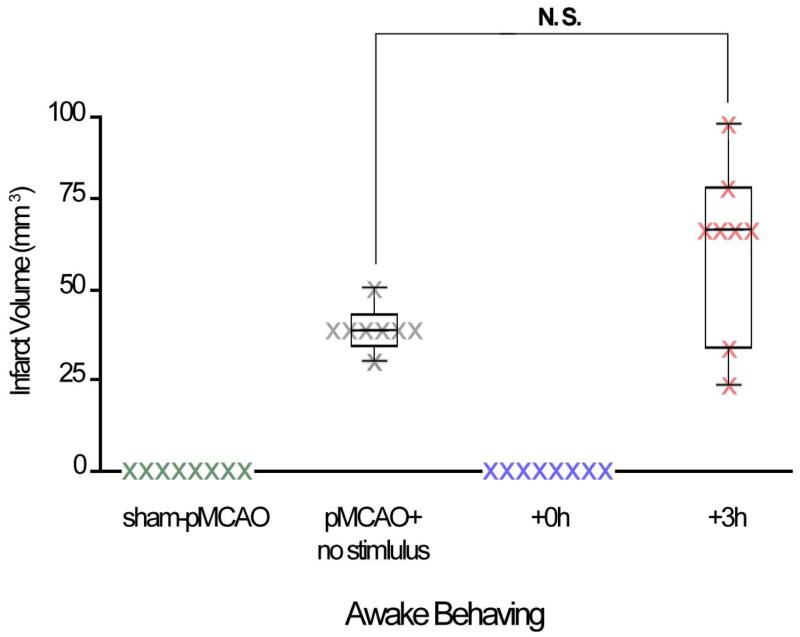
In order to determine whether self-induced sensory-motor activity, independent of anesthesia, also protects the brain from stroke, rats were revived from anesthesia following pMCAO and allowed to freely explore an enriched environment. Rats that were placed immediately into the enriched environment following pMCAO (+0h; Figure 2A) maintained whisker functional representation equivalent to, or greater than, baseline representation and did not sustain infarct. When revived three hours after pMCAO (+3h), enriched environment exposure resulted in an eliminated whisker functional representation in 5 out of 8 rats, and substantial cortical infarct (Figure 2B). The remaining 3 rats, demonstrated a reduced whisker functional representation and small infarct. Control rats which underwent sham-pMCAO and were placed into the enriched environment immediately following surgery (Figure 2C) maintain consistent whisker functional representation. Enriched environment exposure in untreated pMCAO controls (Figure 2D) resulted in diffuse and unlocalized whisker functional representation, and cortical infarct. (Because histological analysis also confirmed ischemic damage in untreated pMCAO controls, we feel confident that the whisker functional representation in these subjects may be categorized as ‘abnormal’, and not simply enlarged or heightened.) Given the diffuse and unquantifiable nature of the whisker functional representation in untreated pMCAO controls when re-assessed 24 hours post pMCAO, this group was excluded from further imaging analysis, and work is currently underway to further elucidate the nature of cortical dysfunction in these rats.
Figure 2.
To quantify these findings between-subject ANOVAs of imaging data were conducted between sham controls, +0h, and +3h rats at baseline, and at 24 hours following treatment. At baseline, there were no between groups differences in area or amplitude of the initial dip (area: F2,21= 0.11, p=0.90; amplitude: F2,21= 0.10, p= 0.91; ANOVA) or overshoot (area: F2,21= 0.24, p= 0.79; amplitude: F2,21= 1.93, p=0.17; ANOVA). Differences were found between groups 24 hours after pMCAO for both the initial dip and overshoot (initial dip area: F2,21= 7.74, p=0.003 & amplitude: F2,21= 11.45, p= 0.0004; overshoot area: F2,21= 8.96, p=0.002 & amplitude: F2,21= 6.73, p= 0.01; ANOVA; Figure 2E,F). Both sham control and +0h groups maintained baseline level whisker functional representation 24 hours after pMCAO. In contrast, the +3h group demonstrated a significant reduction in whisker functional representation 24 hours post pMCAO (initial dip area: F1,21= 19.99, p=0.0002 & amplitude: F1,21= 22.30, p= 0.0001; overshoot area: F1,21= 18.63, p=0.0003 & amplitude: F1,21= 16.57, p= 0.0003).
TTC histology revealed that rats that explored the enriched environment immediately after pMCAO (+0h) did not sustain stroke damage (Figure 3). In contrast, all +3h rats sustained infarct (mean±SE: 45.8±8.2 mm3) at the 24-hr post-pMCAO assessment (Figure 3). Untreated pMCAO controls sustained infarct (38.4±5.5 mm3; Figure 3) equivalent to +3h rats (t(14) = 0.66, p=0.51).
Figure 3.
Experiment 2: Sensory-induced protection is not whisker-specific
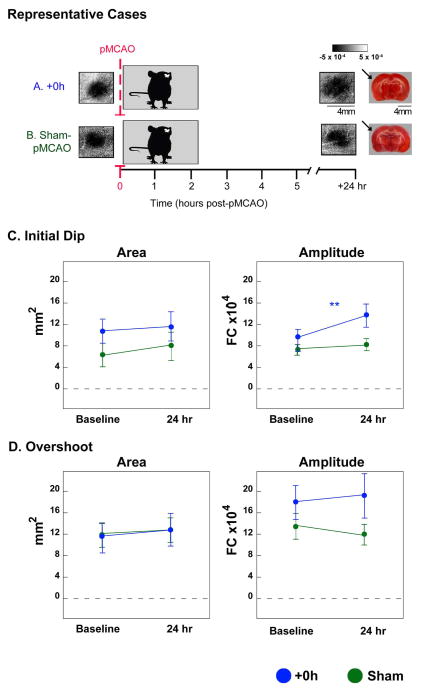
We further sought to address whether sensory-induced neuroprotection in the enriched environment was whisker-specific. Two additional groups of rats, therefore, underwent the same procedures as +0h rats and sham controls, except that after baseline imaging and prior to pMCAO, both whisker arrays were removed (clipped sham controls, and +0h clipped; Figures 1E,F, and 4A,B).
Figure 4.
There were no between groups differences in area or amplitude of the initial dip (area: F1,14= 2.28, p=0.15; amplitude: F1,14= 1.33, p= 0.27; ANOVA), or overshoot (area: F1,14= 0.01, p= 0.91; amplitude: F1,14= 1.61, p=0.22; ANOVA) at baseline. When assessed 24 hours post-pMCAO, there were no between groups differences in area of the initial dip (Figure 4C), area of the overshoot (Figure 4D), or amplitude of the overshoot (initial dip area: F1,14= 0.04, p=0.85; overshoot area: F1,14= 0.0008, p= 0.98 and amplitude: F1,14= 0.08, p= 0.78; ANOVA).
Differences from baseline were found between groups at 24 hours for the amplitude of the initial dip (F1,14= 4.65, p=0.0489, ANOVA). In +0h clipped rats, the amplitude of the initial dip increased compared to baseline assessment (F1,14= 13.08, p=0.003, Figure 4C). This result reproduces previous data demonstrating a significant increase in the initial dip amplitude of rats treated immediately post occlusion with mechanical whisker stimulation in anesthetized conditions (Lay et al., 2010; Davis et al., 2011; Lay et al., 2013).
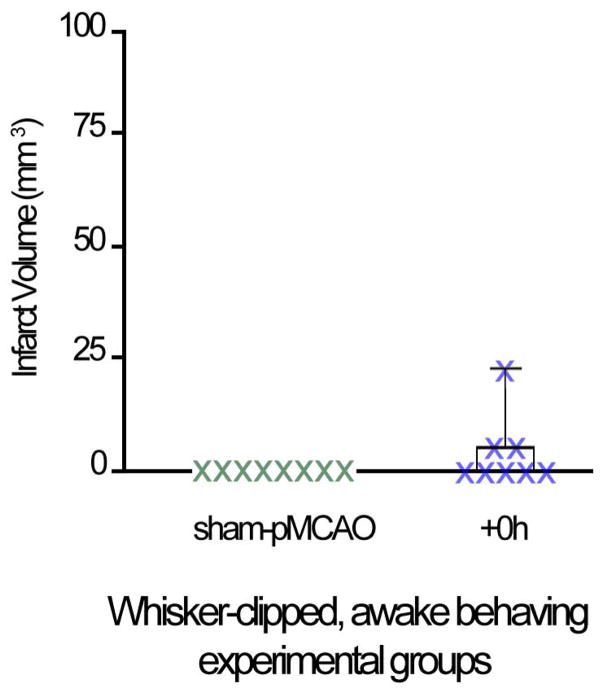
Finally, despite the overall trend of +0h clipped rats to maintain baseline or greater levels of whisker functional representation, a reduction in the area of the initial dip and overshoot was observed in 3/8 rats. Additionally, all 3 of these rats sustained a small infarct (14.7±4.7 mm3; Figure 5). Interestingly, if these animals are excluded from imaging analysis, the initial dip area, in addition to initial dip amplitude, is strengthened (area increases from 11.2±2.5 to 19.4±1.9 mm2, and amplitude significantly increases from 13.1±2.1 to 15.1±2.3 (mean±SE; F1,6= 20.94, p=0.004; ANOVA) in the 5 remaining clipped +0h subjects; therefore expanding the results to include area in addition to amplitude.
Figure 5.
Movement within the enriched environment and its potential relationship to pMCAO outcome
To determine whether revival time from anesthesia or active exploration played a role in the current functional and structural findings, we measured the latency-to-revive from isoflurane during the post-pMCAO observation period (see Methods for details). All experimental groups revived from anesthesia within ~15 minutes of cessation (F5,42= 0.28, p= 0.92; ANOVA). Additionally, work in other labs has shown that whisker clipping reduces whisker guided exploration in rats, even when rats are placed within a novel environment (Sun et al., 2010). We observed a similar result in the +0h clipped rats: un-clipped +0h rats explored for 26.7±5.3 minutes (mean ±SEM), while +0h clipped rats explored for only 8.9±3.6 minutes (t(14) = −2.71, p=0.01). Interestingly, the same 3 subjects that sustained diminished whisker functional representation and small infarct explored the enriched environment for 0, 0, and 5.3 minutes, which on average equals to 20% of their group and therefore among the lowest exploration durations observed. In the +3h group no correlation was found between the level of movement within the enriched environment and the size of infarct as measured by TTC (r=−0.004, n=8, p=0.993).
Discussion
In order to further optimize the pMCAO rat model of ischemic stroke for potential translation, we studied the effects of self-induced sensorimotor activity during ischemia in unrestrained, exploring rats. Based on previous studies in our lab, we hypothesized that sensory-induced activation within the ischemic territory would play a critical role in protecting rats that explored an enriched environment immediately post-pMCAO (+0h), and a harmful role when exploration was initiated 3 hours post-pMCAO (+3h). In order to encourage sensory-motor stimulation via exploratory behavior following pMCAO, we placed revived rats within an enriched environment. An enriched environment promotes exploratory behavior of rodents (Rosenzweig & Bennett, 1996) leading to adaptive cortical plasticity (Xerri, 2012), even in rats (Johansson & Ohlsson, 1996) and mice (Nygren & Wieloch, 2005) which have recently undergone MCA occlusion, thereby supplying the ischemic cortex with sensorimotor activity soon after ischemic onset.
We found that rats were fully protected from ischemic stroke when revived immediately (+0h) following pMCAO presumably by the same sensory evoked mechanism that allows blood flow from collaterals into the permanently occluded MCA and therefore enables reperfusion and protection of the ischemic area (Lay et al. 2010). Furthermore, the protective effect of sensory-evoked cortical activity is not limited to whisker-evoked activity only. Rats that have had their whisker arrays removed (clipped) prior to pMCAO and are placed into the enriched environment immediately post-occlusion also maintain whisker baseline or greater levels of whisker functional representation, contingent upon active exploration. Specifically, the amplitude of the initial dip increased compared with baseline imaging in these rats. Given that sham controls (which also had their whiskers array clipped) display a consistent whisker functional representation, we suggest that the increase in the +0h clipped group’s initial dip amplitude is indicative of neuroprotective plasticity in response to sensory stimulation post-pMCAO (Lay et al., 2010; Davis et al., 2011; Frostig et al., 2013). Through video analysis of all subjects, we found that the great majority of clipped rats explore the enriched environment in a manner very similar to rats with intact whiskers (i.e. clipped rats scan the perimeter of the enriched environment by keeping their body close to the cage walls). As a result, we cannot definitively rule out that experimental subjects did not receive a limited degree of whisker stub sensory stimulation. With this caveat in mind, the whisker array clipped animal data further supports the hypothesis that cortical activation within the MCA ischemic region plays a key role in protection from ischemic insult by demonstrating that in the presence of sensorimotor activity, stroke protection was observed irrespective of either anesthesia or whisker-specific stimulus.
If sensorimotor activity is indeed the critical factor, we further hypothesized that the degree of stroke protection could be modulated by the amount of neuronal activity evoked within the ischemic territory. The data show that this is plausible. Despite the overall trend of +0h whisker array clipped rats to maintain baseline or greater levels of whisker functional representation, a reduction in whisker functional representation was observed in 3/8 rats, and, all three of these rats sustained a small infarct (14.7±4.7 mm3). Additionally, these 3 rats also engaged in active exploration for 20% of the average time of their experimental group. The variability in active exploration in whisker array clipped +0h rats may have played a critical role in cortical activation post-ischemic onset: by remaining stationary for an extended period of time, these rats may have minimized the degree of cortical activation of the ischemic area induced by exploratory activity, and thereby limited its protective effect. Because this sample of 3 animals is small, further research is needed to reveal whether addition of sensory stimulation could compensate for the lack of movement.
Previous studies have suggested that, instead of protecting the cortex, early sensorimotor activation augments physical disability and infarct (Grabowski et al., 1995; Ohlsson & Johansson, 1995; Johansson & Ohlsson, 1996; Humm et al., 1998; Risedal et al., 1999; Risedal et al., 2002; Leasure & Schallert, 2004; Holschneider & Maarek, 2008). Bland et al. 2000, in particular have demonstrated that, in adult male rats, plaster cast immobilization of the unaffected forepaw and forced early use of the affected forepaw resulted in greater physical disability (Bland et al., 2000), and noted a significant cortical damage after 3 hour transient MCA occlusion. While seemingly contradictory to the data presented here, we believe that our findings are in agreement for two primary reasons. First, similar to the Bland et al study, we also find that sensorimotor activity after 3 hours results in ischemic damage to the cortex. Second, Bland et al 2000 also found that subjects who were not immobilized, but were allowed to use both paws freely following MCAO occlusion sustained infarct equivalent to sham-occlusion controls (i.e., no ischemic cortical damage was sustained). While this effect was attributed to the “moderate but not severe” level of ischemia induced in their model, it is possible that those subjects which were allowed to move freely benefited from the same type of sensory induced neuroprotection being described here.
We initiated this study of awake, behaving, ischemic rats in order to further optimize the pMCAO animal stroke model given that most human stroke patients are awake, and un-anesthetized. As in our previous studies which used passive mechanical whisker stimulation, we hypothesized that sensory-induced cortical activity within the ischemic territory plays a central role in protecting the brain from stroke. We found that active exploration after pMCAO has indeed the same complete functional and structural protection effects, findings that fit our previous studies in anesthetized rats and therefore are independent of the level of arousal. We also observed the opposite effect: several cases in which animals, though awake, remained largely stationary after revival and showed decreased protection, as was the case for immobile +0h rats. While seemingly contradictory, each scenario described above might be captured within the theoretical framework of cortical activation: exploration of the enriched environment results in an increase in evoked cortical activity within the ischemic territory. This activity, in turn, protects the cortex from impending ischemic stroke when initiated early, and varying the amount of cortical activity, which is presumably related to sensory-motor activity within the enriched environment, will correspondingly vary the degree of protection. When compared with our previous results, it is evident that these effects are independent of anesthetic effect. The finding that actively exploring +0h rats without whisker arrays were also protected from stroke suggests that their cortical activation of the ischemic area via alternate sensory and motor activity is sufficient to induce neuroprotection, and that this effect is not specific to whisker sensory stimulation.
In the past, treatment for acute ischemic stroke consisted of stabilization, observation, and rehabilitation (Shaikh, 2010). In recent years, new therapies and a better understanding of the pathophysiology have led to the concept of “Time is Brain,” and have placed an emphasis upon early evaluation and treatment (Shaikh, 2010; Frostig et al., 2013; Xerri & Zennou-Azogui, 2014). If proven to be effective in the human clinical population, cortical activation treatment presents the opportunity for the development of a safe and effective treatment option for acute ischemic stroke which could be initiated rapidly, by first responders or informed friends and family, or accompany current clinical procedures such as intravenous thrombolysis using t-PA.
Acknowledgments
The authors wish to thank Quynh Vu, Patricia Vu, Nina Butingan, and Amber Nierode for their contribution to the experiments herein.
Funding
This work was supported by the NIH-NINDS NS-066001, NS-055832, and NRSA 1T32DC010775-01.
Abbreviations
- +0h
whisker stimulation treatment delivered immediately following ischemic onset
- +3h
whisker stimulation treatment delivered 3 hours post-ischemic onset
- ANOVA
analysis of variance
- CCD
charged coupled device
- FC
fractional change
- ISOI
Intrinsic Signal Optical Imaging
- MCA
middle cerebral artery
- pMCAO
permanent middle cerebral artery occlusion
- SEM
standard error mean
- TTC
2,3,5-triphenyltetrazolium chloride
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Contributor Information
Christopher C. Lay, Email: layc@usc.edu.
Ron D. Frostig, Email: rfrostig@uci.edu.
References
- Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC, Feeney DM. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: functional and anatomic outcome. Stroke. 2000;31:1144–1152. doi: 10.1161/01.str.31.5.1144. [DOI] [PubMed] [Google Scholar]
- Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P. Middle cerebral artery occlusion in the young rat. Stroke. 1982;13:855–859. doi: 10.1161/01.str.13.6.855. [DOI] [PubMed] [Google Scholar]
- Davis MF, Lay CC, Chen-Bee CH, Frostig RD. Amount but not pattern of protective sensory stimulation alters recovery after permanent middle cerebral artery occlusion. Stroke. 2011;42:792–798. doi: 10.1161/STROKEAHA.110.607135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MF, Lay CC, Frostig RD. Permanent Cerebral Vessel Occlusion Via Double Ligature and Transection. Journal of Visualized Experimentation. 2013 doi: 10.3791/50418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Chen-Bee CH. The use of intrinsic signal optical imaging for mapping cortical function. In: Brette R, Destexhe A, editors. Handbook of Neuronal Activity Measurements. Cambridge University Press; 2012. [Google Scholar]
- Frostig RD, Lay CC, Davis MF. A rat’s whiskers point the way toward a novel stimulus-dependent, protective stroke therapy. Neuroscientist. 2013;19:313–328. doi: 10.1177/1073858412462607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski M, Sorensen JC, Mattsson B, Zimmer J, Johansson BB. Influence of an enriched environment and cortical grafting on functional outcome in brain infarcts of adult rats. Exp Neurol. 1995;133:96–102. doi: 10.1006/exnr.1995.1011. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hancock AM, Lay CC, Davis MF, Frostig RD. Sensory Stimulation-Based Complete Protection from Ischemic Stroke Remains Stable at 4 Months Post-Occlusion of MCA. J Neurol Disord. 2013;1:135. doi: 10.4172/2329-6895.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschneider DP, Maarek JM. Brain maps on the go: functional imaging during motor challenge in animals. Methods. 2008;45:255–261. doi: 10.1016/j.ymeth.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Ohlsson AL. Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp Neurol. 1996;139:322–327. doi: 10.1006/exnr.1996.0106. [DOI] [PubMed] [Google Scholar]
- Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS One. 2010;5:e11270. doi: 10.1371/journal.pone.0011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation reestablishes cortical function during the acute phase of ischemia. J Neurosci. 2011;31:11495–11504. doi: 10.1523/JNEUROSCI.1741-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay CC, Jacobs N, Hancock AM, Zhou Y, Frostig RD. Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia. Eur J Neurosci. 2013;38:2445–2452. doi: 10.1111/ejn.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Schallert T. Consequences of forced disuse of the impaired forelimb after unilateral cortical injury. Behav Brain Res. 2004;150:83–91. doi: 10.1016/S0166-4328(03)00254-7. [DOI] [PubMed] [Google Scholar]
- Niiro M, Simon RP, Kadota K, Asakura T. Proximal branching patterns of middle cerebral artery (MCA) in rats and their influence on the infarct size produced by MCA occlusion. J Neurosci Methods. 1996;64:19–23. doi: 10.1016/0165-0270(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Nygren J, Wieloch T. Enriched environment enhances recovery of motor function after focal ischemia in mice, and downregulates the transcription factor NGFI-A. J Cereb Blood Flow Metab. 2005;25:1625–1633. doi: 10.1038/sj.jcbfm.9600157. [DOI] [PubMed] [Google Scholar]
- Ohlsson AL, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke. 1995;26:644–649. doi: 10.1161/01.str.26.4.644. [DOI] [PubMed] [Google Scholar]
- Risedal A, Mattsson B, Dahlqvist P, Nordborg C, Olsson T, Johansson BB. Environmental influences on functional outcome after a cortical infarct in the rat. Brain Res Bull. 2002;58:315–321. doi: 10.1016/s0361-9230(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Risedal A, Zeng J, Johansson BB. Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:997–1003. doi: 10.1097/00004647-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Sahakian B, Robbins T, Iversen S. The effects of isolation rearing on exploration in the rat. Learning & Behavior. 1977;5:193–198. [Google Scholar]
- Shaikh S. Anesthesia considerations for the patient with acute ischemic stroke. Semin Cardiothorac Vasc Anesth. 2010;14:62–63. doi: 10.1177/1089253210362794. [DOI] [PubMed] [Google Scholar]
- Sun ML, Zhang XB, Sun X, Zhao MH, Yu YQ. Effect of whisker trimming on behavior and barrel cortex of rat. Chinese Journal of Applied Physiology. 2010;26:354–358. [PubMed] [Google Scholar]
- Ts’o DY, Frostig RD, Lieke EE, Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990;249:417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]
- Xerri C. Plasticity of cortical maps: multiple triggers for adaptive reorganization following brain damage and spinal cord injury. Neuroscientist. 2012;18:133–148. doi: 10.1177/1073858410397894. [DOI] [PubMed] [Google Scholar]
- Xerri C, Zennou-Azogui Yi. Early and Moderate Sensory Stimulation Exerts a Protective Effect on Perilesion Representations of Somatosensory Cortex after Focal Ischemic Damage. PLoS One. 2014;9:e99767. doi: 10.1371/journal.pone.0099767. [DOI] [PMC free article] [PubMed] [Google Scholar]