Abstract
Cholera toxin was obtained in pure form by fractionation on two phosphocellulose columns successively. Cholera toxin and choleragenoid were quantitatively and selectively adsorbed to the first column in 10 mM phosphate buffer, pH 7.0, and were subsequently eluted with buffer of high ionic strength. The toxin was then separated from choleragenoid on the second column by chromatography at pH 8.3. The toxin obtained was highly active and pure as judged by electrophoresis, isoelectric focusing, and various immunological and chemical tests. Pure choleragenoid was by-product of the procedure. The A1 chain of the toxin was obtained in pure form by treating phosphocellulose-bound toxin with urea and a reducing agent. The anionic A1 peptide was thereby released, leaving a complex of the B and A2 chains (A25B) bound to the resin. The latter was then eluted and further purified to obtain nontoxic antigen. The overall yields of cholera toxin and choleragenoid were increased two- to threefold by the use of hypertoxinogenic mutants of Vibrio cholerae.
Full text
PDF

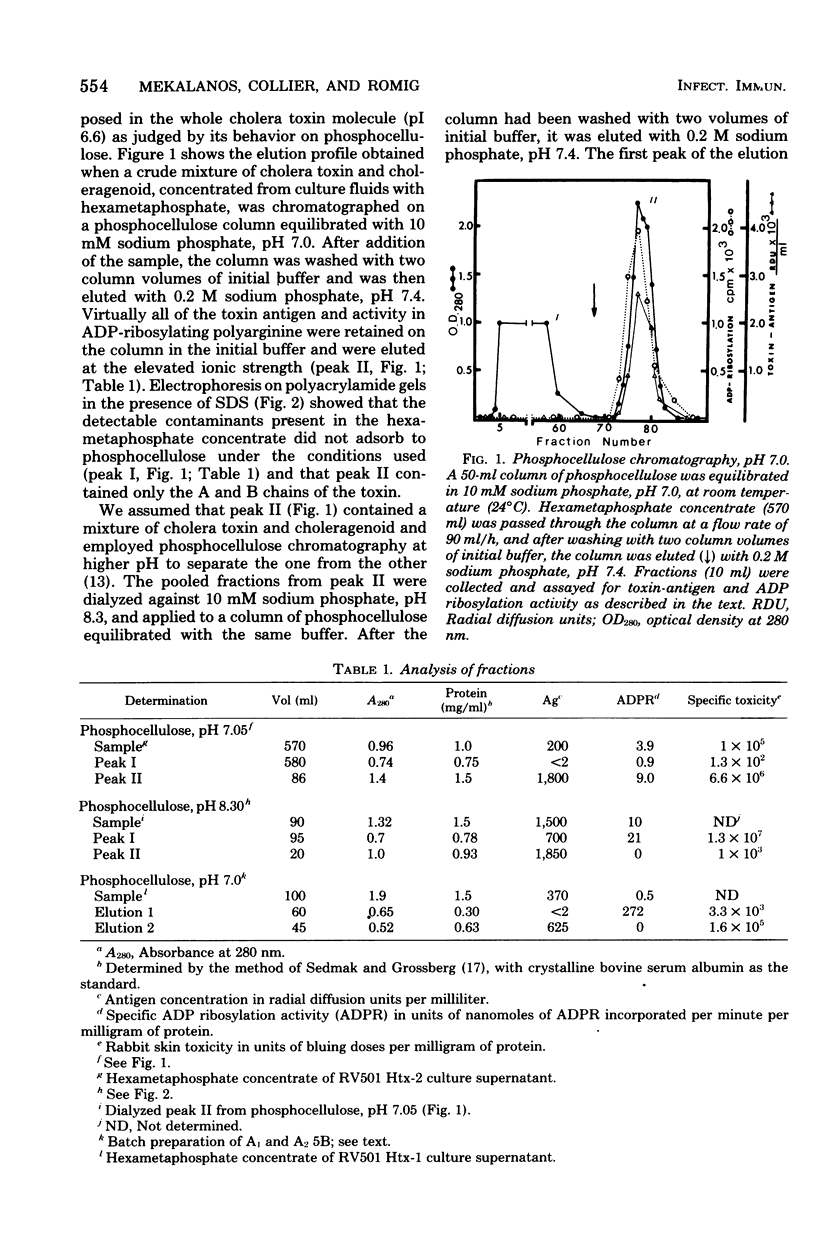
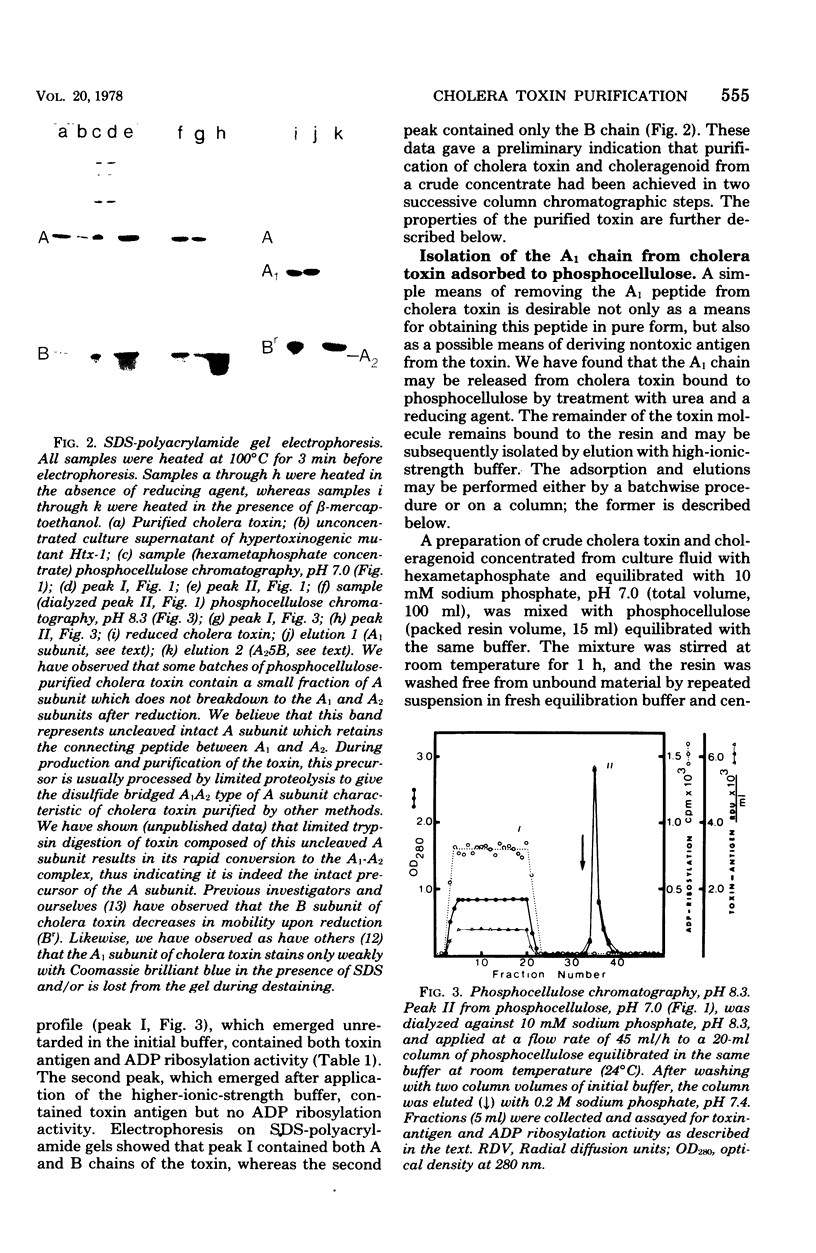



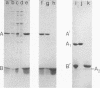
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Vibrio cholerae choleragenoid. Mechanism of inhibition of cholera toxin action. Biochemistry. 1973 Aug 28;12(18):3577–3581. doi: 10.1021/bi00742a034. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A. Properties of cholera exo-enterotoxin (choleragen) and its natural toxoid (choleragenoid). Toxicon. 1972 Aug;10(5):441–450. doi: 10.1016/0041-0101(72)90168-7. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Affinity filters, a new approach to the isolation of tox mutants of Vibrio cholerae. Proc Natl Acad Sci U S A. 1978 Feb;75(2):941–945. doi: 10.1073/pnas.75.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Simple method for purifying choleragenoid, the natural toxoid of Vibrio cholerae. Infect Immun. 1977 Jun;16(3):789–795. doi: 10.1128/iai.16.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Rubin B. A., Tint H. Development of a purified cholera toxoid. I. Purification of toxin. Infect Immun. 1974 Feb;9(2):294–303. doi: 10.1128/iai.9.2.294-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]