SUMMARY
Endothelial cells express several types of integral membrane protein receptors, which upon interaction and activation by their specific ligands, initiate a signaling network that links extracellular cues in circulation to various biological processes within a plethora of cells in the vascular system. A small family of G-protein coupled receptors, termed protease-activated receptors (PAR1–4), can be specifically activated by coagulation proteases, thereby modulating a diverse array of cellular activities under various pathophysiological conditions. Thrombin and all vitamin K-dependent coagulation proteases, with the exception of factor IXa for which no PAR signaling has been attributed, can selectively activate cell surface PARs on the vasculature. Thrombin can activate PAR1, PAR3 and PAR4, but not PAR2 which can be specifically activated by factors VIIa and Xa. The mechanistic details of the specificity of PAR signaling by coagulation proteases are the subject of extensive investigation by many research groups worldwide. However, analysis of PAR signaling data in the literature has proved to be challenging since a single coagulation protease can elicit different signaling responses through activation of the same PAR receptor in endothelial cells. This article is focused on briefly reviewing the literature with respect to determinants of the specificity of PAR signaling by coagulation proteases with special emphasis on the mechanism of PAR1 signaling by thrombin and activated protein C in endothelial cells.
Keywords: protease-activated receptor, thrombin, activated protein C, signaling
Introduction
In addition to their role in the initiation and regulation of the clotting cascade, coagulation proteases regulate diverse cellular functions through the activation of a small family of G-protein coupled receptors (GPCR) called protease-activated receptors (PARs) (1–4). Thus far, four members of the PAR family (PAR1, PAR2, PAR3 and PAR4) have been cloned and characterized (1,5). Protease cleavage of PARs on various cell types exposes new N-termini (tethered ligand) on the extracellular domains of PARs that bind to the second membrane-spanning extracellular loop of the receptors, thereby activating them and eliciting intracellular signaling responses. Vascular PAR signaling, mediated by coagulation proteases, plays key roles in modulating diverse arrays of cellular activities under different pathophysiological conditions including inflammation, cardiovascular disease, tumor growth and metastasis, apoptosis, angiogenesis and tissue remodeling (1–5). Thrombin and all vitamin K-dependent coagulation proteases, with the exception of factor IXa for which no PAR signaling has been attributed, are capable of selectively activating cell surface PARs on various tissues. Thrombin can activate PAR1, PAR3 and PAR4, but not PAR2 (1). PAR2 can be specifically activated by both factor Xa (FXa) and factor VIIa (FVIIa) (1,6,7). Nevertheless, high concentrations of all coagulation proteases may be able to non-specifically cleave all four PARs in vitro or in cellular models. The physiological relevance of such results should be interpreted with some caution. With the exception of PAR3, which has a short cytoplasmic domain and does not appear to be directly involved in signaling, the protease cleavage of other three cell surface PARs can elicit intracellular signaling responses (1). The mechanistic details of the specificity of PAR signaling by plasma proteases are the subject of extensive investigation by many research groups worldwide. Since human umbilical vein endothelial cells (HUVECs) express all four PARs (8–10), this question has been extensively studied using this cell type. However, analysis of PAR signaling data in the literature in HUVECs has proved to be challenging since coagulation proteases can elicit paradoxical signaling responses through activation of the same PAR receptor in these cells. For instance, it has been shown that activation of PAR1 by thrombin induces proinflammatory signaling responses (1,9–12), whereas the activation of the same receptor by activated protein C (APC) elicits antiinflammatory responses in cultured HUVECs (12–15). A PAR1-dependent antiinflammatory activity for APC has also been documented in several animal models of inflammation and severe sepsis. Thus, following a clinical trial, recombinant APC was approved by the FDA as a therapeutic drug for treating adults with severe sepsis (16). However, APC was recently pulled out of the market by the manufacturer (Eli Lilly) due to its apparent lack of significant mortality reducing beneficial effect in a new follow-up clinical study (17). Nevertheless, other controlled clinical trials may be required to fully assess the protective antiinflammatory properties of APC in humans (18). This article will not elaborate on this subject but rather focus on reviewing the literature with respect to the specificity of PAR signaling by coagulation proteases with special emphasis on the mechanism of PAR1 signaling by thrombin and APC in HUVECs.
PAR1 signaling by thrombin
Thrombin activates PAR1 by catalyzing the cleavage of the Arg41-Ser42 peptide bond on the N-terminal extracellular domain (exodomain) of the receptor (1,12). In addition to the P1-Arg41 recognition site, the exodomain of PAR1 also has an acidic hirudin-like sequence at the C-terminus of the scissile bond which constitutes a binding site for basic exosite I of thrombin (1,19). The acidic hirudin-like sequence of PAR1 is also conserved in PAR3, but in neither PAR2 or PAR4 (1,12). The exosite I-dependent interaction of thrombin with this site of PAR1 is primarily responsible for the high specificity of receptor recognition by thrombin, reminiscent of its interaction with thrombomodulin (TM) and fibrinogen (19). PAR1 was first identified as thrombin receptor on the surface of human platelets, which can be effectively cleaved by sub-nanomolar concentrations of thrombin, thereby leading to rapid platelet aggregation in response to vascular injury (1). It is of interest to note that unlike PAR1 on human platelets, PAR4 has been identified as the primary target receptor for thrombin on mouse platelets. In this case, PAR3 has been found to function as a cofactor for PAR4 activation by thrombin on mouse platelets (1). Such species differences in PAR signaling by thrombin have important implications in terms of relating studies conducted in mouse to human that have been nicely discussed in an excellent review article by Coughlin (1).
As a G-protein coupled receptor, the activation of cell surface PAR1 by thrombin facilitates the interaction of the C-terminal intracellular loop of PAR1 with different members of the G protein subfamilies including Gi, Gq and G12/13 (11,20,21). Thrombin elicits proinflammatory signaling responses in endothelial cells through activation and coupling of PAR1 to Gq and/or G12/13 (11,20,21). Thrombin activation of cell surface PAR1 up-regulates the expression of various cytokines (i.e., IL-1, IL-6 and TNF-α) and cell adhesion molecules; E-selectin, P-selectin, intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells (12,22,23). Thrombin also induces apoptosis through the activation of caspases and enhances the barrier permeability of endothelial cells through PAR1-dependent activation of the nuclear factor (NF)-κB and RhoA GTPase (24,25). Noting that the protease cleavage of PAR1 is an irreversible process, termination of signaling is mediated through phosphorylation-dependent internalization of the receptor through coated pits and its subsequent targeting to lysosomes (26,27).
It is worth noting that the expression level of TM on HUVECs is very low in comparison to that on microvascular endothelial cells which express high levels of TM (28). As indicated above, the interaction of thrombin with the hirudin-like exosite of PAR1 is required for the cleavage-dependent activation of the receptor (1,19). Given that both PAR1 and TM compete for binding to thrombin through the same exosite and that thrombin binds to this site on TM with much higher affinity than PAR1 (29,30), further studies will be required to determine whether thrombin can actually bind to and activate PAR1 on capillary endothelial cells which have high levels of TM on their surfaces (28). Furthermore, even if TM-bound thrombin activates PAR1, it is not known if signaling responses elicited by the thrombin-TM complex would be disruptive in nature. We believe that, in addition to its role in regulating the procoagulant function of thrombin, TM also plays a critical role in regulating the PAR1-dependent signaling specificity of thrombin (31,32).
PAR1 signaling by APC
APC activates PAR1 through cleavage of one or two P1-Arg recognition sites (Arg-41 and Arg-46) present on the exodomain of the receptor (33,34). However unlike thrombin, APC is not known to utilize the hirudin-like exosite of PAR1 to activate it, thus APC cleaves the receptor with markedly slower catalytic efficiency (35). Paradoxically, APC activation of PAR1 in endothelial cells evokes cytoprotective and antiinflammatory responses (12,33–37). The PAR1-dependent protective response by APC requires that it forms a complex with endothelial protein C receptor (EPCR) on the surface of endothelial cells (38–40). Thus, it has been demonstrated that the APC-EPCR complex inhibits cytokine-mediated activation of NF-κB and down-regulates expression of proinflammatory cytokines and cell adhesion molecules in endothelial cells. Unlike, thrombin which activates RhoA, APC in complex with EPCR activates Rac1 GTPase and by doing so stabilizes the barrier permeability function of endothelial cells (24,25). It is well established that APC exerts its protective cellular effect through EPCR- and PAR1-dependent up-regulation of the expression of protective genes and down-regulation of the expression of proinflammatory genes (23,24,39). However, noting that thrombin is the only known physiological activator of protein C, and that it can also cleave PAR1 with approximately 3 orders of magnitude higher catalytic efficiency (35), the question that had remained controversial for several years was: how can APC cleavage of PAR1 elicit a protective response in an environment where thrombin is also present? A partial answer for this question came from the observation that the two receptors required for protein C activation by thrombin (TM and EPCR) together with PAR1 are co-localized within lipid-rafts/caveolae microenvironment of endothelial cells (31) (Fig. 1). This finding, together with the observation that TM-bound thrombin cannot activate PAR1 to initiate a proinflammatory response (32), strongly suggests that the activation of protein C and signaling by the APC-EPCR complex is a mechanistically-coupled event occurring locally on specific cell surface microenvironments (41). Interestingly, further studies revealed that EPCR is also associated with caveolin-1 (10). However, when EPCR is bound by the Gla-domain of either protein C or APC (40), EPCR dissociates from caveolin-1, thereby recruiting PAR1 to a protective signaling pathway (10). Remarkably, we have found that, similar to thrombin, Gla-domainless APC also elicits a proinflammatory response in HUVECs (32). However, pretreatment of endothelial cells with the catalytically inactive protein C-S195A switches the PAR1-dependent signaling specificity of both thrombin and Gla-domainless APC from a disruptive to a protective response (32). Thus, it appears that the occupancy of EPCR by protein C results in dissociation of EPCR from caveolin-1, thereby switching the signaling specificity of both proteases (10,32). Further support for this hypothesis was provided by the observation that a meizothrombin/protein C Gla-domain chimera also elicited a protective response in the cytokine-stimulated endothelial cells through the cleavage of PAR1 with an efficiency that was nearly 50-fold higher than APC (10,42). Taken together, these results indicated that the specificity of PAR1 signaling by thrombin and APC is determined by the occupancy of EPCR by its natural ligand independent of the protease cleaving the receptor (42,43). The mechanism by which the occupancy of EPCR alters the specificity of PAR1 signaling by thrombin remains largely unknown and is the subject of investigation in our laboratory.
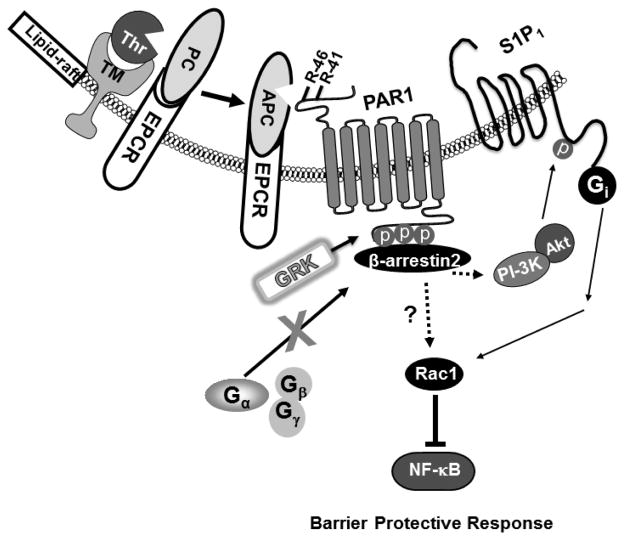
Fig. 1.
Cartoons of protein C activation and APC signaling in the membrane lipid-rafts of endothelial cells. Thrombin binds to thrombomodulin and activates the EPCR-bound protein C zymogen to APC in the membrane lipid-rafts of endothelial cells. APC, remained associated with EPCR, activates PAR1 through the cleavage of Arg-41 and/or Arg-46 on the extracellular domain of the receptor. The activation of PAR1 by APC results in a conformational change on the C-terminal intracellular loop of the receptor, thereby mediating its phosphorylation by a member of G protein coupled receptor kinases (GRK). The phosphorylated receptor recruits β-arrestin2, thereby preventing the interaction of the receptor with α subunit of one of the heterotrimeric G proteins and transmitting the intracellular message via β-arrestin2 biased signaling mechanism. This mode of activation of PAR1 by APC results in the activation of the PI-3K/Akt survival pathway, transactivation of the Gi protein coupled receptor, S1P1 and activation of Rac1 GTPase. These signaling events lead to improvement in the barrier permeability function and inhibition of the NF-κB pathway in endothelial cells.
Biased PAR1 signaling by thrombin and APC
Recently, a new paradigm in GPCR signaling has been discovered in which the transmission of the signal by activated receptor is not mediated through one of the heterotrimeric G proteins, but rather via a biased signaling mechanism through β-arrestins (44,45). In this case, the cytoplasmic domain of GPCR is phosphorylated by GPCR kinases (GRK), thereby recruiting β-arrestins, preventing GPCR interaction with G proteins and directly signaling via β-arrestins (44,45). According to this concept, an interesting recent paper demonstrated that the PAR1-dependent protective activity of APC may not be mediated through either one of the G proteins but via a β-arrestin2 biased signaling mechanism (46) (Fig. 1). The mechanism by which APC but not thrombin can initiate β-arrestin2 biased PAR1 signaling is not known. A hypothesis that may explain β-arrestin2 biased PAR1 signaling by APC was provided in a recent report by Mosnier et al, who demonstrated that APC can cleave a second P1-Arg site (Arg-46) on the exodomain of PAR1 to initiate antiinflammatory signaling responses (33,47) (Fig. 1). This finding indicates that when APC cleaves the alternative recognition site after Arg-46, the activated receptor may differentially recruit β-arrestin2, thereby exerting a protective effect independent of an interaction with one of the G proteins. In light of the observation that unlike APC, the Gla-domainless derivative of APC lacks cytoprotective activity and elicits thrombin-like proinflammatory signaling responses through the cleavage of PAR1 (32), the new findings may suggest that the interaction of APC with EPCR somehow alters the substrate specificity of the protease (by catalytic or topographical mechanisms) so that the EPCR-bound APC specifically cleaves PAR1 at a recognition site that is different from that of thrombin (33,47). Our results, demonstrating a disruptive effect for thrombin in the absence of EPCR occupancy but a protective one for the protease in protein C-S195A-treated cells, may suggest that when EPCR is occupied, thrombin’s PAR1-dependent signaling effect may also be mediated through a β-arrestin2 biased signaling mechanism (Fig. 2). Thus, further studies will be required to test this hypothesis and to determine whether the non-canonical cleavage of PAR1 by APC at Arg-46 site and/or the occupancy of EPCR by the Gla-domain of protein C/APC are responsible for inducing β-arrestin2 biased PAR1 signaling by coagulation proteases. It is worth noting that there is a report showing that the endocytic internalization of the thrombin-bound PAR1 is markedly faster than that of the APC-bound receptor (48), possibility due to exosite I-dependent interaction of thrombin with the hirudin-like exosite of the receptor. Whether such differences in the interaction of APC and thrombin with PAR1 lead to distinct receptor conformations and/or signal-transmission durations which may differentially couple the receptor to either a G-protein or β-arrestin2 requires further investigation.
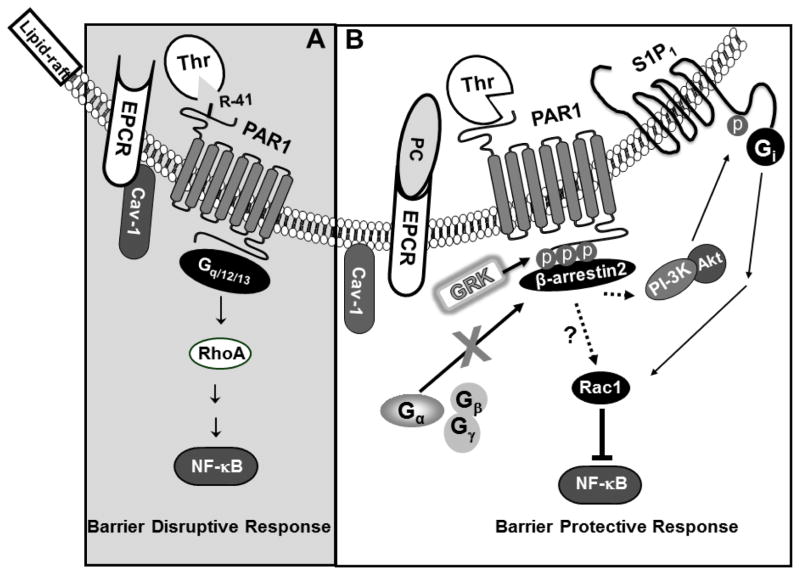
Fig. 2.
Cartoons of PAR1 activation by thrombin in the membrane lipid-rafts of endothelial cells when EPCR is free or occupied by its natural ligand. (A) EPCR interacts with caveolin-1 (Cav-1) within lipid-rafts of endothelial cells when it is not occupied by the Gla-domain of protein C. Under these conditions, thrombin cleavage of PAR1 activates RhoA, up-regulates the NF-κB pathway and elicits disruptive signaling responses through activation of G12/13 and/or Gq proteins. (B) The occupancy of EPCR by protein C leads to dissociation of EPCR from caveolin-1 and a switch in the PAR1-dependent specificity of thrombin signaling, presumably through β-arrestin2 biased signaling and S1P1 transactivation mechanisms as observed with APC signaling.
PAR1 and PAR2 signaling by FVIIa and FXa
FVIIa is the protease of the extrinsic pathway that binds to the cell surface receptor, tissue factor (TF), on negatively charged membrane phospholipids and activates factor X to FXa to initiate the clotting cascade (2,7). The FVIIa-TF complex is also known to activate PAR2 to elicit intracellular signaling responses in various cell types independent of its procoagulant activity (2,7). A strong link between TF-dependent PAR2 signaling by FVIIa and tumor progression/metastasis has been established in several cellular and in vivo models (49,50). It has been demonstrated that activation of PAR2 by FVIIa also modulates the pro-migratory properties of non-cancerous TF- expressing epithelial cells by phosphorylation of the cytoplasmic domain of TF and activation of p38 and ERK1/2 MAP kinases (51–53), suggesting a key role for TF-dependent PAR2 signaling by FVIIa in altering cellular functions.
In addition to TF-dependent signaling through PAR2, FVIIa also activates PAR1 to initiate intracellular signaling responses in endothelial cells (54–56). In a series of interesting recent studies, Rao et al discovered that EPCR functions as a true receptor for FVIIa to modulate the PAR1-dependent signaling specificity of FVIIa (55,56). Similar to APC, FVIIa was shown to bind EPCR with a similar high affinity to effectively activate PAR1, thereby eliciting protective signaling responses in endothelial cells in both in vitro and in vivo model systems (54–56). Thus, FVIIa was shown to prevent the barrier disruptive effect of proinflammatory cytokines in vascular endothelial cells by the EPCR- and PAR1-dependent activation of Rac1, reminiscent of APC-EPCR activation of this GTPase (54,56). The mechanism by which EPCR endows PAR1-dependent protective activity for FVIIa is not known. Further studies will be required to determine whether similar to the APC-EPCR complex, FVIIa-EPCR cleaves the exodomains of PAR1 complex at an alternative non-canonical recognition site and/or the activated receptor signals via the β-arrestin2 biased PAR1 signaling pathway (Fig. 1).
Similar to its activator FVIIa-TF complex, FXa can activate PAR2 to initiate signaling responses in endothelial and other cell types (2,7,57). FXa reportedly also signals through activation of PAR1 (58). In a recent report, it was demonstrated that FXa, through activation of both PAR1 and PAR2, exerts a barrier protective effect in HUVECs in response to proinflammatory stimuli (6). Other studies have reported a proinflammatory role for FXa in similar cellular model systems (59–61). Discrepancies in the results between different studies are not known. In support of a protective PAR2-dependent signaling function for FXa, we demonstrated that FXa can inhibit the barrier disruptive effect of proinflammatory cytokines in HUVECs through activation of PAR2 by a mechanism that was partly independent of the Gla-domain of the protease (62,63). This was evidenced by the observation that both full-length FXa and Gla-domainless FXa exerted a PAR2-dependent protective effect in response to LPS in HUVECs (62). Interestingly, further studies revealed that mutagenesis of two basic residues, Arg-86 and Lys-87, located at the N-terminus of EGF-2 domain of the light chain, abrogated the protective signaling response of FXa, suggesting the interaction of the light chain of the protease with a cell surface receptor/cofactor contributes to the signaling mechanism of the protease (62,63). This observation is consistent with a report that the interaction of 6 residues of the inter-EGF sequence Leu-83 to Leu-88 (Leu-Phe-Thr-Arg-Lys-Leu) of FXa with an endothelial cell surface receptor is required for the signaling function of FXa (64). The physiological significance of FXa signaling in cellular models remains unknown since a high FXa concentration of ~20 nM was required to observe a signaling effect in these studies.
In addition to changing the signaling specificity of PAR1, the occupancy of EPCR by protein C was shown to switch the signaling specificity of PAR2 from a barrier disruptive to a barrier protective effect in endothelial cells (65). This was evidenced by the observation that the PAR2 agonist peptide, SLIGKV, elicited a barrier disruptive response in HUVECs that could be effectively reversed to a protective response if cells were pretreated with the protein C zymogen prior to stimulation by the PAR2 agonist peptide (65). Furthermore, it was discovered that pretreatment of endothelial cells with either protein C or FX zymogen renders the signaling specificity of both PAR1 and PAR2 a protective one in endothelial cells (65). It appears that all coagulation proteases capable of cleaving either PAR1 or PAR2 would elicit only protective signaling responses if cells express protein C and/or the putative factor X/Xa receptors and that the receptors are bound by their physiological ligands. Thus, this mechanism of co-receptor signaling plays a key role in determining the specificity of PAR1 and PAR2 signaling by coagulation proteases in endothelial cells. Based on these results, we believe that the physiological relevance of in vitro studies in cellular models, in particular those monitoring the signaling effects of PAR1 and PAR2 in the absence of co-receptor signaling, should be interpreted with some caution.
PAR4 signaling by coagulation proteases
The activation of PAR4 by coagulation proteases appears to be primarily associated with evoking proinflammatory signaling responses in vascular and arterial endothelial cells (10,66,67). Thrombin has been identified as the primary coagulation protease that is responsible for the activation of cell surface PAR4 in vascular endothelial cells. Thrombin cleavage of PAR4 appears to couple the receptor to Gq, thereby activating RhoA GTPase and signaling via p38 and ERK1/2 MAP kinase pathways (8). In contrast to activation of PAR1, a markedly higher concentration of thrombin is required to cleave PAR4 in order to initiate proinflammatory signaling responses in endothelial cells (10). This is because PAR4 does not possess the hirudin-like sequence of PAR1 on its exodomain in order to facilitate its high affinity interaction with thrombin (1). Thus, TM cannot compete with the activation of PAR4 by thrombin as presumably occurs with the protease activation of PAR1 at the cell surface of endothelial cells. Endothelial cells are known to express both PAR1 and PAR4. Based on the published data, a low concentration of thrombin (<2 nM) is sufficient to activate cell surface PAR1 (10), thus one should be cautious not to attribute PAR4 signaling by higher concentrations of thrombin to PAR1 signaling in endothelial cells. It is of interest to note that even APC signaling turns disruptive and proinflammatory when a non-physiological and high concentration of APC (>100 nM) is employed to activate endothelial cells (10). We have demonstrated that under these conditions, similar to thrombin, APC activates PAR4, thereby losing its antiinflammatory function (10).
PAR signaling crosstalk with other receptors
Communication with a number of other G-protein, non-G-protein coupled receptors and integrins modulates the PAR-dependent signaling specificity of coagulation proteases. The EPCR- and PAR1-dependent cytoprotective and antiinflammatory signaling functions of both APC and thrombin can be inhibited by the siRNA knockdown of either sphingosine 1-phosphate receptor 1 (S1P1) or angiopoietin/Tie2 axis in endothelial cells (21,66,68–70). Moreover, APC-mediated signaling via apolipoprotein E receptor 2 (ApoER2) contributes to its cytoprotective function by a Dab1-dependent activation of the PI3K-Akt survival pathway in U937 cells (71). A study demonstrated that the interaction of APC with integrin CD11b facilitates the PAR1-dependent protective activity of the protease in macrophages by an EPCR-independent mechanism (72). Unlike a protective signaling crosstalk between PAR1 and S1P1, a proinflammatory signaling crosstalk between PAR1 and S1P3 in vascular and dendritic cells have been reported (73), suggesting that tissue-type specific expression of different cell surface receptors can modulate the PAR-dependent signaling specificity of coagulation proteases. Other recent studies have demonstrated that effective EPCR-dependent cytoprotective activity of APC also requires PAR3 in neurons and podocytes (74,75). A PAR3-dependent cytoprotective activity for APC in endothelial cells has also been demonstrated (76). A role for PAR receptor homo- and/or hetero-dimerization, modulating the specificity of PAR signaling in endothelial cells, has also been envisioned (9,77). Finally, in a recent study, we demonstrated that the adenosine receptor signaling through the A2A receptor subtype alters the signaling specificity of thrombin by a cAMP-dependent manner in endothelial cells (78). Thus, pretreatment of HUVECs with adenosine inhibited RhoA activation by thrombin, thereby inhibiting the barrier disruptive effect of thrombin (78). These results underscore the complex nature of the crosstalk that may control the specificity of PAR signaling by coagulation proteases under different pathophysiological conditions. A better understanding of the specificity and mechanism of PAR signaling by coagulation proteases requires systems biology approaches to spatiotemporally measure and integrate crosstalk among multiple signaling networks.
Acknowledgments
I thank Audrey Rezaie for proofreading the manuscript. The research discussed herein was supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Grants No. HL 101917 and HL 62565).
Footnotes
Competing interests
None
References
- 1.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost 2003. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 3.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 4.Antoniak S, Pawlinski R, Mackman N. Protease-activated receptors and myocardial infarction. IUBMB Life. 2011;63:383–389. doi: 10.1002/iub.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu WF, Andersen H, Whitmore TE, et al. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci (USA) 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feistritzer C, Lenta R, Riewald M. Protease-activated receptor-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005;3:2798–805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 7.Awasthi V, Mandal SK, Papanna V, et al. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol. 2007;27:1447–1455. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie E, Saka M, MacKenzie C, et al. Cytokine upregulation of proteinase-activated receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br J Pharmacol. 2007;150:1044–1054. doi: 10.1038/sj.bjp.0707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci (USA) 2007;104:5662–5667. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JS, Yang L, Manithody C, et al. The ligand occupancy of endothelial protein C receptor switches the PAR-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin JN, Shen L, Holinstat M, et al. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 12.Mosnier LO, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 13.Esmon CT. Protein C anticoagulant system--anti-inflammatory effects. Semin Immunopathol. 2012;34:127–132. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiler H. Multiple receptor-mediated functions of activated protein C. Hamostaseologie. 2011;31:185–195. doi: 10.5482/ha-1166. [DOI] [PubMed] [Google Scholar]
- 15.Castellino FJ, Ploplis VA. The protein C pathway and pathologic processes. J Thromb Haemost. 2009;7 (Suppl 1):140–145. doi: 10.1111/j.1538-7836.2009.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Eng J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 17.Mitka M. Drug for severe sepsis is withdrawn from market, fails to reduce mortality. JAMA. 2011;306:2439–2440. doi: 10.1001/jama.2011.1755. [DOI] [PubMed] [Google Scholar]
- 18.Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012;12:678–686. doi: 10.1016/S1473-3099(12)70157-3. [DOI] [PubMed] [Google Scholar]
- 19.Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106:2605–2612. doi: 10.1182/blood-2005-04-1710. [DOI] [PubMed] [Google Scholar]
- 20.Camerer E, Coughlin SR. APC signaling: tickling PAR1 for barrier protection? Blood. 2005;105:3004–3005. [Google Scholar]
- 21.Ossovskaya VS, Bunnett NW. Protease-activated receptors: Contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 22.Kaplanski G, Marin V, Fabrigoule M, et al. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intracellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106) Blood. 1998;92:1259–1267. [PubMed] [Google Scholar]
- 23.Joyce DE, Gelbert L, Ciaccia A, et al. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 24.Finigan JH, Dudek SM, Singleton PA, et al. Activated protein C mediates novel lung endothelial barrier enhancement: Role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 25.Bae JS, Rezaie AR. Thrombin inhibits nuclear factor κB and RhoA pathways in cytokine-stimulated vascular endothelial cells when EPCR is occupied by protein C. Thromb Haemost. 2009;101:513–520. [PMC free article] [PubMed] [Google Scholar]
- 26.Hoxie JA, Ahuja M, Belmonte E, et al. Internalization and recycling of activated thrombin receptors. J Biol Chem. 1993;268:13756–13763. [PubMed] [Google Scholar]
- 27.Hein L, Ishii K, Coughlin SR, et al. Intracellular targeting and trafficking of thrombin receptors. A novel mechanism for resensitization of a G protein-coupled receptor. J Biol Chem. 1994;269:27719–27726. [PubMed] [Google Scholar]
- 28.Busch C, Cancilla PA, DeBault LE, et al. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982;47:498–504. [PubMed] [Google Scholar]
- 29.Ye J, Rezaie AR, Esmon CT. Glycosaminoglycan contributions to both protein C activation and thrombin inhibition involve a common arginine-rich site in thrombin that includes residues arginine 93, 97, and 101. J Biol Chem. 1994;269:17965–17970. [PubMed] [Google Scholar]
- 30.Nieman MT, Schmaier AH. Interaction of thrombin with PAR1 and PAR4 at the thrombin cleavage site. Biochemistry. 2007;46:8603–8610. doi: 10.1021/bi700597p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci (USA) 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 33.Mosnier LO, Sinha RK, Burnier L, et al. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuepbach RA, Madon J, Ender M, et al. Protease-activated receptor-1 cleaved at R46 mediates cytoprotective effects. J Thromb Haemost. 2012;10:1675–1684. doi: 10.1111/j.1538-7836.2012.04825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludeman MJ, Kataoka H, Srinivasan Y, et al. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 36.Toltl LJ, Swystun LL, Pepler L, et al. Protective effects of activated protein C in sepsis. Thromb Haemost. 2008;100:582–592. [PubMed] [Google Scholar]
- 37.Loubele ST, Spronk HM, Ten Cate H. Activated protein C: a promising drug with multiple effects? Mini Rev Med Chem. 2009;9:620–626. doi: 10.2174/138955709788167547. [DOI] [PubMed] [Google Scholar]
- 38.Fukudome K, Esmon CT. Identification, cloning and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 39.Riewald M, Petrovan RJ, Donner A, et al. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 40.Regan LM, Mollica JS, Rezaie AR, et al. The interaction between the endothelial cell protein C receptor and protein C is dictated by the gamma-carboxyglutamic acid domain of protein C. J Biol Chem. 1997;272:26279–26284. doi: 10.1074/jbc.272.42.26279. [DOI] [PubMed] [Google Scholar]
- 41.Feistritzer C, Schuepbach RA, Mosnier LO, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281:20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 42.Bae JS, Rezaie AR. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood. 2011;118:3952–3959. doi: 10.1182/blood-2011-06-360701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the PAR1-dependent signaling specificity of coagulation proteases. IUBMB Life. 2011;63:390–396. doi: 10.1002/iub.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestins in cell signaling: Not just for seven-transmembrane receptors. Molecular Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci (USA) 2011;108:E1372–1380. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouwens EA, Stavenuiter F, Mosnier LO. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost. 2013;11 (Suppl 1):242–253. doi: 10.1111/jth.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuepbach RA, Feistritzer C, Brass LF, et al. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111:2667–2673. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Versteeg HH, Schaffner F, Kerver M, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–199. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Biosci Rep. 2013;33(5):e00064. doi: 10.1042/BSR20130057. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 52.Ott I, Weigand B, Michl R, et al. Tissue factor cytoplasmic domain stimulates migration by activation of the GTPase Rac1 and the mitogen-activated protein kinase p38. Circulation. 2005;111:349–355. doi: 10.1161/01.CIR.0000153333.52294.42. [DOI] [PubMed] [Google Scholar]
- 53.Ahamed J, Niessen F, Kurokawa T, et al. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109:5251–5259. doi: 10.1182/blood-2006-10-051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pendurthi UR, Rao LV. Factor VIIa interaction with endothelial cells and endothelial cell protein C receptor. Thromb Res. 2010;125 (Suppl 1):S19–22. doi: 10.1016/j.thromres.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh S, Pendurthi UR, Steinoe A, et al. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. J Biol Chem. 2007;282:11849–11857. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sen P, Gopalakrishnan R, Kothari H, et al. Factor VIIa bound to endothelial cell protein C receptor activates protease activated receptor-1 and mediates cell signaling and barrier protection. Blood. 2011;117:3199–3208. doi: 10.1182/blood-2010-09-310706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci (USA) 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharjee G, Ahamed J, Pawlinski R, et al. Factor Xa binding to annexin 2 mediates signal transduction via protease-activated receptor 1. Circ Res. 2008;102:457–464. doi: 10.1161/CIRCRESAHA.107.167759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bukowska A, Zacharias I, Weinert S, et al. Coagulation factor Xa induces an inflammatory signalling by activation of protease-activated receptors in human atrial tissue. Eur J Pharmacol. 2013;718:114–123. doi: 10.1016/j.ejphar.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Daubie V, Cauwenberghs S, Senden NH, et al. Factor Xa and thrombin evoke additive calcium and proinflammatory responses in endothelial cells subjected to coagulation. Biochim Biophys Acta. 2006;1763:860–869. doi: 10.1016/j.bbamcr.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Senden NH, Jeunhomme TM, Heemskerk JW, et al. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161:4318–4824. [PubMed] [Google Scholar]
- 62.Rana S, Yang L, Hassanian SM, et al. Determinants of the specificity of protease-activated receptors 1 and 2 signaling by factor Xa and thrombin. J Cell Biochem. 2012;113:977–984. doi: 10.1002/jcb.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manithody C, Yang L, Rezaie AR. Identification of exosite residues of factor Xa involved in recognition of PAR-2 on endothelial cells. Biochemistry. 2012;51:2551–2557. doi: 10.1021/bi300200p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ambrosini G, Plescia J, Chu KC, et al. Activation-dependent exposure of the inter-EGF sequence Leu83-Leu88 in factor Xa mediates ligand binding to effector cell protease receptor-1. J Biol Chem. 1997;272:8340–8345. doi: 10.1074/jbc.272.13.8340. [DOI] [PubMed] [Google Scholar]
- 65.Bae JS, Yang L, Rezaie AR. Factor X/Xa elicits protective signaling responses in endothelial cells directly via PAR-2 and indirectly via endothelial protein C receptor-dependent recruitment of PAR-1. J Biol Chem. 2010;285:34803–34812. doi: 10.1074/jbc.M110.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae JS, Rezaie AR. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb Haemost. 2008;100:101–109. doi: 10.1160/TH08-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slofstra SH, Bijlsma MF, Groot AP, et al. Protease-activated receptor-4 inhibition protects from multiorgan failure in a murine model of systemic inflammation. Blood. 2007;110:3176–3182. doi: 10.1182/blood-2007-02-075440. [DOI] [PubMed] [Google Scholar]
- 68.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 69.Minhas N, Xue M, Fukudome K, et al. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2010;24:873–881. doi: 10.1096/fj.09-134445. [DOI] [PubMed] [Google Scholar]
- 70.Bae JS, Rezaie AR. Thrombin upregulates the angiopoietin-Tie2 Axis: endothelial protein C receptor occupancy prevents the thrombin mobilization of angiopoietin 2 and P-selectin from Weibel-Palade bodies. J Thromb Haemost. 2010;8:1107–1115. doi: 10.1111/j.1538-7836.2010.03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang XV, Banerjee Y, Fernández JA, et al. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci (USA) 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao C, Gao Y, Li Y, et al. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971–1980. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niessen F, Schaffner F, Furlan-Freguia C, et al. Dendritic cell PAR1-S1P3 signaling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 74.Guo H, Liu D, Gelbard H, et al. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 75.Bock F, Shahzad K, Wang H, et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci (USA) 2013;110:648–653. doi: 10.1073/pnas.1218667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burnier L, Mosnier LO. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood. 2013;122:807–816. doi: 10.1182/blood-2013-03-488957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arachiche A, Mumaw MM, de la Fuente M, et al. Protease-activated receptor 1 (PAR1) and PAR4 heterodimers are required for PAR1-enhanced cleavage of PAR4 by α-thrombin. J Biol Chem. 2013;288:32553–32562. doi: 10.1074/jbc.M113.472373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hassanian SM, Dinarvand P, Rezaie AR. Adenosine Regulates the Proinflammatory Signaling Function of Thrombin in Endothelial Cells. J Cell Physiol. 2014;229:1292–1300. doi: 10.1002/jcp.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]