Abstract
Introduction
The role of the serotonin transporter gene polymorphism 5-HTTLPR in attention-deficit/hyperactivity disorder (ADHD) is unclear. Heterogeneity of findings may be explained by gene-environment interactions (GxE), as it has been suggested that S-allele carriers are more reactive to psychosocial stress than L-allele homozygotes. This study aimed to investigate whether 5-HTTLPR genotype moderates effects of stress on ADHD in a multi-site prospective ADHD cohort study.
Methods
5-HTTLPR genotype, as well as the number of stressful life events in the past five years and ongoing long-term difficulties, were determined in 671 adolescents and young adults with ADHD, their siblings, and healthy controls (57.4% male, average age 17.3 years). Linear mixed models, accounting for family relatedness, were applied to investigate the effects of genotype, experienced stress, and their interaction on ADHD severity at time point T2, while controlling for ADHD severity at T1 (mean follow-up time 5.9 years) and for comorbid internalizing problems at T2.
Results
The interaction between genotype and stress significantly predicted ADHD severity at T2 (p=.006), which was driven by the effect on hyperactivity-impulsivity (p=.004). Probing of the interaction effect made clear that S-allele carriers had a significantly more positive correlation between stress and ADHD severity than L-allele homozygotes.
Conclusion
The results show that the interaction between 5-HTTLPR and stress is a mechanism involved particularly in the hyperactivity/impulsivity dimension of ADHD, and that this is independent of comorbid internalizing problems. Further research into the neurobiological mechanisms underlying this interaction effect is warranted.
Keywords: ADHD, gene-environment interaction (GxE), stress, serotonin transporter (5-HTTLPR)
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007), characterized by inattention and/or hyperactive and impulsive behavior (American Psychiatric Association, 2013). Twin and adoption studies show that ADHD has a strong genetic component, with heritability estimated at 76% (Faraone et al., 2005). However, the genes involved remain largely unknown; candidate gene studies have reported small effect sizes and many findings are not being replicated (Gizer, Ficks, & Waldman, 2009). Interaction between genetic and environmental factors (GxE) has been put forward as one possible mechanism responsible for inconsistent findings (Buitelaar, 2005).
The serotonin neurotransmitter system is an attractive target for ADHD genetics studies, as experimentally manipulated serotonin levels lead to ADHD-like behaviors such as impulsive choices, increased motor activity, and delay aversion in both humans and animal models (Brewer & Potenza, 2008). The serotonin transporter gene (SLC6A4, better known as 5-HTT or SERT) is the most widely studied gene involved in serotonin signaling. It is located on the long arm of chromosome 17 (Gelernter, Pakstis, & Kidd, 1995), corresponding to a genomic region that has shown suggestive linkage to ADHD in genome-wide linkage scans (Arcos-Burgos et al., 2004). 5-HTT has a polymorphism in the promoter region (5-HTTLPR), which consists of a 44 base pair deletion/insertion unit, resulting in a 14 repeat short (S) variant and a 16 repeat long (L) variant (D’Souza & Craig, 2006). The S-allele is less transcriptionally active than the L-allele, leading to less availability of serotonin transporter to remove serotonin from the synaptic cleft (Lesch et al., 1996).
Since its discovery, studies into the 5-HTTLPR polymorphism have mostly focused on the S-allele and its association with anxiety-related traits (Lesch et al., 1996). In contrast, studies of ADHD have implicated L-allele homozygosity as a small but statistically significant risk factor (OR≈1.10, p=.004), although there is significant heterogeneity amongst studies (Gizer et al., 2009).
Besides genetic factors, the amount of exposure to stressful conditions in a child’s environment (e.g. severe marital discord of the parents, foster care placement) has been shown to predict ADHD (Biederman et al., 1995; Rutter, Cox, Tupling, Berger, & Yule, 1975). However, individuals show considerable heterogeneity in their response to stress, presumed to be in part due to differences in their genetic make-up (Caspi & Moffitt, 2006).
Numerous animal and human studies have found that 5-HTTLPR genotype moderates the effects of stress (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010). S-allele carriers have stronger reactions, such as increased heart rate and blood flow to limbic brain regions, to emotional stimuli than L-allele homozygotes (Hariri et al., 2002). S-allele carriers also have a stronger positive correlation between the amount of experienced stress and negative outcomes, such as the development of depression and anxiety disorders (Caspi et al., 2003).
While there is considerable variation in the course of ADHD, factors associated with its long-term outcome remain elusive. Only two cross-sectional studies so far have directly investigated a GxE between the 5-HTTLPR and stress with ADHD in adults as outcome measure (Muller et al., 2008; Retz et al., 2008). Both studies reported less ADHD-related problems for S-allele carriers compared to L-allele homozygotes at low stress levels, whereas no group differences were observed at high stress levels. The present study aimed to replicate and expand on these previous findings in a large and well-phenotyped sample of adolescents and young adults with ADHD and non-ADHD controls. In order to study ADHD severity in reaction to stress, we focused on stress experienced between two time points, five years apart, and controlled for ADHD severity at time point one. In addition we also controlled for internalizing problems at time point two, as this GxE has been mostly investigated with anxiety and depression as outcome measures (Caspi et al., 2010).
Methods
Participants
Participants were selected from the Dutch part of the International Multicenter ADHD Genetics (IMAGE) study. The first measurement point of this study, T1, took place between 2003 and 2006, and is extensively described elsewhere (Brookes et al., 2006). At T1, 365 families with at least one child with combined type ADHD and at least one biological sibling (regardless of ADHD diagnosis) were recruited, in addition to 148 control families with at least one child with no ADHD diagnosis in any of the first-degree family members. ADHD families were recruited through ADHD outpatient clinics in the regions Amsterdam, Groningen, and Nijmegen (The Netherlands). Control families were recruited through schools in the same regions. Participants were reassessed at T2, between 2009 and 2012. Mean follow-up period was 5.9 years (SD=.72); follow-up rates were 79% for ADHD families and 80% for control families. Inclusion criteria were the same for both time points: participants had to be of European Caucasian descent, have an IQ ≥ 70, and no diagnosis of autism, epilepsy, general learning difficulties, brain disorders, and known genetic disorders. Participants had to be between 6–18 years old at T1.
Assessment protocol
All measurements were part of a comprehensive assessment protocol. At both time points, testing was carried out either at the VU University Amsterdam and VU University Medical Centre in Amsterdam or at the Radboud University Medical Centre and Donders Institute for Brain, Cognition and Behavior in Nijmegen. The study was approved by the regional ethics committee (CMO Regio Arnhem – Nijmegen; 2008/163; ABR: NL23894.091.08) and the medical ethical committee of the VU University Medical Center. All participants above 12 years of age signed for informed consent/assent (parents signed informed consent for participants under 18 years of age).
ADHD diagnoses were made at T2 on the basis of an algorithm which combined information from behavioral questionnaires (typically filled in by a parent and a second observer) and a structured diagnostic interview performed by a trained professional, using DSM-IV criteria (American Psychiatric Association, 2000). An extensive discussion of this algorithm and the instruments used is provided on www.neuroimage.nl, as well as in the Additional Supporting Information. Of the 671 participants who met the inclusion criteria, 307 received a diagnosis of ADHD, 294 were classified as healthy controls, and 69 had some ADHD symptoms but did not meet the criteria for an ADHD diagnosis and were labeled as having “subthreshold ADHD”.
We used continuous measures of ADHD severity and comorbid internalizing problems as dependent variables, and combined all participants regardless of diagnostic status. At both time points, the participants were assessed on the basis of the Conners rating scales filled in by two informants, as multi-informant scores have been shown to be more accurate for ADHD and comorbid internalizing problems (Jensen et al., 1999). At T1, a parent-rated questionnaire (Conners’ Parent Rating Scale - Revised: Long version; CPRS-R:L) and a teacher-rating (Conners’ Teacher Rating Scale - Revised: Long version; CTRS-R:L) were available. At T2, the parent-rated questionnaire was available again, as well as the teacher-rating for participants <18 years (N= 292) or a self-report for participants ≥ 18 years (Conners’ Adult ADHD Rating Scales - Self-Report:Long Version; CAARS-S:L; N= 371). All three versions have shown good reliability and criterion validity (Conners, Sitarenios, Parker, & Epstein, 1998a; Conners, Sitarenios, Parker, & Epstein, 1998b; Conners et al., 1999).
The Conners questionnaires contain separate subscales for the ‘inattention’ (CPRS-R:L / CTRS-R:L scale L; CAARS-S:L scale E) and ‘hyperactivity/impulsivity’ (CPRS-R:L / CTRS-R:L scale M; CAARS-S:L scale F) domains of ADHD, both with nine items corresponding to each of the ADHD DSM symptoms. We constructed measures of severity of inattention and hyperactivity/impulsivity by counting the number of occurrences where either informant rated an item from these scales as ‘pretty much true/often’ (2) or ‘very much true/very often’ (3). The total ADHD severity measure was constructed by summing the scores on these two domains.
For internalizing problems, the ‘anxious/shy’ subscale of the CPRS-R:L and CTRS-R:L (scale D) was used (the CAARS-S:L lacks a comparable subscale, for participants older than 18 years only the CPRS-R:L was used; N= 371). As comorbid internalizing problems tend to be underreported (Jensen et al., 1999) the highest raw score of either informant was chosen, and transformed to a Z-score.
Assessment of experienced stress
Two questionnaires were used to assess the amount of psychosocial stress the participants had experienced before T2. At T2, parents filled in the Long-Term Difficulties (LTD) questionnaire (Bosch et al., 2012; Oldehinkel, Verhulst, & Ormel, 2008), which contained thirteen items measuring whether their children have been exposed to chronic stressors; see Additional Supporting Information in the online version of this paper for an overview of the items. They were asked to only report chronic, ongoing difficulties. The number of difficulties reported ranged from 0 (40.5%) to 7 (0.3%). In addition, participants themselves filled in a Stressful Live Events (SLE) questionnaire (Bosch et al., 2012; Oldehinkel et al., 2008) at T2 containing eleven items on exposure to specific major stressful events in the past five years, such as death of a loved one, abuse, or failure at something important; see Additional Supporting Information for an overview. The number of events reported ranged from 0 (13.0%) to 10 (0.1%). If participants filled in less than half the items on both questionnaires, they were excluded from further analysis; if they filled in more than half the items on only one of the two questionnaire, then that score was used (2.1% missed the SLE score; 3.3% missed the LTD score). If more than half of the items were filled in, missing items were imputed with ‘no’, i.e. we assumed the major life event had not occurred if not reported. The number of chronic long term difficulties correlated modestly with the number of stressful life events in the past five years; r=0.22. For the composite stress measure, the scores on the questionnaires were transformed to Z-values and combined according to common practice for aggregating similar measures (Ley, 2007).
Genotyping
Blood samples were either used to generate lymphocyte cell lines from which DNA was extracted, or DNA was extracted directly from a portion of the blood sample at Rutgers University Cell and DNA Repository, New Jersey, USA. DNA stocks for the entire data set were collated in London where they were stored and plated out for further analysis. Saliva samples were collected at T2 from those participants whose DNA had not been collected at T1, and sent to the Department of Human Genetics of the Radboud University Medical Centre. DNA was isolated from saliva using Oragene containers (DNA Genotek, Ottawa, Ontario, Canada) according to the protocol supplied by the manufacturer. Genotyping procedures can be found in (Brookes et al., 2006), genotyping of additional samples was performed as described in (Landaas et al., 2010).
Socio-economic status
As a measure of socio-economic status (SES), the last successfully completed education level of the parents was recoded into a measure reflecting years of education. This scale contained nine levels, ranging from 0 (no formal education) to 17 (university) years of education (Buis, 2010). The average of both parents was used, which ranged from 5 to 17 with an average of 11.8.
Statistical analysis
This study investigated a dominant model of the 5-HTTLPR S-allele, wherein S-allele carriers were coded as ‘1’ and L-allele homozygotes were coded as ‘0’. This is in accordance with the majority of studies investigating this GxE (Caspi et al., 2010) and is based on the functional effects of the S and L-allele (Lesch et al., 1996). In addition, L-alleles with the rs25531 C-G single nucleotide polymorphism were recoded as a functional S-allele, in accordance with prior studies (Hu et al., 2006). This led to 14 L-allele homozygotes being recoded. Compliance of genotype distribution with Hardy-Weinberg equilibrium was checked using standard methods.
All data were analyzed using R (R Core Team, 2012). Differences between genotypes in sample demographics were checked with Pearson’s Chi-squared tests for categorical variables and with one-way ANOVA’s for continuous variables. In order to account for the within-family correlation due to the inclusion of siblings in the sample, the data were analyzed with linear mixed models with family as a random factor, estimating a random intercept.
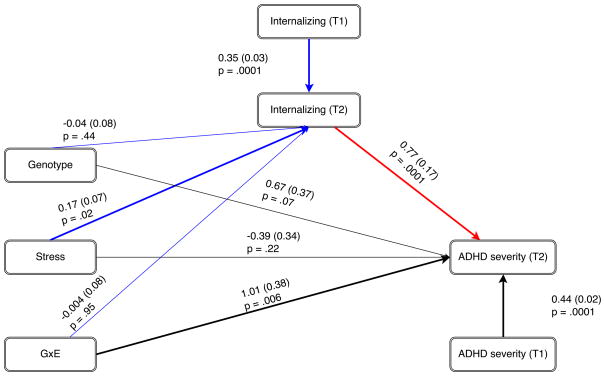
Conditional process analysis was employed in order to investigate the possible role of internalizing problems in the effect of the GxE on ADHD (Hayes, 2013). The model consisted of a direct and an indirect path, as illustrated in Figure 2. The indirect path consisted of: path a) the effect of the interaction between genotype and stress on internalizing problems at T2, while controlling for internalizing problems at T1; and path b) the effect of internalizing problems at T2 on ADHD severity at T2, while controlling for ADHD severity at T1. The direct path (path c′) consisted of the effect of the GxE on ADHD severity at T2, while controlling for ADHD severity at T1 and internalizing problems at T2.
Figure 2.
The full conditional process model. The blue lines indicate path ‘a’, wherein the effects of the GxE on internalizing problems at T2 are investigated, while controlling for internalizing problems at T1. The red line indicates path ‘b’, which represents the correlation between internalizing problems at T2 and ADHD severity at T2. The black lines indicate path c′, used to determine the direct effect of the GxE on ADHD severity while controlling for ADHD severity at T1 and internalizing problems at T2. The parameter estimates are shown next to their paths, with the standard errors displayed behind them between brackets and the corresponding p-values beneath them.
Gender, age, SES, and test location were added as covariates to the model. For continuous predictors, the mean was subtracted from each score. The p-values of the mixed models were estimated through a Markov-Chain Monte Carlo algorithm. Significance of the indirect effects were calculated through percentile bootstrap procedures (Hayes, 2013, pg. 106). Regions of significance (ROS) were obtained through a hierarchical linear model ROS calculator (Preacher, Curran, & Bauer, 2006) which indicates at what stress score the two genotypes differ significantly from each other on ADHD severity.
Sensitivity analyses
Four sensitivity analyses were performed to check whether the findings were due to methodological choices. First, we checked whether the effects of the GxE were driven by either one of the two components of the composite stress score, LTD and SLE, by running the conditional process analysis for each separately. Second, we added a three-way interaction between the GxE and diagnostic status (controls and subthresholds coded as ‘0’, ADHD as ‘1’) to the model to check whether the effects of the GxE differed significantly between diagnostic groups. Third, we checked whether the type of informant used had an effect by adding a binary covariate to the model which coded for type of informant combination used (parent and teacher or parent and child). Fourth, we reran the analysis without covarying for T1 ADHD severity to enable comparison with other GxE studies without longitudinal data.
Results
Demographic characteristics
ADHD severity at T2 was correlated with gender (male coded as ‘0’; r= −.36, p= 2.2 * 10−16) and SES (r= −.16, p= 3.3 * 10−5), but not with age (r= .01, p= .78) or testing location (r= .02, p= .56). No significant differences in gender distribution, age, experienced stress, SES, or testing location were found between S-allele carriers and L-allele homozygotes, as summarized in Table 1. Genotyping frequencies were as expected in the Caucasian population and did not deviate from Hardy-Weinberg Equilibrium (χ2= 0.11, p=.74).
Table 1.
Demographics of the study sample at T2, split by genotype.
| Variable | S-allele carriers | L-allele homozygotes | Test-statistic | P-value |
|---|---|---|---|---|
| Participants | 524 | 147 | ||
| Covariates | ||||
| Amsterdam location | 47.9% | 46.9% | χ =0.01 | .91 |
| Male gender | 59.2% | 51.0% | χ =2.79 | .10 |
| Age in years (SD) | 17.17 (3.21) | 17.77 (3.44) | F=3.89 | .05 |
| Parents’ years of education (SD) | 11.8 (2.44) | 12.11 (2.42) | F=2.40 | .12 |
| Stress Z-score (SD) | 0.02 (1.01) | −0.08 (0.96) | F=1.08 | .30 |
| Stressful live events (SD) | 2.08 (1.54) | 2.12 (1.53) | F=0.08 | .77 |
| Long-term difficulties (SD) | 1.27 (1.45) | 1.07 (1.41) | F=2.22 | .14 |
| Internalizing Z-score at T2 (SD) | 0.01 (1.00) | −0.03 (1.00) | F=0.13 | .72 |
The direct effect of the GxE on ADHD
There was no evidence of conditional main effects of genotype or stress on ADHD. However, the interaction effect was statistically significant (p =.006, see Table 2 and Figure 2), indicating that 5-HTTLPR genotype moderated the effect of stress exposure on T2 ADHD severity, independent of ADHD severity at T1 or internalizing problems.
Table 2.
Results of the regression of stress, 5-HTTLPR genotype and their interaction on ADHD severity at T2.
| Predictor | ADHD | Inattention | Hyperactivity-impulsivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Intercept | 5.45 | 0.40 | .0001 | 3.37 | 0.25 | .0001 | 2.13 | 0.21 | .0001 |
| Genotype | 0.67 | 0.37 | .07 | 0.37 | 0.23 | .10 | 0.28 | 0.20 | .16 |
| Stress | −0.39 | 0.34 | .22 | −0.07 | 0.21 | .61 | −0.30 | 0.18 | .09 |
| Interaction | 1.01 | 0.38 | .006 | 0.43 | 0.23 | .05 | 0.58 | 0.20 | .004 |
| Conners score at T1 | 0.44 | 0.02 | .0001 | 0.47 | 0.03 | .0001 | 0.38 | 0.03 | .0001 |
| Internalizing at T2 | 0.77 | 0.17 | .0001 | 0.52 | 0.11 | .0001 | 0.26 | 0.09 | .006 |
The conditional main effects of genotype or stress also were not significant for either inattention or hyperactivity-impulsivity, when investigated separately. Whereas the GxE was marginally significant for inattention (p = 0.05), it was highly significant for hyperactivity-impulsivity (p = 0.004). Results are summarized in Table 2.
Probing the interaction
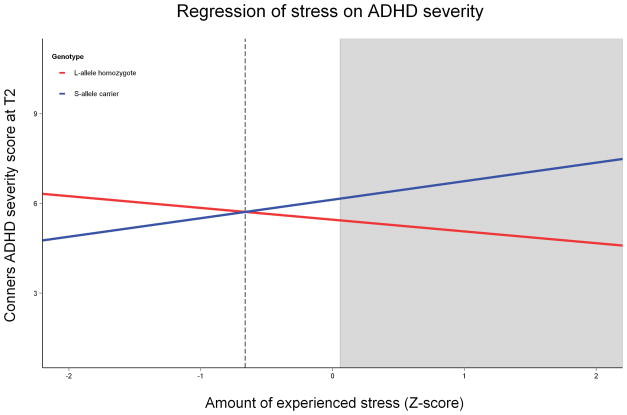
Visualization of the interaction effect on T2 ADHD severity (see Figure 1) made clear that S-allele carriers displayed a positive relation between stress and ADHD severity, whereas L-allele homozygotes did not. This was confirmed by within-group analyses of the effects of stress (S-allele carriers: B=0.67, SE=0.18, p=.0004; L-allele homozygotes: B=−0.55, SE=0.34, p=.11). The two simple slopes, corresponding to the effects of stress on either genotype, were calculated to cross at a stress Z-score of −0.66, well within the data range (74% of participants had higher scores, 26% lower).
Figure 1.
The interaction effect between stress and 5-HTTLPR genotype. Stress score on the X-axis is a composite of two questionnaires asking about ongoing long-term difficulties and stressful live events experienced in the past five years. ADHD severity on the Y-axis was measured through Conners questionnaires filled in by two informants. Whereas the S-allele carriers show a positive correlation between stress and ADHD severity, L-allele homozygotes do not. The shaded area indicates where the two genotypes differ significantly in severity (S>L, at stress levels higher than 0.06).
Regions of significant differences in ADHD severity between the genotypes were found to be at stress Z-scores below −2.67 (L>S) and above 0.06 (S>L). In this dataset, no individuals fell within the lower region of significance (ROS) while 268 participants (41%) had stress scores placing them in the higher ROS.
The role of internalizing problems
Stress was a significant predictor of T2 internalizing problems (p=.02) independent of internalizing problems at T1, whereas genotype was not. The interaction between stress and 5-HTTLPR on T2 internalizing problems was also not statistically significant. Internalizing problems at T2 were highly correlated with ADHD severity at T2 (p=.0001). These results are displayed in Figure 2.
The indirect effect of stress on T2 ADHD severity, by co-occurring internalizing problems, was significant (p=.04); this was not the case for genotype or the GxE. Results are summarized in Table 3.
Table 3.
Results of the conditional process analysis estimating the indirect effect, through internalizing problems, and total effect of the GxE between 5-HTTLPR genotype and stress on ADHD.
| Path | Predictor | Estimate | SE | P |
|---|---|---|---|---|
| Indirect | Genotype | −0.03 | 0.06 | .62 |
| Stress | 0.13 | 0.06 | .04 | |
| Interaction | −0.003 | 0.005 | .55 | |
| Total | Genotype | 0.65 | 0.37 | .09 |
| Stress | −0.25 | 0.34 | .41 | |
| Interaction | 1.03 | 0.38 | .005 |
The total effect of the GxE on ADHD
The total effect of the GxE on ADHD was highly similar to the estimates obtained from the direct path, with no conditional main effects for genotype or stress but a significant interaction between genotype and stress (p=.005), see Table 3.
Sensitivity analyses
The pattern of results remained the same when either the LTD or SLE questionnaire was used by itself as a measure of stress instead of the composite score, with no conditional main effects of genotype or stress, but a significant effect of the GxE on ADHD (LTD: B=0.51, SE=0.26, p=.04 ; SLE: B=0.55, SE=0.24, p=.02).
There was no evidence of a three-way interaction (B=0.29, SE=0.56, p=.53) when diagnostic status was added to the model, indicating that the effects of the GxE did not differ significantly between those with a full ADHD diagnosis versus healthy controls and subthreshold cases.
When the covariate coding for type of informant was added to the model, the GxE effect remained significant (B=0.99, SE=0.37, p=.006), and the main effect of informant was not significant (B = −0.48, SE = 0.45, p=.29), indicating that type of informant combination used did not significantly explain our findings.
Dropping the T1 ADHD severity measure as a covariate made the effect of the GxE on T2 ADHD severity more significant (B=1.60, SE=0.46, p=.0004). The conditional effects of stress and 5-HTTLPR genotype remained non-significant. Running this model with hyperactivity and inattention scores as outcome measure separately led to similar increases in significance (hyperactivity/impulsivity: B=0.80, SE=0.23, p=.0006; inattention: B=0.78, SE=0.27, p=.003), with, as in our main analyses, a much stronger effect for hyperactivity/impulsivity than inattention.
Discussion
Our study was able to confirm earlier reports on an association of the gene-environment interaction between the 5-HTTLPR polymorphism and psychosocial stress with ADHD (Muller et al., 2008; Retz et al., 2008), and therefore added to the evidence that this specific GxE is a mechanism involved in ADHD. We also extended previous work by using a moderated mediation model, enabling us to focus on the effect of stressors between two time points while adjusting for baseline ADHD severity and internalizing problems.
We found that S-allele carriers were more reactive to stress than L-allele homozygotes, following the findings of previous studies investigating this specific GxE (Caspi et al., 2010). One suggested mechanism underlying this GxE is that stress causes methylation of 5-HTTLPR, thereby reducing its transcriptional activity (Beach, Brody, Todorov, Gunter, & Philibert, 2010). High stress levels may therefore shift serotonin transporter levels in low-expressing S-allele carriers to a pathologically low level whereas such a downward shift would have less negative consequences for the higher expressing L-allele homozygotes. The outcome of the ROS calculation is in agreement with this, as it showed that S-allele carriers had significantly more severe ADHD than L-allele homozygotes at high stress levels, whereas at low stress levels there was no significant difference. This pattern of results is in accordance with the classic diathesis-stress framework (Monroe & Simons, 1991) whereby the S-allele is characterized as a ‘vulnerability gene’. An alternative paradigm, the differential susceptibility theory, has received much attention in recent years. Proponents of this framework state that 5-HTTLPR should instead be viewed as a ‘plasticity gene’, i.e. S-allele carriers should not only be viewed as more susceptible to negative outcomes under stressful conditions, but also as more likely to benefit from favorable conditions (Belsky & Pluess, 2009). As our study only contained measures of stress, these two theories could not be directly contrasted. Further research into this GxE should therefore also contain data on beneficial conditions in participants’ lives.
Our results suggest that serotonergic neurotransmission, as determined by the interplay between stress and serotonin transporter genotype, is more influential on the outcome of hyperactivity/impulsivity severity in adolescence and young adulthood than it is for inattention. This is in accordance with many findings in the literature which indicate that serotonergic neurotransmission is associated with increased motor activity and impulsive behavior (Walderhaug et al., 2002; Brunner & Hen, 1997) whereas studies describing a link between inattention and serotonin levels are scarce. Serotonergic genes have also been implicated specifically in severity of hyperactivity/impulsivity, but not severity of inattention, by our group (Bralten et al., 2013).
We also found an indirect effect of stress on ADHD mediated by internalizing problems. Neuroimaging studies have shown that stress has detrimental effects on the structure and functioning of the prefrontal cortex (PFC), specifically the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC, Hart & Rubia, 2012). Decreased control of the PFC over subcortical areas has been put forward as an important mechanism underlying both internalizing problems and ADHD (Arnsten & Rubia, 2012). The detrimental effects of stress on these PFC regions may thus be a shared mechanism in the etiology of ADHD and internalizing problems, which brought about a significant mediation effect in this study.
Despite converging evidence that 5-HTTLPR moderates the effects of stress on anxiety and depression (Caspi et al., 2010), we did not replicate these findings in this sample. Previous studies were performed either in healthy populations or in samples with high rates of depression or anxiety, whereas the current study sample was focused on individuals with ADHD. The lack of GxE effects on internalizing problems in this study may indicate that the internalizing problems in ADHD differ from ‘pure’ internalizing problems; these problems in ADHD may be more a manifestation of “emotional impulsivity” (Barkley & Fischer, 2010). Although both ADHD and anxiety or depression likely involve a top-down deficit in regulating emotional responses to events, it is possible that emotion dysregulation in mood disorders such as anxiety and depression may be more due to deviant frontolimbic activity (e.g. subgenual ACC and amygdala, cf. Pezawas et al., 2005), while emotional problems in ADHD may stem from deviant orbitofrontal activity. Future neuroimaging studies contrasting the two may provide more clarity.
Limitations of this study include the retrospective assessment of stress and its correlational nature, which prevents strong inferences about causality; any pre-existing differences correlated with ADHD may have caused more experienced stress, i.e. the presence of gene-environment correlations. We also did not have measures of serotonin levels nor of epigenetic signatures, therefore discussion of the potential molecular mechanisms underlying the GxE remains speculative. Strengths include a large sample size, use of multiple informants, the longitudinal design and use of a moderated mediation analysis controlling for internalizing problems.
This study confirmed an interaction effect between 5-HTTLPR and stress on ADHD in a sample of adolescents. This underlines the importance of including possible moderating genetic and environmental factors when investigating determinants of ADHD. Future studies aimed at investigating the biological pathways involved, for instance through neuroimaging, can shed more light on ADHD etiology and ultimately guide attempts at developing more targeted and effective therapies.
Supplementary Material
Key points.
Both stress and 5-HTTLPR genotype have been associated with increased risk for ADHD, yet findings across studies are inconsistent. This inconsistency may, in part, be due to failure to take gene-environment interaction effects into account.
We found that the interaction between stress and 5-HTTLPR genotype significantly predicted ADHD, in the absence of main effects of stress or genotype.
S-allele carriers were found to be more reactive to stress than L-allele homozygotes.
The interaction effect was independent of comorbid internalizing problems and stronger for hyperactivity/impulsivity than inattention.
Analysis of gene-environment interactions can provide a more nuanced understanding of ADHD etiology than investigations of main effects. This may aid in the development of personalized approaches to prevention and treatment.
Acknowledgments
This work was supported by NIH Grant R01MH62873 (to Stephen V. Faraone), NWO Large Investment Grant 1750102007010 (to Jan Buitelaar) and grants from Radboud University Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam.
Footnotes
- In the past year, Dr. Faraone received consulting income, travel expenses and/or research support from Akili Interactive Labs, Alcobra, VAYA Pharma, and SynapDx and research support from the National Institutes of Health (NIH). His institution is seeking a patent for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health and Oxford University Press: Schizophrenia: The Facts.
- Jan Buitelaar has been in the past 3 years a consultant to / member of advisory board of / and/or speaker for Janssen Cilag BV, Eli Lilly, Shire, Novartis, Roche and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies.
- Jaap Oosterlaan has received an unrestricted investigator initiated research grant from Shire pharmaceuticals.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental health disorders: DSM-5. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Pineda D, Lopera F, Palacio JD, Palacio LG, et al. Attention-deficit/hyperactivity disorder in a population isolate: Linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. American Journal of Human Genetics. 2004;75(6):998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(5):503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: An examination of the iowa adoptee sample. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2010;153B(2):710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, et al. Family-environment risk factors for attention-deficit hyperactivity disorder. A test of rutter’s indicators of adversity. Archives of General Psychiatry. 1995;52(6):464–470. doi: 10.1001/archpsyc.1995.03950180050007. [DOI] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, et al. Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. the TRAILS study. Psychoneuroendocrinology. 2012;37(9):1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Bralten J, Franke B, Waldman I, Rommelse N, Hartman C, Asherson P, et al. Candidate genetic pathways for attention-deficit/hyperactivity disorder (ADHD) show association to hyperactive/impulsive symptoms in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(11):1204–1212. e1. doi: 10.1016/j.jaac.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: Relationships to drug addictions. Biochemical Pharmacology. 2008;75(1):63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: Association signals in DRD4, DAT1 and 16 other genes. Molecular Psychiatry. 2006;11(10):934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Buis ML. Inequality of Educational Outcome and Opportunity in the Netherlands during the 20th Century. Faculty of Social Sciences, VU-University; Amsterdam: 2010. Scaling levels of education. [Google Scholar]
- Buitelaar JK. ADHD: Strategies to unravel its genetic architecture. Journal of Neural Transmission Supplementum. 2005;(69):1–17. doi: 10.1007/3-211-31222-6_1. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews Neuroscience. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science (New York, NY ) 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, Sparrow E. Self-ratings of ADHD symptoms in adults I: Factor structure and normative data. Journal of Attention Disorders. 1999;3(3):141–151. [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised conners’ parent rating scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998a;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the conners teacher rating scale (CTRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998b;26(4):279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- D’Souza UM, Craig IW. Functional polymorphisms in dopamine and serotonin pathway genes. Human Mutation. 2006;27(1):1–13. doi: 10.1002/humu.20278. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Pakstis AJ, Kidd KK. Linkage mapping of serotonin transporter protein gene SLC6A4 on chromosome 17. Human Genetics. 1995;95(6):677–680. doi: 10.1007/BF00209486. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Human Genetics. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science (New York, NY ) 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Rubio-Stipec M, Canino G, Bird HR, Dulcan MK, Schwab-Stone ME, et al. Parent and child contributions to diagnosis of mental disorder: Are both informants always necessary? Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(12):1569–1579. doi: 10.1097/00004583-199912000-00019. [DOI] [PubMed] [Google Scholar]
- Landaas ET, Johansson S, Jacobsen KK, Ribases M, Bosch R, Sanchez-Mora C, et al. An international multicenter association study of the serotonin transporter gene in persistent ADHD. Genes, Brain, and Behavior. 2010;9(5):449–458. doi: 10.1111/j.1601-183X.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY ) 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Ley P. Quantitative aspects of psychological assessment: An introduction. Duckworth Publishers; 2007. [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Muller DJ, Mandelli L, Serretti A, DeYoung CG, De Luca V, Sicard T, et al. Serotonin transporter gene and adverse life events in adult ADHD. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008;147B(8):1461–1469. doi: 10.1002/ajmg.b.30706. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Verhulst FC, Ormel J. Low heart rate: A marker of stress resilience. the TRAILS study. Biological Psychiatry. 2008;63(12):1141–1146. doi: 10.1016/j.biopsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. The American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Retz W, Freitag CM, Retz-Junginger P, Wenzler D, Schneider M, Kissling C, et al. A functional serotonin transporter promoter gene polymorphism increases ADHD symptoms in delinquents: Interaction with adverse childhood environment. Psychiatry Research. 2008;158(2):123–131. doi: 10.1016/j.psychres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Rutter M, Cox A, Tupling C, Berger M, Yule W. Attainment and adjustment in two geographical areas. I--the prevalence of psychiatric disorder. The British Journal of Psychiatry : The Journal of Mental Science. 1975;126:493–509. doi: 10.1192/bjp.126.6.493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.