Abstract
OBJECTIVES:
To describe the technical feasibility, safety, and clinical outcomes of coil-assisted retrograde transvenous obliteration (CARTO) in treating portal hypertensive non-esophageal variceal hemorrhage.
METHODS:
From October 2012 to December 2013, 20 patients who received CARTO for the treatment of portal hypertensive non-esophageal variceal bleeding were retrospectively evaluated. All 20 patients had at least 6-month follow-up. All patients had detachable coils placed to occlude the efferent shunt and retrograde gelfoam embolization to achieve complete thrombosis/obliteration of varices. Technical success, clinical success, rebleeding, and complications were evaluated at follow-up.
RESULTS:
A 100% technical success rate (defined as achieving complete occlusion of efferent shunt with complete thrombosis/obliteration of bleeding varices and/or stopping variceal bleeding) was demonstrated in all 20 patients. Clinical success rate (defined as no variceal rebleeding) was 100%. Follow-up computed tomography after CARTO demonstrated decrease in size with complete thrombosis and disappearance of the varices in all 20 patients. Thirteen out of the 20 had endoscopic confirmation of resolution of varices. Minor post-CARTO complications, including worsening of esophageal varices (not bleeding) and worsening of ascites/hydrothorax, were noted in 5 patients (25%). One patient passed away at 24 days after the CARTO due to systemic and portal venous thrombosis and multi-organ failure. Otherwise, no major complication was noted. No variceal rebleeding was noted in all 20 patients during mean follow-up of 384±154 days.
CONCLUSIONS:
CARTO appears to be a technically feasible and safe alternative to traditional balloon-occluded retrograde transvenous obliteration or transjugular intrahepatic portosystemic shunt, with excellent clinical outcomes in treating portal hypertensive non-esophageal variceal bleeding.
INTRODUCTION
Traditional balloon-occluded retrograde transvenous obliteration (BRTO) is a well-accepted method of treating gastric variceal bleeding (GVB) in the setting of portal hypertension, with a very high clinical success rate of 95% of ceasing variceal bleeding.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 It is even more valuable when considering that GVB is associated with a higher mortality rate and poorer outcomes, as endoscopic treatment or conservative management are ineffective in treating GVB.11, 12, 13, 14, 15 With an increased incidence of GVB, due to effective endoscopic treatment of esophageal varices redistributing more portal flow toward the gastric varices, BRTO becomes an attractive addition to the armamentarium to treat GVB.
Currently, BRTO requires the use of an indwelling occlusive balloon inflated for hours with injection of sclerosing agents such as ethanolamine oleate or sodium tetradecal sulfate to cause sclerosis of varices and efferent shunts. An indwelling inflated balloon results in lengthy procedure times, additional hospital resources, and logistics (e.g., intensive care unit bed, additional patient transport, additional interventional radiology suite time, and staff) and the innate complications associated with balloons (e.g., balloon rupture). The sclerosing agents are also associated with some of the serious complications of BRTO, including: pulmonary edema, disseminated intravascular coagulation, portal vein thrombosis, severe renal dysfunction, and anaphylactic reaction.3,4,9,16, 17, 18, 19, 20, 21, 22, 23, 24
Recently, modified BRTO was proposed and reported by Gwon et al.,25 where an indwelling balloon was replaced with a vascular plug to minimize some of the complications and logistical issues associated with the balloon. However, it has its own limitations (e.g., size limit). In this study, we describe a novel method of performing modified BRTO with coils and gelfoam (coil-assisted retrograde transvenous obliteration (CARTO)) instead of indwelling balloons or vascular plugs and sclerosing agents. The aim of the study is to evaluate the short and long-term safety and effectiveness of CARTO in the treatment of non-esophageal variceal bleeding, including GVB.
METHODS
Patient demographics
This retrospective study was approved by the institutional review board. We retrospectively reviewed medical records in 43 patients who received modified BRTO between October 2012 and December 2013. Forty-three patients underwent some type of BRTO for variceal bleeding and/or hepatic encephalopathy. All 43 patients demonstrated either gastrorenal or splenorenal shunt, which was accessible from the renal vein. The patients without an appropriate shunt received transjugular intrahepatic portosystemic shunt (TIPS; n=5). Of the 43, 20 who had undergone CARTO for the treatment of acute or recent variceal bleeding were included in this study. Of the rest of the 43 patients not included in this study, 15 patients received CARTO for the treatment of hepatic encephalopathy and 8 patients received PARTO (Plug-assisted Retrograde Transvenous Obliteration) for the treatment of GVB. The entry criterion was follow-up of ≥6 months. All the 20 patients had recorded follow-up data of at least 6 months. Demographic, clinical, and laboratory data were collected and reviewed retrospectively. Thirteen out of the 20 patients had an endoscopic evaluation confirming an active bleeding site or stigmata of recent bleeding/pending rupture. Seventeen out of the 20 patients had recent preprocedural triple-phase computed tomography (CT) or magnetic resonance imaging, with evidence supporting the presence of efferent gastrorenal shunt. All the 20 patients had recorded follow-up data of at least 6 months, which included CT and/or endoscopy and clinic visits.
Technique of CARTO
An illustration of CARTO procedure is shown in Figure 1. All CARTO procedures were performed with moderate sedation, except for three patients who required general anesthesia due to hemodynamic instability, caused by acute hypotensive crisis. Prior to CARTO procedure, a triple- or dual-phase contrast-enhanced CT of the abdomen was reviewed to confirm the presence and size of an efferent shunt-draining varices to the systemic venous circulation. After preprocedural planning with CT images, either the right internal jugular or right common femoral venous access was achieved. An 8 or 14 Fr vascular sheath (Cook Inc. Bloomington, IN) was placed in the inferior vena cava or renal vein for stable access. Using a 0.035 glidewire and 4 or 5 Fr glide or C2 catheter, the efferent shunt was accessed. The catheter was exchanged with an occlusion balloon (Fogarty or Equalizer balloon), and a balloon-occluded retrograde venogram was performed to assess and confirm the varices and the size of the shunt. After removing the balloon, the efferent shunt was accessed using a double microcatheter system (Figure 2) with a combination of Renegade STC (Boston Scientific Co., Natick, MA) and Renegade Hi-Flo (Boston Scientific Co.) with either 0.014 or 0.016 microwires. The Renegade Hi-Flo microcatheter was advanced distally into or adjacent to the gastric varices to perform a variceal/shunt venogram and to perform Gelfoam embolization (Figure 2a). Through the more proximally placed microcatheter within the efferent shunt, correctly sized detachable coils (Interlock detachable coils, Boston Scientific Co.; average 11.1±5.3, range 5–22) were deployed, and complete occlusion of the efferent shunt was confirmed with a venogram (Figure 2a). Then, through the distally placed microcatheter, gelfoam (gelfoam sponge sizes from approximately 2 to 5 mm3) mixed with contrast agent was injected to embolize the entire efferent shunt, varices, collaterals, and afferent veins (average number of Gelfoam packs=6.3±3.5 packs, range 3–13 packs) (Figure 2b). Postembolization venography was performed using the distally placed microcatheter again to confirm complete occlusion of the efferent shunt and complete stasis within the varices. After venographic confirmation of completion, the microcatheters were removed. During the procedure, the patient's blood pressure, pulse, electrocardiogram, and arterial oxygen saturation were monitored. Serial laboratory values, including liver function tests, complete blood counts, and ammonia, were monitored until discharge. All patients had a triple-phase contrast-enhanced CT of the abdomen within 2–3 days prior to discharge to evaluate: (1) obliteration of the efferent shunt and gastric varices, and (2) evidence of immediate complications, including renal vein thrombosis, inferior vena cava thrombosis, or portal vein thrombosis. All patients were clinically assessed for pulmonary embolism.
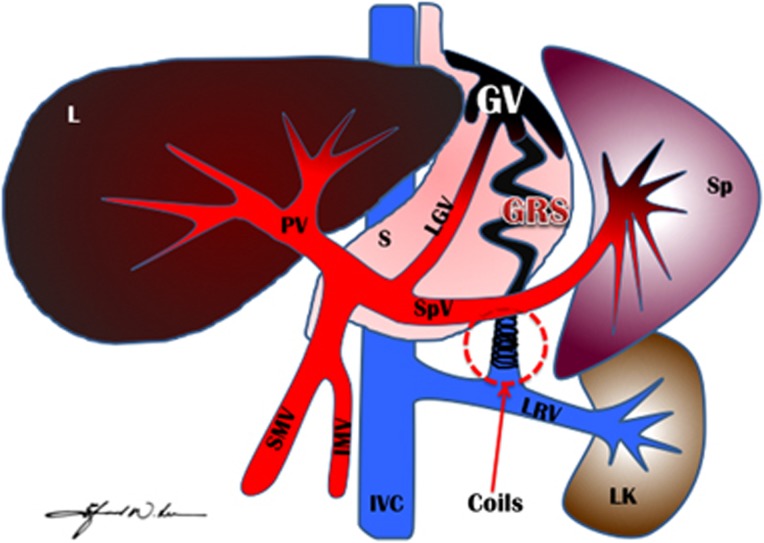
Figure 1.
An illustration of coil-assisted retrograde transvenous obliteration (CARTO) procedure. This illustration demonstrates complete obliteration/thrombosis of GV and GRS (in black color) post-CARTO procedure after deploying multiple coils in the GRS and filling the GRS and GV via retrograde fashion using gelfoam slurry. Coils, coils in the GRS; GRS, gastrorenal shunt; GV, gastric varices; IMV, inferior mesenteric vein; IVC, inferior vena cava; L, Liver; LGV, left gastric vein (coronary vein); LK, left kidney; LRV, left renal vein; PV, portal vein; S, Stomach; SMV, superior mesenteric vein; Sp, Spleen; SpV, splenic vein.
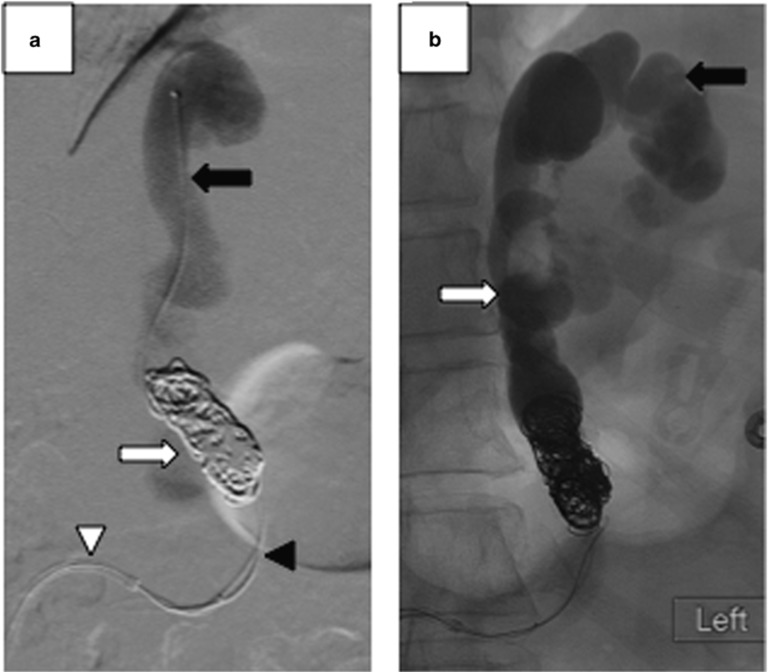
Figure 2.
Fluoroscopic images of coil-assisted retrograde transvenous obliteration (CARTO). (a) A double microcatheter system with the proximal microcatheter (black arrowhead) is used for coil deployment, and the distal microcatheter (black arrow) is used for gelfoam injection and gastric variceal (GV) and gastrorenal shunt (GRS) venogram. Multiple detachable coils (white arrow) are used in place of an indwelling occlusive balloon used in a conventional balloon-occluded retrograde transvenous obliteration. (b) Technically successful CARTO demonstrating complete stasis and opacification of GRS (white arrow) and GV (black arrow).
Upon discharge, all 20 patients underwent 1-, 3- and/or 6-month triple-phase CT and/or follow-up upper gastrointestinal endoscopy. Follow-up clinical and laboratory data were collected from all the 20 patients and reviewed.
Definitions and statistical analysis
Technical success was defined as successfully achieving complete occlusion of efferent shunts using embolization coils with complete gelfoam-induced thrombosis/obliteration of bleeding varices and/or immediate stopping of variceal bleeding. Clinical success was defined as the lack of variceal rebleeding in any clinical follow-up, including endoscopically visualized bleeding or clinical evidence of hematemesis, hematochezia, or melena. Complete thrombosis/obliteration of the varices was defined as the complete disappearance of targeted varices, as seen on follow-up CT or endoscopy 1 month following CARTO or at any time thereafter. Complications were classified as “major” and “minor” according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee.26 “Minor” complications were defined as those requiring no or nominal therapy, including overnight admission for observation only. “Major” complications were defined as those necessitating major therapy, an unplanned increase in the level of care or prolonged hospitalization (>48 h), or those resulting in permanent adverse sequelae or death.
Paired-sample t-test was used to compare pre- and post-CARTO serum ammonia levels. The statistical analysis was performed using the SPSS software (version 15.0. SPSS, Chicago, IL), with P-values <0.05 considered to be statistically significant.
RESULTS
Patients
There were 12 men and 8 women with a mean age of 58 years (range 17–75 years). The indications for CARTO were GVB (n=14), stomal variceal bleeding (n=3), duodenal variceal bleeding (n=1), jejunal variceal bleeding (n=1), and rectal variceal bleeding (n=1). Seven patients presented with acute variceal bleeding and 13 patients had a history of recent bleeding within 3 months at the time of presentation. Table 1 summarizes patient demographics.
Table 1. Patient characteristics.
| Patient demographics | |
| Male: female | 12: 8 |
| Age (years) | 58 (17–75) |
| Underlying liver disease | |
| Alcoholic cirrhosis | 6 (30.0%) |
| Hepatitis C cirrhosis | 6 (30.0%) |
| Hepatitis B cirrhosis | 3 (15.0%) |
| Cryotogenic cirrhosis | 2 (10.0%) |
| Primary sclerosing cholangitis cirrhosis | 2 (10.0%) |
| Non-alcoholic steatohepatitis | 1 (5.0%) |
| Pre-CARTO MELD | 12.2±5.2 |
| Concomitant malignancy | |
| Hepatocellular carcinoma | 1 (5.0%) |
| Metastatic neuroendocrine tumor | 1 (5.0%) |
CARTO, coil-assisted retrograde transvenous obliteration; MELD, model for end-stage liver disease.
Technical outcomes
Successful coil occlusion of efferent shunt with complete gelfoam embolization and thrombosis of the shunt was achieved in all 20 patients and was confirmed with post-CARTO venography in all the patients. Fifteen patients received CARTO via transfemoral approach and five patients received transjugular approach. The mean procedure time from venous access to the end of procedure (removal of sheath and start of venotomy hemostasis with manual compression) was 2.82±0.56 h. Technical success was achieved in all 20 patients with complete resolution of variceal bleeding during immediate follow-up by both clinical (hemodynamic stability, no transfusion requirement, no hematemesis, or melena) and radiological (CT evidence of disappearance of shunt and varices) evidence (technical success rate=100%).
Seven patients presented with severe hypotension requiring 2–3 vasopressors at the beginning of the procedure. However, their hemodynamic status normalized at the end of procedure or within a day after the procedure. No procedure-related immediate complications or death were identified in any patients. However, one patient passed away at 24 days post-CARTO with severe systemic venous thrombosis and multi-organ failure (n=1).
Clinical outcomes
Clinical success rate was 100%. Complete thrombosis of the efferent shunts and varices were seen in all the patients as early as 2 days after CARTO, and sequential disappearance of targeted varices was noted at 1-, 3-, and 6-month follow-up CTs in 19 patients (Figure 3). 8/20 patients had a 1-year follow-up CT with consistent resolution of varices. In all, 13/20 patients also underwent periodic endoscopic follow-up at 1, 2, 3, and/or 6 months and showed complete resolution of intra-luminal gastric varices (Figure 4). No patients had clinical symptoms related to variceal bleeding such as hemodynamic instability, decreasing hemoglobin, hematemesis, anemia or melena, except for one patient who had asymptomatic anemia (H/H=5.8/19.9 from 8.0/27.6) discovered incidentally during a regular hepatology visit. He was transfused with packed red blood cell without further anemia, and no etiology of anemia was found following a full evaluation.
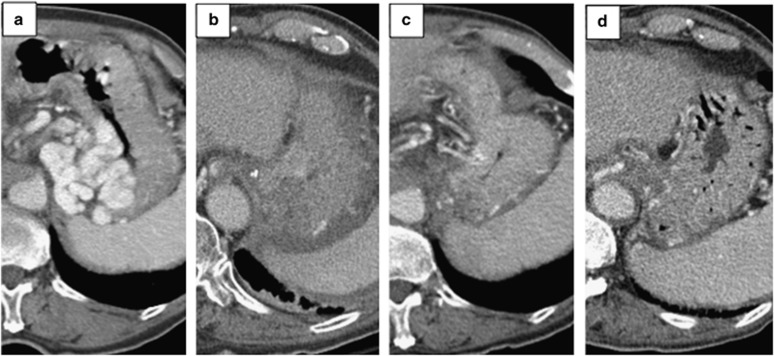
Figure 3.
Contrast-enhanced portal-venous phase axial computed tomography images of gastric varices in (a) pre-CARTO (pre-coil-assisted retrograde transvenous obliteration) with huge transmural gastric variceals (GVs) in the gastric fundus with a recent history of acute upper gastrointestinal bleeding, (b) Two-day post-CARTO demonstrating non-enhancing/hypoenhancing gastric fundal wall consisted with completely thrombosed GV, (c) 1-month post-CARTO demonstrating mild mucosal hypoenhancement of gastric fundus without evidence of GVs, and (d) 6-month post-CARTO showing complete disappearance of GVs with normal gastric fundal enhancement/anatomy.
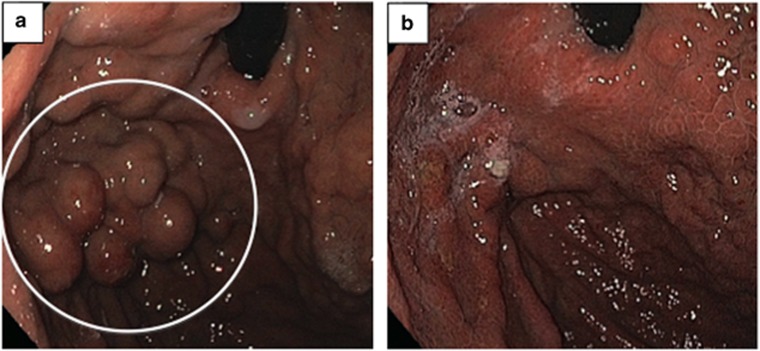
Figure 4.
Endoscopic images of the gastric fundus. (a) Pre-CARTO (pre-coil-assisted retrograde transvenous obliteration) image demonstrating massive gastric variceals (GVs; white circle) in the gastric fundus with red spots, stigmata of recent bleeding, and (b) 6-month post-CARTO image demonstrating complete resolution of previously seen GVs.
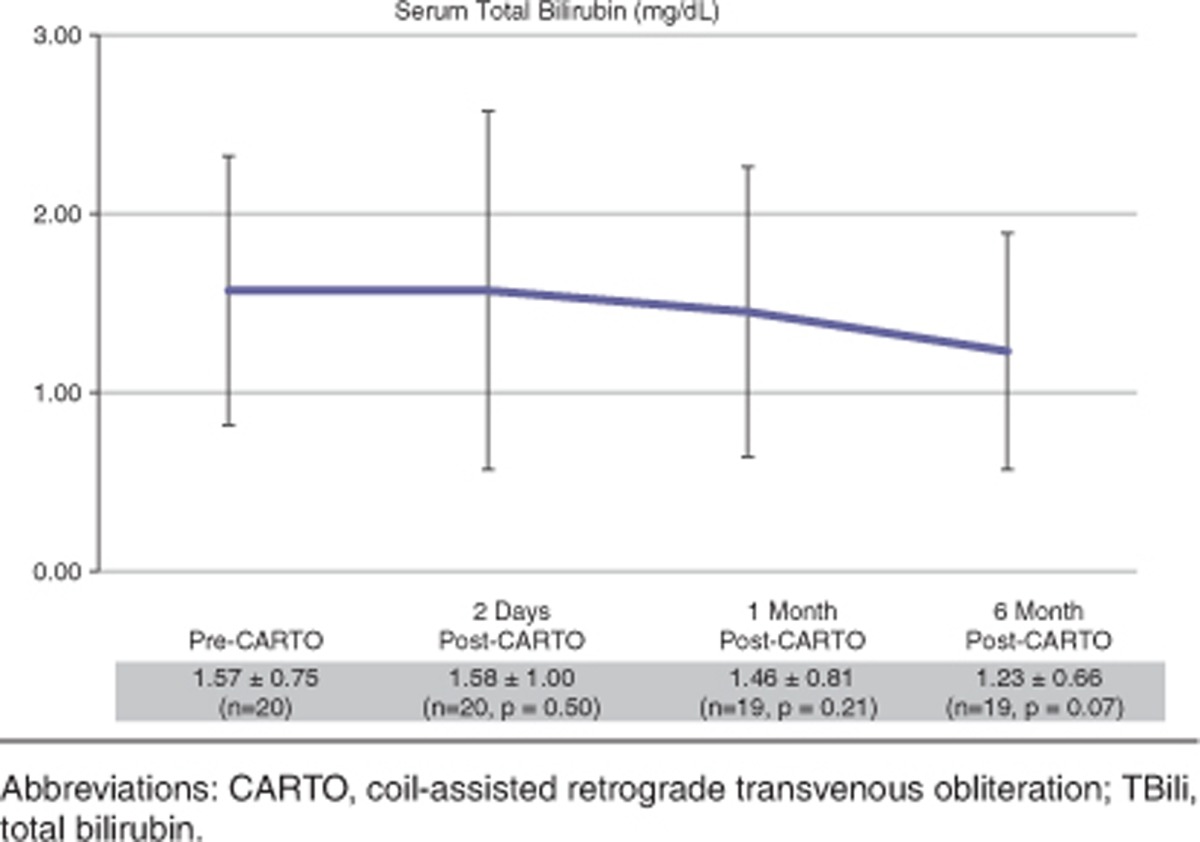
Transient increase in serum total bilirubin (TBili) level was noted in 12 patients with normalizing or down-trending levels within 2 days and complete normalization at 1 month follow-up in 10 patients. The mean pre-CARTO TBili in 10 patients was 1.3±0.6 and post-CARTO TBili was 1.5±0.6 at 2 days and 1.2±0.5 at 1 month. Two patients had increased and persistently elevated TBili at 1 month without improvement. Both patients had higher baseline TBili values of 2.8 and 6.7 mg/dl, respectively. Conversely, eight patients had immediate improvement in TBili at 2 days and of these eight, six patients had persistently improved TBili at 1 month.
Of note, the mean serum ammonia level in patients tested (n=12) pre-CARTO was 124.5±35.2 and post-CARTO was 91.0±28.7 at 2 days and 77.1±14.0 at 1 month. Table 2 demonstrates serum Tbili level changes over the course of follow-up.
Table 2. Serum Tbili level changes over the course of follow-up.
Follow-up and complications
The mean follow-up period was 384±154 days (range 24–622 days). Of the 13 patients who had known esophageal varices, 3 had endoscopic evidence of worsened esophageal varices compared with pre-CARTO evaluation. However, to date, no patients have experienced esophageal variceal bleeding. Of the 20 patients, 4 patients had episodes of upper gastrointestinal bleeding during the follow-up, which were all caused by portal hypertensive gastropathy without variceal bleeding (20%). Five patients had new ascites/hydrothorax or worsened ascites after CARTO (25%). One patient passed away at 24 days with a diagnosis of systemic and portal venous thrombosis and multi-organ failure. Otherwise, no evidence of portal or splenic vein thrombosis, renal vein or caval thrombosis, renal failure, or pulmonary embolization was observed in 19 patients. No patients showed signs of hepatic encephalopathy. Nineteen patients are alive as of June 2014.
DISCUSSION
In this study, we demonstrated that BRTO/CARTO can effectively treat portal hypertensive variceal bleeding, including GVB, without using an indwelling balloon catheter. Without an indwelling balloon catheter, we were able to modify and perform CARTO in 20 patients with immediate cessation of variceal bleeding. Our study showed that CARTO can achieve a technical success rate of 100% and a clinical success rate of 100% at 6-month follow-up in 19 patients, which is concordant with previously reported results for conventional BRTO and modified BRTO.1, 2, 3,7, 8, 9, 10,17,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 CARTO was successfully used to treat any non-esophageal variceal bleeding, including gastric, duodenal, jejunal, and stomal variceal bleeding.
The indwelling balloon in BRTO performs two functions: (1) it provides complete stasis within the gastrorenal shunt to promote sclerosis, and hence it promotes thrombosis of the shunt and varices, and (2) it provides complete blockage of gastrorenal shunt flow into the systemic circulation to prevent any sclerosant leakage. However, due to long balloon indwelling time (4–48 h), the indwelling balloon may be a nidus for complications such as increased risk of balloon rupture, access site complications, and infection, as well as logistical challenges for hospitals such as patient discomfort/inconvenience, intensive care unit or higher level monitored bed requirement, and additional staff.1, 2, 3,7, 8, 9, 10,17,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Therefore, it inevitably increases the periprocedural/postprocedural cost. Some of these issues were resolved with a recently proposed modified BRTO using a vascular plug.25 By using a more permanent vascular occlusive device, the vascular plug, the complications and logistical issues with an indwelling balloon catheter are successfully resolved. However, the vascular plug has its own limitations, including (1) the fact that the vascular plug may not create complete occlusion of a large gastrorenal shunt with very rapid blood flow due to its mesh material, and (2) the vascular plug is only available up to 22 mm, which can block gastrorenal shunts with diameters up to 16–18 mm, but cannot be used for larger sizes. In our experience, coils can be used for shunts measuring up to 25–30 mm size, and with enough coils, a complete occlusion can be achieved without leakage. Like the vascular plug, coils also create permanent vascular occlusion while avoiding some of the complications and challenges associated with BRTO and its usage of indwelling balloon catheters.
In addition to replacing indwelling balloon catheters with detachable coils, we also used gelfoam slurry instead of sclerosing agents to reduce some of the previously reported serious complications associated with BRTO using sclerosing agents. These include death, pulmonary embolism, pulmonary edema, portal vein and/or splenic vein thrombosis, renal vein thrombosis, renal failure, and anaphylactic reaction.3,4,9,16, 17, 18, 19, 20, 21, 22, 23, 24 In our study, although we had one death post-CARTO, which is likely due to the patient's underlying liver disease and co-morbilities (multiple medical problems, including chronic renal failure and diabetes), we had no major complications associated with gelfoam slurry. One can argue that gelfoam slurry in the gastrorenal shunt and gastric varices may have triggered a cascade of hypercoagulapathy in this patient that may have led to systemic and portal venous thrombosis. However, the gelfoam has been utilized in other numerous embolization studies in our institution and have not shown to trigger such complications. Unfortunately, we were unable to prove this patient's hypercoagulable state as the patient's family refused a postmortem autopsy. Physiologically expected complications of BRTO were noted in a small number of patients who underwent CARTO, including transient elevation of liver function tests, including TBili and worsening of ascites and/or hydrothorax. The liver function tests have normalized to baseline levels in all the patients except for 2 patients who had relatively higher levels of initial TBili prior to the CARTO procedure. No patients developed hepatic encephalopathy, which is commonly associated with TIPS placement, an alternative treatment for variceal bleeding. Due to re-direction of portal flow from gastric varices to esophageal varices after CARTO/BRTO, several patients had endoscopically visualized enlarged esophageal varices, but no patient has experienced esophageal variceal bleeding at the time of review. In their 10-year follow-up study, Akahoshi et al.2 demonstrated up to 30% esophageal bleeding after BRTO.
The short- and long-term follow-up clinical data for CARTO is acceptable in our cohort. The rebleeding rate is the most important clinical parameter for the treatment of gastric varices as it is associated with a high mortality rate. In our study with a limited sample size, CARTO demonstrated a 0% rebleeding rate at 1 year in 8 patients and at 6 months in 11 patients. This is comparable to previously reported outcomes from Asia with traditional BRTO.2,3,8,10,17,25,26,29,30,33, 34, 35, 36 Four patients with episodes of upper gastrointestinal bleeding were found to have portal hypertensive gastropathy and demonstrated slow oozing from the gastric lumen without variceal bleeding. These patients were all conservatively managed. As it appears, CARTO may have survival benefits in these selected patients with acute variceal bleeding. All the 19 patients are alive, and 11 patients remain on the liver transplant list at our institution at the time of manuscript submission. This survival benefit should be further evaluated with larger sample sizes and longer follow-up.
At our institution, any patients with active upper gastrointestinal bleeding would receive an upper endoscopy first to identify the location of bleeding source. Once it is found to be a GVB, the abdominal CT is utilized to identify the gastrorenal shunt, and CARTO would be performed to stop the bleeding. Especially in patients with a high model for end-stage liver disease (MELD) score (>19), isolated GVB, and high probability or fear of hepatic encephalopathy, CARTO is a preferred treatment method. However, in patients with simultaneous esophageal and GVB that is not controlled with endoscopic treatment, TIPS is considered.
Our study has some limitations. Due to its retrospective nature, the conclusion and outcomes of this study could be subject to bias and are not as definitive as a prospective trial. However, this study's purpose is to report a novel technique that may be added to our current armamentarium of treating patients with portal hypertension and the sequelae of variceal bleeding. Although only 20 patients were evaluated, based on our results, this technique appears to be an excellent alternative to currently practiced traditional BRTO or modified BRTO. Additional studies such as a multi-centered, randomized controlled trial may be performed to further evaluate the outcome of this study, such as comparing clinical outcomes of TIPS vs. CARTO and longer-term follow-up of CARTO.
In summary, with our preliminary outcomes data, including efficacy and complications, CARTO appears to be a safe and effective treatment option for the treatment of portal hypertensive variceal bleeding, including gastric, duodenal, jejunal, stomal, and rectal varices. The effectiveness appears to be comparable to previously reported modified BRTO and traditional BRTO.
Study Highlights
Guarantor of the article: Edward W. Lee, MD, PhD.
Specific author contributions: Edward W. Lee, MD, PhD—conceiving, initiating, and writing up; Sammy Saab, MD; Antoinette S. Gomes, MD; Ronald Busuttil, MD, PhD; Justin McWilliams, MD; Francisco Durazo, MD; Steven-Huy Han, MD; Leonard Goldstein, MD; Bashir A. Tafti, MD; John Moriarty, MD; Christopher T. Loh, M; and Stephen T. Kee, MD—conceiving and writing up
Financial support: None.
Potential competing interests: None.
References
- Akahane T, Iwasaki T, Kobayashi N, et al. Changes in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration. Am J Gastroenterol. 1997;92:1026–1030. [PubMed] [Google Scholar]
- Akahoshi T, Hashizume M, Tomikawa M, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol. 2008;23:1702–1709. doi: 10.1111/j.1440-1746.2008.05549.x. [DOI] [PubMed] [Google Scholar]
- Cho SK, Shin SW, Lee IH, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007;189:W365–W372. doi: 10.2214/AJR.07.2266. [DOI] [PubMed] [Google Scholar]
- Choi SY, Won JY, Kim KA, et al. Foam sclerotherapy using polidocanol for balloon-occluded retrograde transvenous obliteration (BRTO) Eur Radiol. 2011;21:122–129. doi: 10.1007/s00330-010-1895-3. [DOI] [PubMed] [Google Scholar]
- Kanagawa H, Mima S, Kouyama H, et al. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11:51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Koito K, Namieno T, Nagakawa T, et al. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996;167:1317–1320. doi: 10.2214/ajr.167.5.8911204. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Hamamoto N, Nomura T, et al. Balloon-occluded retrograde transvenous obliteration of high risk gastric fundal varices. Am J Gastroenterol. 1999;94:643–649. doi: 10.1111/j.1572-0241.1999.00928.x. [DOI] [PubMed] [Google Scholar]
- Ninoi T, Nishida N, Kaminou T, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340–1346. doi: 10.2214/ajr.184.4.01841340. [DOI] [PubMed] [Google Scholar]
- Sabri SS, Swee W, Turba UC, et al. Bleeding gastric varices obliteration with balloon-occluded retrograde transvenous obliteration using sodium tetradecyl sulfate foam J Vasc Interv Radiol 201122309–316.quiz 316. [DOI] [PubMed] [Google Scholar]
- Sonomura T, Sato M, Kishi K, et al. Balloon-occluded retrograde transvenous obliteration for gastric varices: a feasibility study. Cardiovasc Intervent Radiol. 1998;21:27–30. doi: 10.1007/s002709900206. [DOI] [PubMed] [Google Scholar]
- de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645–663. doi: 10.1016/s1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Pagan JC, Barrufet M, Cardenas A, et al. Management of gastric varices. Clin Gastroenterol Hepatol. 2013;12:919–928. doi: 10.1016/j.cgh.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Lopera JE. Gastric varices. Radiographics. 2013;33:100–101. doi: 10.1148/rg.3311125194. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Agarwal SR.Gastric varices and portal hypertensive gastropathy Clin Liver Dis 20015727–767., x. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- Bellary SV, Isaacs P. Disseminated intravascular coagulation (DIC) after endoscopic injection sclerotherapy with ethanolamine oleate. Endoscopy. 1990;22:151. doi: 10.1055/s-2007-1012824. [DOI] [PubMed] [Google Scholar]
- Chikamori F, Kuniyoshi N, Shibuya S, et al. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001;129:414–420. doi: 10.1067/msy.2001.112000. [DOI] [PubMed] [Google Scholar]
- Hirota S, Matsumoto S, Tomita M, et al. Retrograde transvenous obliteration of gastric varices. Radiology. 1999;211:349–356. doi: 10.1148/radiology.211.2.r99ma25349. [DOI] [PubMed] [Google Scholar]
- Lee JY, Moon SH, Lee SM, et al. A case of noncardiogenic pulmonary edema by ethanolamine oleate. Korean J Intern Med. 1994;9:125–127. doi: 10.3904/kjim.1994.9.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad WE, Sabri SS. Balloon-occluded retrograde transvenous obliteration (BRTO): technical results and outcomes. Semin Intervent Radiol. 2011;28:333–338. doi: 10.1055/s-0031-1284460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SK, Shin SW, Do YS, et al. Development of thrombus in the major systemic and portal veins after balloon-occluded retrograde transvenous obliteration for treating gastric variceal bleeding: its frequency and outcome evaluation with CT. J Vasc Interv Radiol. 2008;19:529–538. doi: 10.1016/j.jvir.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Akasaka T, Shibata T, Isoda H, et al. Septic complication after balloon-occluded retrograde transvenous obliteration of duodenal variceal bleeding. Cardiovasc Intervent Radiol. 2010;33:1257–1261. doi: 10.1007/s00270-009-9740-2. [DOI] [PubMed] [Google Scholar]
- Shimoda R, Horiuchi K, Hagiwara S, et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging. 2005;30:306–313. doi: 10.1007/s00261-004-0270-8. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shiozawa K, Ikehara T, et al. Short-term effects and early complications of balloon-occluded retrograde transvenous obliteration for gastric varices. ISRN Gastroenterol. 2012;2012:919371. doi: 10.5402/2012/919371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwon DI, Ko GY, Yoon HK, et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration—a primary report. Radiology. 2013;268:281–287. doi: 10.1148/radiol.13122102. [DOI] [PubMed] [Google Scholar]
- Arai H, Abe T, Shimoda R, et al. Emergency balloon-occluded retrograde transvenous obliteration for gastric varices. J Gastroenterol. 2005;40:964–971. doi: 10.1007/s00535-005-1654-4. [DOI] [PubMed] [Google Scholar]
- Araki T, Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices from unconventional systemic veins in the absence of gastrorenal shunts. Tech Vasc Interv Radiol. 2012;15:241–253. doi: 10.1053/j.tvir.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Cho SK, Shin SW, Yoo EY, et al. The short-term effects of balloon-occluded retrograde transvenous obliteration, for treating gastric variceal bleeding, on portal hypertensive changes: a CT evaluation. Korean J Radiol. 2007;8:520–530. doi: 10.3348/kjr.2007.8.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Yoon CJ, Park JH, et al. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4:109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12:327–336. doi: 10.1016/s1051-0443(07)61912-5. [DOI] [PubMed] [Google Scholar]
- Haruta I, Isobe Y, Ueno E, et al. Balloon-occluded retrograde transvenous obliteration (BRTO), a promising nonsurgical therapy for ectopic varices: a case report of successful treatment of duodenal varices by BRTO. Am J Gastroenterol. 1996;91:2594–2597. [PubMed] [Google Scholar]
- Hashimoto N, Akahoshi T, Yoshida D, et al. The efficacy of balloon-occluded retrograde transvenous obliteration on small intestinal variceal bleeding. Surgery. 2010;148:145–150. doi: 10.1016/j.surg.2009.10.052. [DOI] [PubMed] [Google Scholar]
- Hiraga N, Aikata H, Takaki S, et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007;42:663–672. doi: 10.1007/s00535-007-2077-1. [DOI] [PubMed] [Google Scholar]
- Jang SY, Kim GH, Park SY, et al. Clinical outcomes of balloon-occluded retrograde transvenous obliteration for the treatment of gastric variceal hemorrhage in Korean patients with liver cirrhosis: a retrospective multicenter study. Clin Mol Hepatol. 2012;18:368–374. doi: 10.3350/cmh.2012.18.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto M, Imamura M, Kamada K, et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002;178:1167–1174. doi: 10.2214/ajr.178.5.1781167. [DOI] [PubMed] [Google Scholar]
- Kumamoto M, Toyonaga A, Inoue H, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25:1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Torii N, Yatsuji S, et al. Long-term follow up of esophageal varices after balloon-occluded retrograde transvenous obliteration for gastric varices. Hepatol Res. 2008;38:340–347. doi: 10.1111/j.1872-034X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Okugawa H, Maruyama H, Kobayashi S, et al. Therapeutic effect of balloon-occluded retrograde transvenous obliteration for gastric varices in relation to haemodynamics in the short gastric vein. Br J Radiol. 2009;82:930–935. doi: 10.1259/bjr/28956799. [DOI] [PubMed] [Google Scholar]