Abstract
Autism spectrum disorder (ASD) has been characterized by atypical socio-communicative behavior, sensorimotor impairment and abnormal neurodevelopmental trajectories. DTI has been used to determine the presence and nature of abnormality in white matter integrity that may contribute to the behavioral phenomena that characterize ASD. Although atypical patterns of sensory responding in ASD are well documented in the behavioral literature, much less is known about the neural networks associated with aberrant sensory processing. To address the roles of basic sensory, sensory association and early attentional processes in sensory responsiveness in ASD, our investigation focused on five white matter fiber tracts known to be involved in these various stages of sensory processing: superior corona radiata, centrum semiovale, inferior longitudinal fasciculus, posterior limb of the internal capsule, and splenium. We acquired high angular resolution diffusion images from 32 children with ASD and 26 typically developing children between the ages of 5 and 8. We also administered sensory assessments to examine brain-behavior relationships between white matter integrity and sensory variables. Our findings suggest a modulatory role of the inferior longitudinal fasciculus and splenium in atypical sensorimotor and early attention processes in ASD. Increased tactile defensiveness was found to be related to reduced fractional anisotropy in the inferior longitudinal fasciculus, which may reflect an aberrant connection between limbic structures in the temporal lobe and the inferior parietal cortex. Our findings also corroborate the modulatory role of the splenium in attentional orienting, but suggest the possibility of a more diffuse or separable network for social orienting in ASD. Future investigation should consider the use of whole brain analyses for a more robust assessment of white matter microstructure.
Highlights
-
•
First study of ASD to link observed sensory behaviors to white matter integrity
-
•
Findings suggest that aberrant limbic connectivity is related to tactile defensiveness.
-
•
Findings corroborate modulatory role of splenium in orienting.
-
•
Findings suggest a more diffuse or separable network for social orienting in ASD.
1. Introduction
1.1. DTI studies of typical white matter development and abnormalities in ASD
Autism spectrum disorder (ASD) has been characterized by atypical socio-communicative behavior, sensorimotor impairment and abnormal neurodevelopmental trajectories. Diffusion tensor imaging (DTI), which measures the displacement of water molecules in the brain (Basser et al., 1994; Le Bihan et al., 2001) and is used to characterize white matter microstructure, has been used to describe both typical and aberrant white matter development. White matter volume increases with typical development in all four major lobes of the brain, with the most rapid increases occurring before age 10 (Giedd et al., 1999; Giedd, 2004; Iwasaki et al., 1997; Pfefferbaum et al., 1994; Rivkin, 2000) and progressing in parallel with regional maturation of function. Higher fractional anisotropy (FA, a measure that reflects the orientational coherence of fiber tracts) and a lower apparent diffusion coefficient (ADC, an intravoxel measure of diffusion magnitude) tend to reflect more developed tracts with higher signal transmission speeds (Basser & Pierpaoli, 2011; Bonekamp et al., 2007; Cascio et al., 2007).
DTI has been used to determine the presence and nature of white matter abnormalities that may contribute to the behavioral phenomena that characterize ASD. This literature has been reviewed recently (Aoki et al., 2013; Travers et al., 2012), and suggests that although widespread differences in white matter integrity have been reported (Cheng et al., 2010; Shukla et al., 2011), the most commonly replicated findings involve the corpus callosum, cingulum bundle, superior longitudinal fasciculus, and temporal white matter tracts. A lack of a clear consensus likely reflects methodological differences, including means of addressing data quality, choice of comparison groups, and inclusion criteria such as age and developmental level. One large scale study suggested that when groups were carefully matched on degree of motion, the only apparent FA differences were in the inferior longitudinal fasciculus (Koldewyn et al., 2014). In addition to controlling for motion, another important way to clarify white matter differences specific to ASD is to control for age and development. While this is best accomplished with large scale longitudinal studies, another approach is to use cross-sectional studies with a focus on narrow age ranges. This approach ameliorates the masking of differences that could occur through averaging a range of developmental white matter profiles into a single sample.
1.2. Sensory symptoms of ASD and putative neural correlates
Sensory processing abnormalities have been reported in ASD since the earliest clinical and autobiographical accounts (Cesaroni & Garber, 1991; Grandin & Scariano, 1986; Kanner, 1943), and have been added to the diagnostic criteria for ASD in the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association, 2013). Among the specific sensory symptoms featured in the DSM-5 are sensory hyper-responsiveness (an oversensitivity to sensory stimuli that often include a defensive reaction such as covering one's ears to an innocuous sound) and sensory hypo-responsiveness (a depressed sensitivity that includes failure to orient to salient stimuli, e.g., pain; Baranek et al., 2006; Ben-Sasson et al., 2009). These sensory patterns relate to both the social communication impairments (Brock et al., 2012; Foss-Feig et al., 2012; Watson et al., 2011) and restricted and repetitive behaviors that characterize ASD (Baranek et al., 1997; Boyd et al., 2010; Foss-Feig et al., 2012; Wiggins et al., 2009). A third pattern of sensory responding in ASD – sensory seeking (unusual interest in sensory properties of environmental stimuli) – is less understood, but has been theorized to serve as a compensatory mechanism for both hypo-responsiveness (e.g., seeking to increase sensory input to overcome high thresholds; Dunn, 1997) and hyper-responsiveness (e.g., seeking limited, repetitive sensory stimuli to soothe over-arousal; Liss et al., 2006).
Although these patterns of sensory responding in ASD are well documented in the behavioral literature, much less is known about the neural networks associated with processing basic sensory stimuli in ASD. A recent fMRI study using simple auditory and visual stimuli showed increased activation in the primary sensory cortices, as well as in limbic areas related to emotion processing and regulation in children with ASD, relative to controls. These findings suggest atypical lower (i.e., at the level of primary or association sensory cortex) and higher (i.e., at the level of attentional or limbic cortices) order processing of sensory stimuli in ASD (Green et al., 2013). On the contrary, previous fMRI studies investigating both visual (Hadjikhani et al., 2004) and auditory (Gomot et al., 2008) stimuli report intact processing in primary sensory regions. Similarly, ERP studies routinely note higher order processing abnormalities (e.g., Ceponiene et al., 2003), with a subset also showing early (lower order) sensory differences (Donkers et al., 2013). The complexity of the sensory stimulus (Bertone & Faubert, 2003; Bertone et al., 2005) and the degree of social relevance (Greene et al., 2011) also play important roles in neural processing, and behavioral data further suggest a potentially important distinction between social and nonsocial sensory orienting in ASD (Baranek et al., 2013).
1.3. White matter tracts for sensory processing and orienting
In this study, our goal was to focus on white matter tracts with known roles in sensorimotor processing, and in early attentional processes, including alerting and orienting, which are relevant to aberrant sensory behaviors seen in ASD. The superior corona radiata (SCR) and centrum semiovale (CS) contain both motor and sensory fibers projecting to and from the anterior parietal and posterior frontal lobes. The integrity of the fibers contained in these pathways may modulate the transmission of cortical sensory signals and subsequently impact reactivity patterns in ASD, such as hypo- or hyper-responsiveness, implicating primary involvement of early sensory processing, rather than attention or limbic processes. The inferior longitudinal fasciculus (ILF) carries fibers between the occipital, temporal, and parietal sensory association cortex (Martino & De Lucas, 2014; Schmahmann et al., 2007) and may be important for linking integrated sensory input with limbic structures for the evaluation of affective significance, thus its integrity in ASD might reflect the degree to which higher order processing drives sensory abnormalities.
Each of three component functional processes in attention – alerting, orienting and executive function – have been linked to a unique neural network (Fan et al., 2009; Posner & Petersen, 1990; Posner & Rothbart, 2007; Posner et al., 2006; Raz & Buhle, 2006). Fibers carried by the posterior limb of the internal capsule (PLIC) are associated with the function and modulation of attentional alerting (Callejas et al., 2005; Fan et al., 2009; Fan et al., 2005; Fimm et al., 2001; Rueda et al., 2004; Sturm & Willmes, 2001; Yin et al., 2012), while the splenium of the corpus callosum (SPLEN) is heavily linked to orienting (Luders et al., 2009; Noudoost et al., 2006; Weber et al., 2005). Niogi et al. (2010) found correlations between FA in the SPLEN and orienting, and between FA in the PLIC and alerting. Thus, we focused our investigation on these five tracts (SCR, CS, ILF, PLIC, SPLEN) in order to address the roles of basic sensory (SCR, CS), sensory association (ILF), and early attentional processes (PLIC, SPLEN) in sensory hyper- and hypo-responsiveness in ASD.
2. Methods
2.1. Participant characterization
Thirty-two children with ASD and 26 typically developing (TD) children between the ages of 5 and 8 years completed this study. After excluding participants with poor image quality resulting from excessive motion (n = 13) and scanner/acquisition errors(n = 4), the final sample resulted in 19 children with ASD (7.34 years ± 0.72; 17 males) and 22 children with TD (7.10 years ± 1.11; 18 males). Within each group, included and excluded participants did not differ in chronological age, mental age, or autism severity as measured by the ADOS (all ps > .1). Participants in the ASD group were recruited from the university medical center and surrounding community, and a diagnosis of ASD was confirmed with research-reliable administration of the Autism Diagnostic Observation Schedule (ADOS; Gotham et al., 2007) and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), as well as the judgment of a licensed clinical psychologist based on DSM (4th ed.; DSM-IV; American Psychiatric Association (2000)) criteria. Participants in the TD control group were excluded if they had a diagnosed psychiatric or learning disorder or had a first-degree relative with ASD. Additionally, control participants were screened using the Social Communication Questionnaire (SCQ; Berument et al., 1999; Rutter et al., 2003) and the Child Behavior Checklist (CBCL; Achenbach et al., 2001) to confirm that ASD and other psychiatric symptomatology did not reach an at-risk level for diagnosis. All participants were screened and excluded for any genetic and neurological problems, had not experienced head injuries, and were free of all MRI contraindications.
2.2. Cognitive and sensory assessments
Participants' cognitive ability was assessed by trained research assistants using the Kaufman Brief Intelligence Test, Second Edition (KBIT-2; Kaufman & Kaufman, 2004) or Mullen Scales of Early Learning (MSEL; Mullen, 1995), dependent on the language level of the participant. Nonverbal and verbal mental age scores were calculated using mental age equivalents provided in the KBIT-2 and MSEL manuals. Although the groups did not differ on chronological age, mental age was significantly higher in the TD group (ASD: 7.01 ± 2.11, TD: 8.94 ± 2.37, t(41) = −2.81, p = 0.008), which was driven by verbal mental age (ASD: 6.47 ± 1.59, TD: 9.00 ± 1.98, t(41) = −4.5, p < 0.001). Nonverbal mental age, however, did not differ significantly between groups (ASD: 7.55 ± 3.22, TD: 8.87 ± 3.04, t(41) = −1.38, p = 0.17). See Table 1 for a summary of participant characteristics.
Table 1.
Participant characterization and sensory scores for ASD and TD groups. For each group (ASD = autism spectrum disorder; TD = typically developing), means and standard deviations are reported for participant characteristics, including chronological age, sex and mental age (calculated using the KBIT-2 or the MSEL). Ranges are also reported for chronological and mental age. Group mean sensory scores (calculated using the SPA and TDDT-R) are also reported. For each variable, between-group comparison values (t- or χ2 tests) are reported with corresponding p values.
| Group | Age | % Male | Mental age (Mean ± SD) |
Sensory score (mean rank) |
||||
|---|---|---|---|---|---|---|---|---|
| Average | Nonverbal | Verbal | Social Orienting |
Nonsocial Orienting |
Tactile Defesnsive |
|||
| ASD (N = 19) | 7.34 (±0.72) | 89.47% | 6.96 (±2.22) | 7.49 (±3.40) | 6.43 (±1.68) | 25.03 | 21.97 | 26.37 |
| Range | 5.9–8.4 | 3.0–13.0 | 3.17–18.5 | 2.63–9.5 | ||||
| TD (N = 22) | 7.1 (±1.11) | 81.81% | 9.10 (±1.92) | 9.06 (±2.98) | 9.14 (±1.92) | 17.52 | 20.16 | 16.36 |
| Range | 5.3–8.9 | 5.0–14.0 | 5.0–16.0 | 5.83–13.0 | ||||
| Test statistic | t = 0.843 | χ2 = 0.478 | t = –2.98 | t = –1.56 | t = –4.69 | U = 132.5 | U = 190.5 | U = 107 |
| p-value | 0.404 | 0.489 | 0.005 | 0.127 | <0.001 | 0.033a | 0.622 | 0.008a |
Statistically significant between-group difference in sensory score.
Participants completed two structured sensory assessments — the Sensory Processing Assessment (SPA; Baranek, 1999) and the Tactile Defensiveness and Discrimination Test-Revised (TDDT-R; Baranek, 2010), administered by trained personnel and consensus coded by blind raters under the supervision of a team member who had achieved reliability with the author of the instruments. Both assessments are play-based and involve toys and activities that have specific sensory features. The SPA measures response to sensory stimuli across multiple sensory domains, with novel toys presented to measure both sensory avoidance and sensory fascination/repetitive engagement, while simultaneously presenting both social (name call, tapping of shoulder, and hand wave) and nonsocial (sound stick, air puff to the back of the neck, and a light flash) distracter items to observe orientation and habituation patterns to such salient stimuli. The TDDT-R includes self-directed activities and experimenter-administered items to assess sensory defensiveness and seeking, specifically limited to the tactile domain. Scores from four sensory measures of interest were included in this study: two general sensory orientation measures from the SPA (‘social orienting’ and ‘non-social orienting’) and two tactile-specific measures from the TDDT-R (‘tactile seeking’ and ‘tactile defensiveness’). High scores on each measure are associated with more atypical sensory processing patterns.
2.3. Image processing
All images were acquired during a single scan session on a 3 Tesla Philips Achieva MRI scanner (Philips Healthcare, Inc., Best, Netherlands), located at the Vanderbilt University Institute of Imaging Science. During scanning procedures, participants wore foam earplugs in both ears and Philips headphones to attenuate noise, and watched a video of their choice for the duration of the scan. A high-resolution T1-weighted anatomical volume (TR = 9 ms, TE = 4.6 ms, FOV = 256 mm2, 1 mm isotropic voxels, 170 sagittal slices, 6 min 30 s duration) was collected to provide a template for image registration. Diffusion weighted data were acquired using a high angular-resolution diffusion imaging (HARDI) sequence (2.5 mm2 isotropic voxels, 50 axial slices, 14 min 34 s). We collected 92 diffusion directions (b = 1600 s/mm2) and one T2-weighted volume(b = 0 s/mm2).
A novel image processing pipeline was developed to measure FA and ADC in the SCR, CS, ILF, PLIC and SPLEN of individual brains (Fig. 1). All images were visually inspected for common artifacts such as fat shift and ghosting and underwent standard preprocessing and quality assurance procedures that incorporated head motion, artifact propensity, variance, and bias of estimated measures (Lauzon et al., 2013). A QA rating between 1 and 5 was assigned based on these measures and only scans with ratings above 3 were included in the analysis. HARDI data were eddy current and motion corrected, and skull stripped in FMRIB Software Library (FSL; Jenkinson et al., 2012; Smith et al., 2004). Raw T1-weighted images were re-oriented along the anterior commissure–posterior commissure (ACPC) line in Brain Voyager (Formisano et al., 2005; Goebel et al., 2006), then skull stripped in FSL. Each subject's brain-extracted HARDI and T1W/3D images were coregistered, and a tensor fit was performed for each ACPC-oriented HARDI image in DTI Studio (Jiang et al., 2006). Pixel-based outlier rejection was used to eliminate noisy pixels by the following threshold criteria: “Minimum bad area” = 80 (based on recommended value of 30 pixels per 1 mm2), “Minimum Z-value” = 3 (standard deviations from global mean signal), and “Minimum B0-value” = 100 (intensity threshold to remove floor noise). The proportion of rejected pixels did not differ significantly between groups (t(39) = 1.06, p = .299; see also Supplementary Table S1). Tensor fit output files were used as input in Reproducible Objective Quantification Scheme (ROQS), a software-based tool to obtain regional white matter measurements of diffusion tensor imaging parameters (Niogi et al., 2007).
Fig. 1.
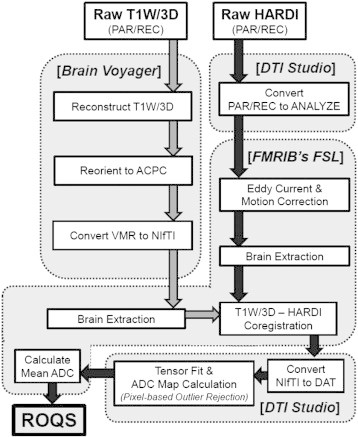
Image processing pipeline. Raw T1W/3D (3D T1-weighted image; PAR/REC = Philips image file format) image was reconstructed and reoriented to ACPC (anterior commissure–posterior commissure orientation) using Brain Voyager software (VMR = file format inherent to Brain Voyager software); following, the T1W/3D image was brain extracted in FMRIB's FSL software program. HARDI (high angular-resolution diffusion image) data were converted to Analyze format in DTI Studio and motion corrected, eddy current corrected and brain extracted in FSL. Preprocessed T1W/3D images were coregistered with preprocessed HARDI data using FSL. Coregistered images were subsequently converted to DAT file format in DTI Studio where tensor fit and ADC calculation was then performed, incorporating pixel-based outlier rejection. The mean ADC map, calculated using FSL, and other files resulting from the tensor fit were used as input files to perform image analysis in Reproducible Objective Quantification Scheme (ROQS) software.
ROQS exploits fiber information from the diffusion tensor to semi-automatically segment anatomically distinct WM fiber tracts for quantitative DTI analysis. ROQS is able to segment WM fiber tracts faster than manual delineation and with better reproducibility and accuracy. For each brain, nine WM fiber tracts were delineated on a best-fit 2D slice: SPLEN, and bilaterally CS, SCR, PLIC, and ILF (Fig. 2, Supplementary Fig. S1). Bilateral fiber tracts were delineated separately for each side. We obtained measures of FA and ADC (mean diffusivity) from each tract, for each individual, calculated in native space. For the TD group and the ASD group separately, within each tract, individual outliers (having an individual FA or ADC value greater or less than 3 standard deviations from the group mean) were excluded for quality assurance.
Fig. 2.
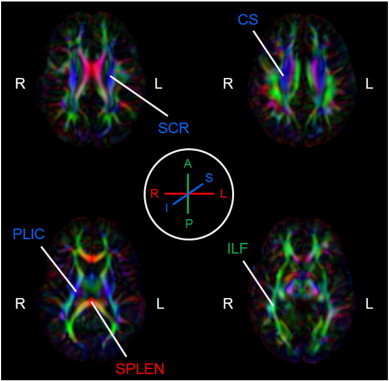
White matter fiber tracts identified in representative participant. White matter fiber tracts include superior corona radiata (SCR), centrum semiovale (CS), posterior limb of the internal capsule (PLIC), splenium (SPLEN) and inferior longitudinal fasciculus (ILF). Tracts are shown on an FA color map of a representative participant, giving fiber orientation – red (right–left), green (anterior–posterior), blue (superior–inferior) – indicated by legend (encircled in middle of figure). Brain is in radiological orientation, as indicated by right (R) and left (L) hemispheric labels.
2.4. Statistical analyses
Group differences in sensory behavior were analyzed using a multivariate analysis of covariance (MANCOVA) test, with group as the independent variable and each of the four sensory behavior scores (nonsocial orienting, social orienting, tactile seeking, tactile defensiveness), as dependent variables. Mental age was used as a covariate because it has been shown to influence sensory responses (Baranek et al., 2006, 2013) and differed between groups. For the DTI data, FA and ADC were analyzed as separate dependent variables, using analysis of covariance (ANCOVA) tests. Laterality (left, right, commissural) and tract (SPLEN, CS, SCR, PLIC, ILF) were within-subject variables while group was the between-subjects variable. Because there was a trend for a group difference (p = 0.0549, see Supplementary Table S1 for details) in image quality rating even after our rigorous QA procedure, the QA rating was included as a covariate. Post hoc, independent samples two-tailed t-tests or Mann–Whitney U tests (for variables where data were not normally distributed, given by a Shapiro–Wilk test) were used to assess between-group differences for each of the four sensory behavior scores and both FA and ADC in each white matter fiber tract.
A Spearman rank correlation test was used to evaluate correlations between significant DTI parameters and sensory behavior scores in the ASD group. Spearman rank was chosen for correlation testing to address the non-normal distribution of most of the sensory variables.
3. Results
3.1. Sensory assessment
There was an overall significant effect of group (F(4,34) = 7.27, p < 0.001), but no effect of mental age (F(4,34) = 1.074, p = 0.384) on sensory scores. ASD group mean scores were higher across all four variables, consistent with more aberrant sensory responsiveness. Follow-up tests revealed significant effects of group for three of the four sensory scores (social orienting: F(1,40) = 4.26, p = 0.046; tactile seeking F(1,40) = 23.93, p < 0.001; and tactile defensiveness: F(1,40) = 4.51, p = 0.041). There was no significant effect of group on nonsocial orienting (F(1,40) = 0.438, p = 0.512).
3.2. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) in white matter fiber tracts
An ANCOVA, with laterality (left, right, or commissural fiber) and individual tract (SPLEN, CS, ILF, SCR, and PLIC) as within-subject variables, group as a between-subjects variable, QA rating as a covariate, and FA as the dependent variable, revealed main effects of group (F(1, 346) = 8.75, p = 0.003) and tract (F(3,346) = 225.5, p < 0.001) as well as a group by tract interaction (F(3,346) = 3.96, p = 0.008). There was not a significant main effect of laterality, or the QA rating covariate (F(1,346) = 1.48, p > .1) nor any other significant interactions. These results indicate tract-specific differences in FA in children with ASD.
Because there was no effect of laterality, right and left FA values for the four bilateral tracts (CS, ILF, SCR, PLIC) were then collapsed into average bilateral values to reduce the number of post-hoc comparisons. A Shapiro–Wilk test for normality revealed normal distributions of FA values within each tract, within each group, with the exceptions of CS in the ASD group (p = 0.041) and PLIC in the TD group (p = 0.049). An analysis of group means with QA rating included as a covariate revealed that FA was significantly lower for the ASD group than the TD group in two tracts (Fig. 3): SPLEN (F(1,38) = 5.36, p = 0.026, ηp2 = .12) and ILF (F(1,36) = 6.14, p = 0.018, ηp2 = .17). There was also a nonsignificant trend for lower FA in the CS (F(1,38) = 3.66, p = 0.063, ηp2 = .14). There was no significant effect of QA rating on any of these tests (all ps > .1). Mean FA values for each collapsed tract in each group and post-hoc analyses are summarized in Table 2.
Fig. 3.
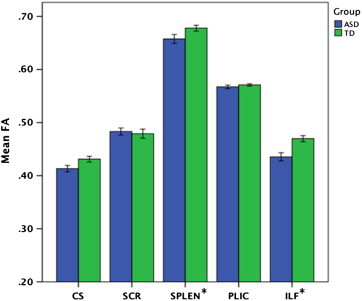
Mean FA by group and tract. Mean fractional anisotropy (FA) for the ASD (blue, N = 19) and TD (green, N = 22) groups for the centrum semiovale (CS), superior corona radiata (SCR), splenium (SPLEN), posterior limb of the internal capsule (PLIC), and inferior longitudinal fasciculus (ILF). Error bars: ±1 SE. * = Statistically significant between-group difference in tract-specific FA.
Table 2.
Mean FA values for ASD and TD groups. For each of the five target tracts, the mean fractional anisotropy (FA) is shown for each group (ASD = autism spectrum disorder; TD = typically developing; F = between-group F-test value with QA rating covaried out; p = p-value). Bilateral tracts have been collapsed due to no main effect of laterality.
| Tract | Fractional anisotropy |
F | p | |
|---|---|---|---|---|
| ASD | TD | |||
| SPLEN | .658 (±.04) | .678 (±.03) | 5.358 | 0.026a |
| CS | .413 (±.03) | .431 (±.03) | 3.663 | 0.063 |
| SCR | .483 (±.03) | .479 (±.05) | 0.28 | 0.599 |
| PLIC | .567 (±.02) | .572 (±.01) | 0.042 | 0.839 |
| ILF | .434 (±.04) | .467 (±.04) | 6.138 | 0.018a |
Statistically significant between-group difference in tract-specific FA.
A separate ANCOVA with ADC as the dependent variable revealed similar main effects. There were significant main effects of group (ASD > TD; F(1, 349) = 4.67, p = 0.031), tract (F(3, 349) = 188.6, p < 0.001), and laterality (F(1, 349) = 20.4, p < 0.001). There was no effect of QA rating and no significant interactions, suggesting generalized increases in ADC in children with ASD. Mean ADC values for each tract in each group are summarized in Table 3.
Table 3.
Mean ADC values for ASD and TD groups. For each of the five target tracts, the mean apparent diffusion coefficient (ADC) is shown for each group (ASD = autism spectrum disorder; TD = typically developing). Bilateral tracts showed a significant main effect of laterality and are therefore displayed individually (R = right; L = left).
| Apparent diffusion coefficient |
||
|---|---|---|
| Tract | ASD | TD |
| SPLEN | 8.65E−04 (±6.20E−05) | 8.48E−04 (±5.19E−05) |
| R CS | 6.68E−04 (±2.35E−05) | 6.70E−04 (±2.26E−05) |
| L CS | 6.84E−04 (±2.80E−05) | 6.79E−04 (±2.39E−05) |
| R SCR | 6.44E−04 (±2.57E−05) | 6.42E−04 (±1.68E−05) |
| L SCR | 6.57E-04 (±1.79E−05) | 6.49E−04 (±1.75E−05) |
| R PLIC | 6.50E−04 (±1.43E−05) | 6.40E−04 (±1.26E−05) |
| L PLIC | 6.60E−04 (±2.00E−05) | 6.47E−04 (±1.62E−05) |
| R ILF | 7.32E−04 (±3.95E−05) | 7.26E−04 (±3.30E−05) |
| L ILF | 7.59E−04 (±2.99E−05) | 7.52E−04 (±4.32E−05) |
3.3. Sensory assessment correlations with WM integrity
We used the Spearman Rank correlations (ρ = correlation coefficient) within groups to test for relationships between the four sensory scores obtained from behavioral observations and FA in three tracts: SPLEN and ILF (the two regions that showed significant differences in post-hoc tests), and CS (considered exploratory as the effect of group did not reach statistical significance). In the ASD group, nonsocial orienting was found to significantly correlate with FA in the SPLEN (ρ = −0.49; p = 0.03) and tactile defensiveness significantly correlated with FA in the ILF (ρ = −0.57; p = 0.01), such that lower FA was associated with more abnormal scores (less orienting and more tactile defensiveness, respectively). These relations between sensory response and FA are depicted in Fig. 4. No significant ASD group correlations were found for FA in the CS. No significant correlations were found within the TD group for any measure.
Fig. 4.
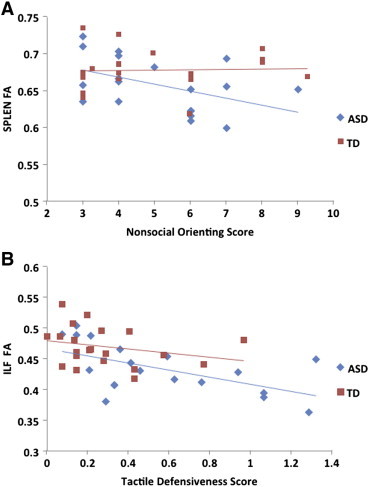
Mean FA by group and tract. Scatter plots depicting relation of (A) nonsocial sensory orienting score and FA value in the SPLEN, and (B) tactile defensiveness and FA value in the ILF. Higher scores on sensory measures indicate more atypical behavior and values were calculated according to the SPA and TDDT-R manuals.
4. Discussion
Consistent with the growing literature supporting pervasive sensory processing impairments in ASD (Marco et al., 2011; Rogers & Ozonoff, 2005), the ASD group scored higher on all sensory variables measured by behavioral observation, suggesting impairments related to tactile processing and sensory orienting across modalities. Failure to orient to salient stimuli is commonly observed in individuals with ASD and has been shown to predict deficits in social-communication abilities (Dawson et al., 2004). In the current study, although social orienting was significantly decreased in the ASD group, nonsocial orienting did not show a difference between groups. This finding suggests some degree of specificity to these commonly observed behavioral deficits, in agreement with previous work (Baranek et al., 2013). It will be important for future studies to examine social and nonsocial orienting separately in order to better understand the scope of orienting deficits in ASD.
A lack of tract-specific differences in ADC suggests a global increase in intravoxel diffusion in the ASD group, consistent with current evidence describing global white matter abnormality in ASD (Alexander et al., 2007; Barnea-Goraly et al., 2004; Brito et al., 2009; Keller et al., 2007; Lee et al., 2007; Shukla et al., 2010; Sundaram et al., 2008). The measurement of ADC is influenced by the complexity of fiber architecture, where higher values indicate simpler configurations such as a single dominant fiber orientation or multiple fibers that cross at a smaller angle (Vos et al., 2012). Under this assumption, globally increased ADC may reflect an aberrantly simple neuroarchitecture in ASD. This supports the idea that, rather than being limited to socio-communicative networks, impairments in ASD affect a range of sensorimotor, socio-communicative and cognitive domains.
There was also a main effect of group for FA whereby FA was decreased in the ASD group. A reduction in FA reflects a loss of white matter integrity caused by underlying microstructural abnormalities that may be influenced by decreased fiber density and/or reduced directional coherence of fiber bundles related to demyelination and/or compromised axonal integrity (Basser & Pierpaoli, 2011; Pierpaoli & Basser, 1996). In contrast to ADC findings, the group by tract interaction for FA and particular brain–behavior relationships suggest tract-specific differences in FA among children with ASD.
Reduced FA in the SPLEN is consistent with previous findings in ASD (Egaas et al., 1995; Frazier & Hardan, 2009; Hardan et al., 2009; Piven et al., 1997; Shukla et al., 2010), and a disruption in SPLEN myelination would support the neurophysiologic profile of ASD as a late information processing disorder (Minshew et al., 1997; Novick et al., 1980). In the ASD group, decreased FA in the SPLEN was related to decreased nonsocial orienting, consistent with a modulatory role for the splenium in orienting patterns (Luders et al., 2009; Noudoost et al., 2006; Weber et al., 2005), although a similar relation was not seen in the TD group. The association between SPLEN FA and nonsocial orienting in ASD corroborates recent evidence that inefficient visual orienting and associated SPLEN white matter integrity reduction may be early markers of risk for ASD (Elison et al., 2013). Decreased FA in this region has also been associated with sensory inattention in a sample of children with sensory processing disorder (Owen et al., 2013), which may relate to the current finding of reduced SPLEN FA and orienting in ASD. Although the association between sensory orienting behaviors and the SPLEN (Niogi et al., 2010) and its relevance for ASD (Elison et al., 2013) have been reported previously, the specific relationship to nonsocial (and not to social) orienting was surprising. In particular, even though behavioral evidence suggested specificity related to social orienting deficits in ASD, the brain–behavior relationship revealed a pattern specific to nonsocial orienting in ASD. Imaging studies have shown a number of brain regions that are preferentially involved in social orienting, including the extrastriate cortex (Engell et al., 2010; Greene et al., 2009; Hietanen et al., 2006; Tipper et al., 2008), inferior frontal gyrus (Engell et al., 2010), medial frontal cortex (Tipper et al., 2008), and superior temporal sulcus (Kingstone et al., 2004). Therefore, it is possible that a more diffuse network is involved in orientation to social stimuli and relies less on the specific modulatory role of the SPLEN.
Reduced white matter integrity in the ILF is consistent with previous studies (Jou et al., 2011; Koldewyn et al., 2014; Shukla et al., 2011). The ILF primarily comprises association fibers that connect ventral temporal and occipital regions (Schmahmann et al., 2007). It has been heavily associated with social functions (Peters et al., 2011) that are affected in ASD, such as face processing (Philippi et al., 2009; Tavor et al., 2014). Reduced ILF FA in the ASD group may reflect decreased myelination or diminished microstructural integrity of these white matter fibers, suggesting differences at the level of sensory association and limbic processing. Although reduced FA in the ILF in ASD replicated previous studies, the correlation between FA in the ILF and tactile defensiveness in the ASD group was a novel finding. The vertical branch of the ILF connects temporal limbic structures with the inferior parietal lobule (Schmahmann et al., 2007; Seltzer & Pandya, 1986), which is a multimodal sensory association region (Banat et al., 2000) that integrates input from the somatosensory association cortex and is important for bodily perception and agency (Hargreaves et al., 2012; Yang et al., 2011). Thus, the relation between FA in this pathway and negative emotional reaction to touch in the ASD group may reflect an aberrant connection between the inferior parietal cortex and limbic structures deep within the temporal lobe. As with orienting, the variability of defensiveness scores in the TD group was restricted, which may have limited our ability to detect a similar relation in this group.
The current study has several strengths. Our use of validated observational sensory measures with blind raters, rather than parent report, was a unique strength, eliminating some of the drawbacks of parent report such as response bias and variability in interpretation of questionnaire items. The integration of this rich sensory data with neuroimaging data is also a strength of the study. Our data are gathered from younger children than many neuroimaging studies, allowing a snapshot of the brain at a time when sensory features are more prominent than later in life. The narrow age range of our sample also minimizes the “blurring” that comes with obtaining cross-sectional behavioral and neuroimaging measures across many developmental stages. A 92-direction acquisition provides high signal to noise ratio.
Regarding limitations of the current study, ROQS uses semi-automated tract selection for anatomically reliable definition; using TBSS or tractography in a whole-brain analysis may provide a more robust assessment of white matter microstructure and the opportunity for additional metrics such as tract volume and fiber density. Further, to investigate the potential of aberrant connections, such as that between the inferior parietal cortex and temporal limbic structures, tractography would provide a means for the identification of innervated cortical regions. Finally, a potential limitation was that our processing pipeline did not allow for re-orientation of the b matrix, which may have introduced bias in our results (Leemans and Jones, 2009).
5. Conclusion
We used high angular-resolution diffusion imaging in children with and without ASD to investigate a brain–behavior relationship in white matter tracts with known roles in sensorimotor and early attentional processing. We targeted the centrum semiovale (CS), superior corona radiata (SCR), inferior longitudinal fasciculus (ILF), splenium of the corpus callosum (SPLEN) and posterior limb of the internal capsule (PLIC), which we predicted all might be relevant to aberrant sensory behaviors seen in young children with ASD. At the time of publication, this is the first known study of ASD to link sensory variables in directly observed behaviors to white matter integrity. The relationship between increased tactile defensiveness and reduced FA may reflect an aberrant connection between limbic structures in the temporal lobe and the inferior parietal cortex. Our findings also corroborate the modulatory role of the SPLEN in orienting deficits in ASD, but suggest the possibility that a more diffuse or separable network may underlie the social orienting deficits that are more specific to ASD. Future investigation should consider the use of whole brain analyses, including tractography, for a more robust assessment of white matter microstructure. In summary, our findings suggest a modulatory role of ILF and SPLEN in atypical sensorimotor and early attention processes in ASD.
Acknowledgments
This work was supported by the National Institute of Mental Health (K01 MH090232 awarded to C.J.C.), the Landreth Family Discovery Grant, and the Nicholas Hobbs Society of the Vanderbilt Kennedy Center. The National Center for Advancing Translational Sciences (UL1 TR000445) provided database support and supplemental funding for image acquisition (VR 2719 awarded to C.J.C.). The laboratory of Dr. Grace Baranek (University of North Carolina, Chapel Hill) provided access to sensory assessments and training in their administration and scoring. The authors thank Dr. Bruce McCandliss for providing software and training for the ROQS analysis, and Micheal Gmaz Sandbank for behavioral training assistance for the scanner in a subset of participants.
Appendix A. Supplementary data
Supplementary material.
References
- Achenbach T.M., Rescorla L.A. Manual for the ASEBA School-age Forms and Profiles. Research Center for Children, Youth and Families; Burlington, VT: 2001. [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Boudos R., DuBray M.B., Oakes T.R., Lainhart J.E. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. 17023185 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fourth edition, text rev. Author; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fifth edition. Psychiatric Publishing; Arlington, VA, American: 2013. [Google Scholar]
- Aoki Y., Abe O., Nippashi Y., Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Molecular Autism. 2013;4(1):25. doi: 10.1186/2040-2392-4-25. 23876131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati R.B., Goerres G.W., Tjoa C., Aggleton J.P., Grasby P. The functional anatomy of visual–tactile integration in man: a study using positron emission tomography. Neuropsychologia. 2000;38(2):115–124. doi: 10.1016/s0028-3932(99)00074-3. 10660224 [DOI] [PubMed] [Google Scholar]
- Baranek, G.T. (1999), Sensory processing assessment for young children (SPA). Unpublished Manuscript
- Baranek, G.T. (2010), Tactile defensiveness and discrimination test — revised (TDDT-R). Unpublished Manuscript
- Baranek G.T., David F.J., Poe M.D., Stone W.L., Watson L.R. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. 16712636 [DOI] [PubMed] [Google Scholar]
- Baranek G.T., Foster L.G., Berkson G. Tactile defensiveness and stereotyped behaviors. American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 1997;51(2):91–95. doi: 10.5014/ajot.51.2.91. 9124275 [DOI] [PubMed] [Google Scholar]
- Baranek G.T., Watson L.R., Boyd B.A., Poe M.D., David F.J., McGuire L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology. 2013;25(2):307–320. doi: 10.1017/S0954579412001071. 23627946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss A.L. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. 14744477 [DOI] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. 8130344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. Journal of Magnetic Resonance (San Diego, Calif.: 1997) 2011;213(2):560–570. doi: 10.1016/j.jmr.2011.09.022. 22152371 [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A., Carter A.S., Briggs-Gowan M.J. Sensory over-responsivity in elementary school: prevalence and social–emotional correlates. Journal of Abnormal Child Psychology. 2009;37(5):705–716. doi: 10.1007/s10802-008-9295-8. 19153827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone A., Faubert J. How is complex second-order motion processed? Vision Research. 2003;43(25):2591–2601. doi: 10.1016/s0042-6989(03)00465-6. 14552801 [DOI] [PubMed] [Google Scholar]
- Bertone A., Mottron L., Jelenic P., Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain: A Journal of Neurology. 2005;128(10):2430–2441. doi: 10.1093/brain/awh561. 15958508 [DOI] [PubMed] [Google Scholar]
- Berument S.K., Rutter M., Lord C., Pickles A., Bailey A. Autism screening questionnaire: diagnostic validity. The British Journal of Psychiatry. The Journal of Mental Science. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bonekamp D., Nagae L.M., Degaonkar M., Matson M., Abdalla W.M.A., Barker P.B., Horská A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. 17092743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd B.A., Baranek G.T., Sideris J., Poe M.D., Watson L.R., Patten E., Miller H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research: Official Journal of the International Society for Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. 20437603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito A.R., Vasconcelos M.M., Domingues R.C., Hygino da Cruz L.C., Jr, Rodrigues L.deS., Gasparetto E.L., Calçada C.A.B.P. Diffusion tensor imaging findings in school-aged autistic children. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2009;19(4):337–343. doi: 10.1111/j.1552-6569.2009.00366.x. 19490374 [DOI] [PubMed] [Google Scholar]
- Brock M.E., Freuler A., Baranek G.T., Watson L.R., Poe M.D., Sabatino A. Temperament and sensory features of children with autism. Journal of Autism and Developmental Disorders. 2012;42(11):2271–2284. doi: 10.1007/s10803-012-1472-5. 22366913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas A., Lupiàñez J., Funes M.J., Tudela P. Modulations among the alerting, orienting and executive control networks. Experimental Brain Research. 2005;167(1):27–37. doi: 10.1007/s00221-005-2365-z. 16021429 [DOI] [PubMed] [Google Scholar]
- Cascio C.J., Gerig G., Piven J. Diffusion tensor imaging: application to the study of the developing brain. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(2):213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Ceponiene R., Lepistö T., Shestakova A., Vanhala R., Alku P., Näätänen R., Yaguchi K. Speech–sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni L., Garber M. Exploring the experience of autism through firsthand accounts. Journal of Autism and Developmental Disorders. 1991;21(3):303–313. doi: 10.1007/BF02207327. 1938776 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chou K.-H., Chen I.-Y., Fan Y.-T., Decety J., Lin C.-P. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50(3):873–882. doi: 10.1016/j.neuroimage.2010.01.011. 20074650 [DOI] [PubMed] [Google Scholar]
- Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. 14979766 [DOI] [PubMed] [Google Scholar]
- Donkers F.C.L., Schipul S.E., Baranek G.T., Cleary K.M., Willoughby M.T., Evans A.M., Belger A. Attenuated auditory event-related potentials and associations with atypical sensory response patterns in children with autism. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1948-y. 24072639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. The impact of sensory processing abilities on the daily lives of young children and their families: a conceptual model. Infants & Young Children. 1997;9:23–25. [Google Scholar]
- Egaas B., Courchesne E., Saitoh O. Reduced size of corpus callosum in autism. Archives of Neurology. 1995;52(8):794–801. doi: 10.1001/archneur.1995.00540320070014. 7639631 [DOI] [PubMed] [Google Scholar]
- Elison J.T., Paterson S.J., Wolff J.J., Reznick J.S., Sasson N.J., Gu H., Piven J. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. American Journal of Psychiatry. 2013;170(8):899–908. doi: 10.1176/appi.ajp.2012.12091150. 23511344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell A.D., Nummenmaa L., Oosterhof N.N., Henson R.N., Haxby J.V., Calder A.J. Differential activation of frontoparietal attention networks by social and symbolic spatial cues. Social Cognitive and Affective Neuroscience. 2010;5(4):432–440. doi: 10.1093/scan/nsq008. 20304864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Gu X., Guise K.G., Liu X., Fossella J., Wang H., Posner M.I. Testing the behavioral interaction and integration of attentional networks. Brain and Cognition. 2009;70(2):209–220. doi: 10.1016/j.bandc.2009.02.002. 19269079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Fossella J., Flombaum J.I., Posner M.I. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. 15907304 [DOI] [PubMed] [Google Scholar]
- Fimm B., Zahn R., Mull M., Kemeny S., Buchwald F., Block F., Schwarz M. Asymmetries of visual attention after circumscribed subcortical vascular lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71(5):652–657. doi: 10.1136/jnnp.71.5.652. 11606678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E., Di Salle F., Goebel R. Fundamentals of data analysis methods in functional MRI. Signal Processing and Communications. 2005;27:481–503. [Google Scholar]
- Foss-Feig J.H., Heacock J.L., Cascio C.J. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Research in Autism Spectrum Disorders. 2012;6(1):337–344. doi: 10.1016/j.rasd.2011.06.007. 22059092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier T.W., Hardan A.Y. A meta-analysis of the corpus callosum in autism. Biological Psychiatry. 2009;66(10):935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021(1):77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27(5):392–401. doi: 10.1002/hbm.20249. 16596654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M., Belmonte M.K., Bullmore E.T., Bernard F.A., Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain: A Journal of Neurology. 2008;131(9):2479–2488. doi: 10.1093/brain/awn172. 18669482 [DOI] [PubMed] [Google Scholar]
- Gotham K., Risi S., Pickles A., Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. 17180459 [DOI] [PubMed] [Google Scholar]
- Grandin T., Scariano M. Emergence: Labelled Autistic. Arena; Novato, CA: 1986. [Google Scholar]
- Greene D.J., Colich N., Iacoboni M., Zaidel E., Bookheimer S.Y., Dapretto M. Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage. 2011;56(1):354–362. doi: 10.1016/j.neuroimage.2011.02.031. 21334443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D.J., Mooshagian E., Kaplan J.T., Zaidel E., Iacoboni M. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychological Research. 2009;73(4):499–511. doi: 10.1007/s00426-009-0233-3. 19350270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.A., Rudie J.D., Colich N.L., Wood J.J., Shirinyan D., Hernandez L., Totenham N., Dapretto M., Bookheimer S.Y. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N., Joseph R.M., Snyder J., Chabris C.F., Clark J., Steele S., Tager-Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. 15219586 [DOI] [PubMed] [Google Scholar]
- Hardan A.Y., Pabalan M., Gupta N., Bansal R., Melhem N.M., Fedorov S., Minshew N.J. Corpus callosum volume in children with autism. Psychiatry Research: Neuroimaging. 2009;174(1):57–61. doi: 10.1016/j.pscychresns.2009.03.005. 19781917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves I.S., Leonard G.A., Pexman P.M., Pittman D.J., Siakaluk P.D., Goodyear B.G. The neural correlates of the body–object interaction effect in semantic processing. Frontiers in Human Neuroscience. 2012;6:22. doi: 10.3389/fnhum.2012.00022. 22375111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen J.K., Nummenmaa L., Nyman M.J., Parkkola R., Hämäläinen H. Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage. 2006;33(1):406–413. doi: 10.1016/j.neuroimage.2006.06.048. 16949306 [DOI] [PubMed] [Google Scholar]
- Iwasaki N., Hamano K., Okada Y., Horigome Y., Nakayama J., Takeya T., Nose T. Volumetric quantification of brain development using MRI. Neuroradiology. 1997;39(12):841–846. doi: 10.1007/s002340050517. 9457706 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Jiang H., van Zijl P.C.M., Kim J., Pearlson G.D., Mori S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. 16413083 [DOI] [PubMed] [Google Scholar]
- Jou R.J., Mateljevic N., Kaiser M.D., Sugrue D.R., Volkmar F.R., Pelphrey K.A. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. AJNR. American Journal of Neuroradiology. 2011;32(9):1607–1613. doi: 10.3174/ajnr.A2558. 21799040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbance of affective contact. Nervous Child. 1943;2:217–250. [Google Scholar]
- Kaufman A., Kaufman N. Kaufman Brief Intelligence Test. second edition. Pearson; London, UK: 2004. [Google Scholar]
- Keller T.A., Kana R.K., Just M.A. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18(1):23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kingstone A., Tipper C., Ristic J., Ngan E. The eyes have it!: an fMRI investigation. Brain and Cognition. 2004;55(2):269–271. doi: 10.1016/j.bandc.2004.02.037. 15177792 [DOI] [PubMed] [Google Scholar]
- Koldewyn K., Yendiki A., Weigelt S., Gweon H., Julian J., Richardson H., Kanwisher N. Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proceedings of the National Academy of Sciences of the United States of America. 2014;111 doi: 10.1073/pnas.1324037111. 201324037 24449864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon C.B., Asman A.J., Esparza M.L., Burns S.S., Fan Q., Gao Y., Anderson A.W., Davis N., Cutting L.E., Landman B.A. Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS One. 2013;8(4):e61737. doi: 10.1371/journal.pone.0061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J.F., Poupon C., Clark C.A., Pappata S., Molko N., Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging: JMRI. 2001;13(4):534–546. doi: 10.1002/jmri.1076. 11276097 [DOI] [PubMed] [Google Scholar]
- Lee J.E., Bigler E.D., Alexander A.L., Lazar M., DuBray M.B., Chung M.K., Lainhart J.E. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424(2):127–132. doi: 10.1016/j.neulet.2007.07.042. 17714869 [DOI] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;61:1336–1349. doi: 10.1002/mrm.21890. 19319973 [DOI] [PubMed] [Google Scholar]
- Liss M., Saulnier C., Fein D., Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism: the International Journal of Research and Practice. 2006;10(2):155–172. doi: 10.1177/1362361306062021. 16613865 [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview — Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. 7814313 [DOI] [PubMed] [Google Scholar]
- Luders E., Narr K.L., Hamilton L.S., Phillips O.R., Thompson P.M., Valle J.S., Levitt J.G. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65(1):84–88. doi: 10.1016/j.biopsych.2008.08.027. 18842255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E.J., Hinkley L.B.N., Hill S.S., Nagarajan S.S. Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research. 2011;69(5 Pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. 21289533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J., De Lucas E.M. Subcortical anatomy of the lateral association fascicles of the brain: a review. Clinical Anatomy (New York, N.Y.) 2014;27(4):563–569. doi: 10.1002/ca.22321. 24453050 [DOI] [PubMed] [Google Scholar]
- Minshew N.J., Goldstein G., Siegel D.J. Neuropsychologic functioning in autism: profile of a complex information processing disorder. Journal of the International Neuropsychological Society: JINS. 1997;3(4):303–316. 9260440 [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Niogi S., Mukherjee P., Ghajar J., McCandliss B.D. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Frontiers in Neuroanatomy. 2010;4:2. doi: 10.3389/neuro.05.002.2010. 20204143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., McCandliss B.D. Diffusion tensor imaging segmentation of white matter structures using a Reproducible Objective Quantification Scheme (ROQS) Neuroimage. 2007;35(1):166–174. doi: 10.1016/j.neuroimage.2006.10.040. 17208014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B., Afraz S.-R., Vaziri-Pashkam M., Esteky H. Visual spatial integrity in the absence of splenium. Brain Research. 2006;1076(1):177–186. doi: 10.1016/j.brainres.2006.01.020. 16476417 [DOI] [PubMed] [Google Scholar]
- Novick B., Vaughan H.G., Jr, Kurtzberg D., Simson R. An electrophysiologic indication of auditory processing defects in autism. Psychiatry Research. 1980;3(1):107–114. doi: 10.1016/0165-1781(80)90052-9. 6934552 [DOI] [PubMed] [Google Scholar]
- Owen J.P., Marco E.J., Desai S., Fourie E., Harris J., Hill S.S., Arnett A.B., Mukherjee P. Abnormal white matter microstructure in children with sensory processing disorders. NeuroImage. Clinical. 2013;2:844–853. doi: 10.1016/j.nicl.2013.06.009. 24179836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.U., Kaufmann W.E., Bacino C.A., Anderson A.W., Adapa P., Chu Z., Wilde E.A. Alterations in white matter pathways in Angelman syndrome. Developmental Medicine and Child Neurology. 2011;53(4):361–367. doi: 10.1111/j.1469-8749.2010.03838.x. 21121904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Mathalon D.H., Sullivan E.V., Rawles J.M., Zipursky R.B., Lim K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. 8080387 [DOI] [PubMed] [Google Scholar]
- Philippi C.L., Mehta S., Grabowski T., Adolphs R., Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. 19955360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Basser P.J. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. 8946355 [DOI] [PubMed] [Google Scholar]
- Piven J., Bailey J., Ranson B.J., Arndt S. An MRI study of the corpus callosum in autism. American Journal of Psychiatry. 1997;154(8):1051–1056. doi: 10.1176/ajp.154.8.1051. 9247388 [DOI] [PubMed] [Google Scholar]
- Posner M.I., Petersen S.E. The Attention System of the Human Brain. Annual Review of Neuroscience. 1990;13(1):25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58(1):1–23. doi: 10.1146/annurev.psych.58.110405.085516. 17029565 [DOI] [PubMed] [Google Scholar]
- Posner M.I., Sheese B.E., Odludaş Y., Tang Y. Analyzing and shaping human attentional networks. Neural Networks: the Official Journal of the International Neural Network Society. 2006;19(9):1422–1429. doi: 10.1016/j.neunet.2006.08.004. 17059879 [DOI] [PubMed] [Google Scholar]
- Raz A., Buhle J. Typologies of attentional networks. Nature Reviews. Neuroscience. 2006;7(5):367–379. doi: 10.1038/nrn1903. 16760917 [DOI] [PubMed] [Google Scholar]
- Rivkin M.J. Developmental neuroimaging of children using magnetic resonance techniques. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(1):68–80. doi: 10.1002/(SICI)1098-2779(2000)6:1<68::AID-MRDD9>3.0.CO;2-9. 10899799 [DOI] [PubMed] [Google Scholar]
- Rogers S.J., Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. 16313426 [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. 15093142 [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Social Communication Questionnaire (SCQ) Western Psychological Services; Los Angeles, CA.: 2003. [Google Scholar]
- Schmahmann J.D., Pandya D.N., Wang R., Dai G., D'Arceuil H.E., de Crespigny A.J., Wedeen V.J. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain: A Journal of Neurology. 2007;130(3):630–653. doi: 10.1093/brain/awl359. 17293361 [DOI] [PubMed] [Google Scholar]
- Seltzer B., Pandya D.N. Posterior parietal projections to the intraparietal sulcus of the rhesus monkey. Experimental Brain Research. 1986;62(3):459–469. doi: 10.1007/BF00236024. 3720878 [DOI] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Lincoln A.J., Müller R.-A. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging studyJournal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1269–1278. doi: 10.1016/j.jaac.2010.08.018. 21093776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Müller R.-A. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. 21073464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. 15501092 [DOI] [PubMed] [Google Scholar]
- Sturm W., Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14(1):S76–S84. doi: 10.1006/nimg.2001.0839. 11373136 [DOI] [PubMed] [Google Scholar]
- Sundaram S.K., Kumar A., Makki M.I., Behen M.E., Chugani H.T., Chugani D.C. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cerebral Cortex (New York, N.Y.: 1991) 2008;18(11):2659–2665. doi: 10.1093/cercor/bhn031. 18359780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I., Yablonski M., Mezer A., Rom S., Assaf Y., Yovel G. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. Neuroimage. 2014;86:123–130. doi: 10.1016/j.neuroimage.2013.07.085. 23933304 [DOI] [PubMed] [Google Scholar]
- Tipper C.M., Handy T.C., Giesbrecht B., Kingstone A. Brain responses to biological relevance. Journal of Cognitive Neuroscience. 2008;20(5):879–891. doi: 10.1162/jocn.2008.20510. 18201123 [DOI] [PubMed] [Google Scholar]
- Travers B.G., Adluru N., Ennis C., Tromp D.P.M., Destiche D., Doran S., Alexander A.L. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Research: Official Journal of the International Society for Autism Research. 2012;5(5):289–313. doi: 10.1002/aur.1243. 22786754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S.B., Jones D.K., Jeurissen B., Viergever M.A., Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage. 2012;59(3):2208–2216. doi: 10.1016/j.neuroimage.2011.09.086. 22005591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L.R., Patten E., Baranek G.T., Poe M., Boyd B.A., Freuler A., Lorenzi J. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech Language and Hearing Research. 2011;54(6):1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Treyer V., Oberholzer N., Jaermann T., Boesiger P., Brugger P., Marzi C.A. Attention and interhemispheric transfer: a behavioral and fMRI Study. Journal of Cognitive Neuroscience. 2005;17(1):113–123. doi: 10.1162/0898929052880002. 15701243 [DOI] [PubMed] [Google Scholar]
- Wiggins L.D., Robins D.L., Bakeman R., Adamson L.B. Brief report: sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. Journal of Autism and Developmental Disorders. 2009;39(7):1087–1091. doi: 10.1007/s10803-009-0711-x. 19283461 [DOI] [PubMed] [Google Scholar]
- Yang J., Shu H., Bi Y., Liu Y., Wang X. Dissociation and association of the embodied representation of tool-use verbs and hand verbs: an fMRI study. Brain and Language. 2011;119(3):167–174. doi: 10.1016/j.bandl.2011.06.001. 21741081 [DOI] [PubMed] [Google Scholar]
- Yin X., Zhao L., Xu J., Evans A.C., Fan L., Ge H., Liu S. Anatomical substrates of the alerting, orienting and executive control components of attention: focus on the posterior parietal lobe. PloS One. 2012;7(11):e50590. doi: 10.1371/journal.pone.0050590. 23226322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


