Abstract
Myelin water imaging provides a novel strategy to assess myelin integrity and corresponding clinical relationships in psychosis, of particular relevance in frontal white matter regions. In the current study, T2 myelin water imaging was used to assess the myelin water fraction (MWF) signal from frontal areas in a sample of 58 individuals experiencing first-episode psychosis (FEP) and 44 healthy volunteers. No differences in frontal MWF were observed between FEP subjects and healthy volunteers; however, differences in normal patterns of associations between frontal MWF and age, education and IQ were seen. Significant positive relationships between frontal MWF and age, North American Adult Reading Test (NAART) IQ, and years of completed education were observed in healthy volunteers. In contrast, only the relationship between frontal MWF and NAART IQ was significant after Bonferroni correction in the FEP group. Additionally, significant positive relationships between age and MWF in the anterior and posterior internal capsules, the genu, and the splenium were observed in healthy volunteers. In FEP subjects, only the relationship between age and MWF in the splenium was statistically significant. Frontal MWF was not associated with local white matter volume. Altered patterns of association between age, years of education, and MWF in FEP suggest that subtle disturbances in myelination may be present early in the course of psychosis.
Keywords: Myelin water fraction, First-episode psychosis, Schizophrenia, Frontal white matter
Abbreviations: ALIC, anterior limb internal capsule; FEP, first episode psychosis; MWF, myelin water fraction; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, version IV; NAART, North American Adult Reading Test
Highlights
-
•
Myelin Water Fraction (MWF) Imaging is a new technique for white matter assessment
-
•
MWF imaging revealed abnormal myelination patterns in first-episode psychosis (FEP)
-
•
Abnormal frontal MWF associations to age, education and IQ were most apparent in FEP
-
•
These findings suggest aberrant frontal myelination occur early in the illness
1. Introduction
Given the frequency of reported white matter abnormalities in psychotic disorders, recent hypotheses regarding the etiology of schizophrenia have begun to focus more closely on the possibility of abnormal myelination of late developing frontal white matter tracts (Whitford et al., 2012). Until recently, our ability to explore abnormal myelination and subsequent frontal mis- or disconnection as an underlying central mechanism in the emergence of schizophrenia has been hampered by technological limitations. The advent of new magnetic resonance imaging (MRI) modalities has made it possible to explore little characterized facets of underlying structural deficits in psychotic disorders and to more fully explore the hypothesis of disconnectivity in schizophrenia and schizophrenia spectrum disorders. Disconnectivity or aberrant connectivity may be a common feature of the psychopathologies associated with schizophrenia including decreased information processing speed, difficulties with memory, poor attention and passive withdrawal from normal activities (Girard et al., 2010; Hunter and Barry, 2012). White matter deficits and white matter disarray (i.e. axonal disorganization) have been well established based on in vivo imaging of older, more chronic schizophrenia patients; however, the evidence for similar deficits early in the illness (i.e. at first episode) is more equivocal, with some reports of reduced white matter volumes or altered white matter integrity (Chan et al., 2010; Hao et al., 2006). Some fronto-medial regions (particularly the superior frontal gyri and the entorhinal cortex) appear to have both reduced volume and abnormal tissue integrity in first-episode psychosis patients, based on correlations between findings from standard structural imaging and magnetization transfer imaging (Price et al., 2010). A number of diffusion tensor studies have reported decreased fractional anisotropy in schizophrenia, particularly in the frontal and temporal white matter, providing corollary evidence of white matter abnormalities as a feature of this illness (Debette and Markus, 2010; Lee et al., 2013). In contrast, other diffusion tensor imaging studies of white matter alterations in first-episode schizophrenia have failed to demonstrate differences between first-episode patients and healthy age and gender matched volunteers (Kong et al., 2011). It is postulated that the observed alterations in FA are a result of alterations in the myelin and that a subtle form of axonopathy underlies reduction in anisotropy with relative sparing of the normal white matter histology (Highley et al., 2002).
Numerous white matter tracts have been implicated in schizophrenia, including aberrant interhemispheric connectivity through the corpus callosum (Crow et al., 2007), corticothalamocerebellar circuits (Andreasen, 1999), and fronto-temporal pathways (Friston and Frith, 1995). The frontal white matter tracts coursing through the anterior limb of the internal capsule (ALIC) are of particular interest as they contain corticothalamic projections that subserve sensory processing, sensory gating and cognitive processes such as memory, attention, and psychomotor control (Buchsbaum et al., 2006). Imaging investigations using diffusion as a tool for exploration have suggested that white matter integrity and/or quantity in patients with schizophrenia are reduced, particularly in frontal white matter regions (Du et al., 2012; Garver et al., 2008). Whether the observed changes in frontal white matter integrity are clearly associated with myelin deficits in schizophrenia has not yet been established. Moreover, any potential deficits in myelin need to be interpreted in the context of normal progression of myelination in the developing human brain over the lifespan. Both early and more recent studies have shown that myelination continues well in the 3rd decade of life (Benes et al., 1994; Yakelov and Lecours, 1967). Aberrant developmental trajectories that could contribute to the emergence of schizophrenia may result in differential relationships between myelination and age in patients compared to the normal population.
We are able to apply a newer, highly specific magnetic resonance (MR) methodology developed to assess the myelin-associated water fraction (MWF) in white matter (Mackay et al., 2009; Meyers et al., 2009; Sirrs et al., 2007). This novel technique exploits the rate of signal decay from water within specific components of tissue; as such MWF imaging is well suited to differentiating the specific T2 water signal associated with myelin only, as opposed to water signal from extracellular, neuronal or cerebral spinal fluid compartments (Kolind et al., 2009). Myelin water imaging was validated as a measure of myelin content in fixed brain samples (Laule et al., 2006), and has been used to follow myelination changes in multiple sclerosis (Laule et al., 2003) and phenylketonuria (Sirrs et al., 2007). In a previous study, MWF was successfully used to obtain index measures of frontal white matter integrity in chronic schizophrenia patients and healthy normal volunteers (Flynn et al., 2003). In healthy subjects, both age and education were found to have significant positive associations with measures of myelin integrity (Flynn et al., 2003). These relationships were not seen in chronic schizophrenia patients (Flynn et al., 2003). Both chronicity of illness and long-term exposure to medications may contribute to these observations, as it has been posited that some antipsychotic medications may act to at least partially restore myelin integrity (Garver et al., 2008). It is predicted that 1) first-episode schizophrenia patients will have reduced frontal MWF and 2) positive relationships between age and years of education with MWF will be present in healthy volunteers, but not in FEP subjects. MWF is not expected to be associated with local white matter volume, as the MWF signal is not dependent upon white matter volume.
2. Methods
2.1. Subjects
Ethics approval for this study was granted by the University of British Columbia's Research Ethics Board in accordance with the Canadian Federal Tri-council Policy Statement. All subjects provided full written consent. First-episode psychosis (FEP) subjects were recruited as part of a longitudinal study of the interactions of environment, genetics and neurodevelopment in schizophrenia (The NET-EPI Study: PI — WG Honer). For the current analysis of white matter integrity, a sample of 58 FEP subjects and 44 age and gender-matched healthy volunteers were included. Entry criteria for the current study included a current episode of psychosis, ability to give written and verbal consent in English, normal vision with or without correction, minimum IQ of 80, age 14–40, and ability to tolerate MR scanning. Exclusion criteria for psychosis subjects included any prior exposure to antipsychotic medications, a history of meningitis or other CNS infections, history of head injury leading to loss of consciousness for more than 5 min, DSM-IV drug dependence, severe claustrophobia, and presence of metal surgical implants or metal dental devices. Healthy age and gender-matched volunteers who had no history of psychosis or any other major DSM-IV disorder were recruited by poster advertisement within the local community. Inclusion and exclusion criteria for healthy volunteers were similar. FEP subjects were within the first 15 weeks of their first psychotic episode. All subjects had no more than 10 weeks of lifetime exposure to antipsychotic medications (see Table 1 for mean weeks treatment and average daily doses). At time of baseline scans 24 subjects were antipsychotic naive, 19 were treated with risperidone, 12 were receiving olanzapine and 1 was receiving quetiapine. Mean lifetime doses are provided in Table 1. Diagnoses were made using DSM-IV criteria and included review of a structured clinical interview and longitudinal clinical notes (WGH). Diagnoses were 41 schizophrenia, 15 schizoaffective, and 2 schizophreniform.
Table 1.
Summary clinical and demographic variables.
| Variable | FEP subjects (N = 58) | Healthy volunteers (N = 44) |
|---|---|---|
| Gender | ||
| Male | 40 | 25 |
| Female | 18 | 19 |
| Age (mean years) | 21.7 (range 14.9–35.7: sd 9.8) | 22.7 (range 15.1–38.9: sd 6.5) |
| Ethnicity | ||
| Caucasian | 43 | 34 |
| Asian | 4 | 4 |
| Other | 11 | 6 |
| Education (mean years) | 11.4 (range 7–17: sd 2.1) | 13.9 (range 10–18: sd 2.5) |
| NAART score (mean) | 99.1 (range 84–118: sd 8.5) | 107 (range 90–119: sd 7.9) |
| PANSS total score (mean) | 74.6 (range 49-117: sd 14.3) | |
| Mean medication dose/day | ||
| Mean weeks medicationa | 7.7 weeks | |
| Risperidone (N = 18) | 1.24 | N/A |
| Olanzapine (N = 13) | 10.4 | |
| Quetiapine (N = 1) | .25 |
sd: standard deviation.
At time of scan 26 of 58 FEP subjects were antipsychotic naive.
2.2. Demographic and clinical measures
Demographic and clinical data (age, gender, ethnicity, IQ, years of education, diagnosis, symptom severity) were assessed at intake (see Table 1). All subjects were given a full diagnostic interview by a clinician (GWM or LCK) at intake (baseline). Psychiatric symptoms were assessed with the Positive and Negative Syndromes Scale (PANSS). PANSS scores were available for 57 of 58 subjects, as one subject was unavailable for symptom assessment at study entry. Additionally, one FEP subject did not complete IQ assessments at time of entry. Intelligence (IQ) was assessed by the Kaufman Brief Intelligence Test (KBIT) (Kaufman and Kaufman, 1990) premorbid IQ was assessed with the National American Adult Reading Test (NAART) (Blair and Spreen, 1989).
2.3. Imaging
All images were inspected for motion artifact and susceptibility artifact (DJL, WS) to determine suitability for inclusion in the study. Scans with visible artifacts were excluded.
2.3.1. Myelin water fraction (MWF) imaging
Magnetic resonance images (MRIs) optimized for assessment of the MWF in white matter were acquired on a 1.5 T GE, Signa EchoSpeed scanner (software version 5.7). After a sagittal localizer (TR = 350 ms, TE = 14 ms, 15 slices), single-slice myelin water imaging data were acquired using a 48-echo CPMG sequence, consisting of a 90° slice selective pulse followed by 48 rectangular composite 180° pulses flanked by slice-selective crusher gradient pulses for elimination of signal from outside the selected slice (TR = 3800 ms, echo spacing = 10 ms for the first 32 echoes and 50 ms for the next 16 echoes, BW = 31 kHz, FOV = 22 cm, thickness = 10 mm, matrix 256 × 128, averages = 4). For the 20 central lines of k-space, the repetition time was 3.8 s and was ramped linearly to 2.12 s at the extremities of k-space. The reduction in repetition time had a negligible effect on the estimated T2 distributions but substantially decreased the acquisition time (Laule et al., 2007). The myelin water image slice was positioned transversely to the slice parallel to the Anterior Commissure–Posterior Commissure line to optimize simultaneous visualization of frontal white matter, basal ganglia structures, thalamic nuclei and posterior white matter. A 3D SPGR sequence (graphic prescription, minimum TR, TE = 5 ms, FOV = 22 cm, matrix 256 × 256, 124 continuous 1.5 mm thick slices, flip angle = 45°) was acquired for volumetric assessments.
2.3.2. Volumetric structural imaging
Parallel T1-weighted FSPGR IR 3-D structural images were acquired with the following parameters: TR = 11.5 ms, TE = 5 ms, FA 250, b value = 1000, FOV 24 cm2, NEX = 1, acquisition and reconstruction matrices = 256 × 192 and 256 × 256 respectively, voxel dimensions = 0.9375 mm × 0.9375 mm × 2 mm, and interslice thickness = 1 mm. DTI was performed with the following parameters; TR = 13,000 ms, TE = 72.8 ms, NEX = 2, voxel dimensions = 1.25 mm × 1.25 mm × 2.5 mm, FOV = 32 × 32 cm, and acquisition and reconstruction matrices = 128 × 128 and 256 × 256 respectively.
2.3.3. Image analysis — MWF
Six bilateral white matter regions of interest – ROIs – (genu, minor forceps, anterior internal capsule, posterior internal capsule, splenium and major forceps) were manually selected by a trained rater (EY) and averaged over 3 trials (see Fig. 1). T2 relaxation decay curves from these regions were decomposed into an unspecified number of exponentials by using a non-negative least squares algorithm (Whittall et al., 1997) and myelin water fraction was defined as the signal with T2 below 50 ms divided by the total signal in the T2 distribution (Mackay et al., 1994). All ROIs were analyzed using a pixel-by-pixel analysis based on MWF maps (Mackay et al., 1994; Meyers et al., 2009) whereby the average of the pixels within each ROI was ascertained to obtain an MWF value for each ROI.
Fig. 1.
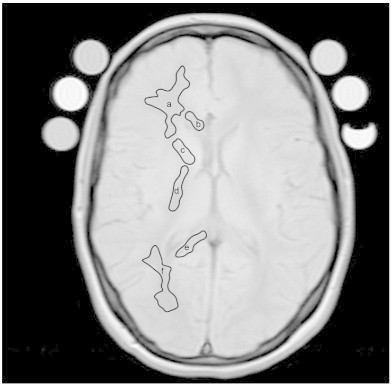
Sample image with manual segmentation of regions of interest (ROI) on the first echo of the T2 relaxation experiment used to determine myelin water fraction. ROIs were manually outlined bilaterally, as demonstrated below on the single hemisphere. Note that regions of interest were conservatively selected to ensure exclusion of non-white tissues. Regions: a) Minor forceps (frontal white matter), b) genu, c) anterior internal capsule, d) posterior internal capsule, e) splenium, and f) major forceps (parieto-occipital white matter).
2.3.4. Image analysis — T1 SPGR 3D structural MRI frontal white matter volume
Frontal white matter segmentation was performed using a standard processing pipeline. Raw scanner DICOM (Digital Imaging & Communications in Medicine) images were converted to NIfTI (Neuroimaging Informatics Technology Initiative) file format using the dcm2nii tool from MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/) prior to processing. FSL 4.1 software (i.e. the FSL BET — Brain Extraction Tool) was used to perform skull-stripping and then to register extracted whole-brain images to standard MNI152 space. Subsequently, using the FAST v4.1 tool in FSL, all brain images were parcellated into gray matter, white matter and cerebrospinal fluid (CSF). Individual lobar volumes were calculated on the basis of lobar masks from the standard MNI whole brain template.
2.4. Statistical analysis
Summary clinical and demographic data are shown in Table 1. Preliminary comparisons included an independent t-test of age between groups, a 1-way ANOVA to explore diagnosis by age interactions on years of education completed and Chi-square analysis of gender distribution between groups. Medication dosages were converted from raw lifetime doses to chlorpromazine equivalents in accordance with standard pharmacokinetic D2 occupancy rates to investigate potential relationships between medication dose and MWF (Virani et al., 2012). Left and right MWF values of the major white matter regions were summed to obtain pooled measures, as preliminary comparisons by repeated measures t-tests of left and right sides did not reveal hemispheric differences for any regions of interest (all p-values > 0.10). Relationships between age, education, IQ, PANSS score and frontal white matter (i.e. minor forceps) MWF measures were examined with Pearson's linear correlations within each group. Data were normally distributed and did not violate the assumptions of the ANOVA model. Standard Bonferroni correction was applied to linear correlations. Omnibus ANCOVA models were use to compare MWF scores between groups with age entered as a co-variate and gender entered as an independent factor, as both age and gender are associated with alterations in myelination (De Bellis et al., 2001). The ANCOVA model Bonferroni correction level for comparisons of MWFs across regions was set to .025 as our a priori specifically hypothesized differences between groups in the frontal white matter (minor forceps), with the investigation of other regions being more exploratory in nature. Omnibus group comparisons of frontal white matter volumes included total age, gender and total brain volume as covariates. Linear Pearson's correlation and univariate regression models were used to explore relationships between white matter volume and frontal MWF scores on the left and right sides in both groups. Standard Bonferroni correction was applied for multiple comparisons (significance set at the p = 0.008 level).
2.5. Results
Healthy volunteers and FEP subjects were similar in age, t(df 100) = 1.23, p = 0.11, and had similar levels of educational attainment, F(df 100) = 0.01, p = 0.90 (note: education data were not available for one FEP subject). Gender distribution across groups was significantly different, with females making up a greater proportion of the healthy volunteer group compared to the FEP group, χ2(df 1) = 6.82, p = 0.01. No within group differences in MWF scores were seen between male and female FEP subjects or healthy volunteers (all p-values > .01). Healthy volunteers also had greater crystallized IQ as assessed by the NAART; F(1,99) = 22.23, p ≤ 0.0001. No relationships between mean baseline medication dose (chlorpromazine equivalents per day) or total lifetime chlorpromazine dose and MWF in any of the six regions of interest were observed in the FEP sample (all p-values > 0.05). Analysis of covariance did not reveal differences in MWF scores for any region of interest between FEP subjects and healthy volunteers (all p-values > 0.10).
In healthy volunteers, significant positive relationships were found between frontal white matter MWF and age (r-value .48, p = .0005), NAART IQ (r-value .43, p = .0045) and years of completed education (r-value .52, p = 0.0003) (see Fig. 2). These relationships remained significant after Bonferroni correction (p-level ≤ .008). Healthy volunteers also had positive relationships between age and MWF in the anterior and posterior internal capsules, the genu, and the splenium (all p-values < 0.008), but not with years of completed education or NAART IQ (all p-values > .008). In FEP subjects, correlation of frontal white MWF and age (r-value .34, p = 0.009) did not remain significant after Bonferroni correction, nor did the correlation of frontal MWF and years of education completed (r-value .33, p = 0.01) (see Fig. 2, A–C). However, the relationship of frontal MWF and NAART IQ remained significant in the FEP group (r-value .38, p = .004). Additionally, a significant correlation between age and MWF of the splenium in FEP subjects was observed (r-value .35, p = 0.0006). In FEP subjects, no significant relationships between age, NAART IQ and years of completed education were observed for all other regions of interest.
Fig. 2.
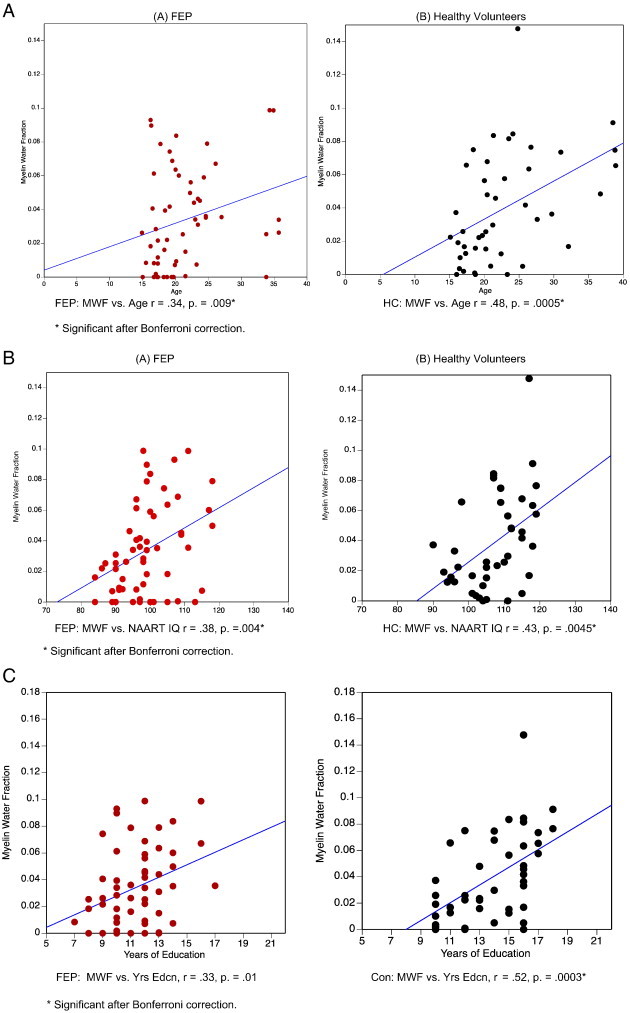
Myelin water fractions and regional associations with myelin water fractions. Panel A: Linear correlation of age and frontal white matter myelin water fraction in FEP subjects (red) and healthy volunteers (black). Significant Bonferroni-corrected values shown with asterisk. * Significant after Bonferroni correction. Panel B. Linear correlations of NAART IQ and frontal myelin water fraction in FEP subjects (red) and healthy volunteers (black). * Significant after Bonferroni correction. Panel C. Linear correlations of years of education and frontal myelin water fraction in FEP subjects (red) and healthy volunteers (black). * Significant after Bonferroni correction.
No between-group differences in frontal lobe white matter volume were observed for either the left or right side (all p-values > 0.50), nor were there left versus right side difference in frontal white volumes within either group (all p-values > 0.40). Neither left nor right frontal MWF was associated with ipsilateral frontal white matter volumes in either patients or healthy volunteers (all p-values > 0.10). No relationships between NAART IQ or age and frontal white matter volumes were observed for either left or right side in FEP subjects or healthy volunteers (all p-values > 0.30). In FEP subjects, positive relationships between frontal white volume and education were observed on the left (r = .34, F(1,55) = 7.40, p = 0.009) and the right (r = .28, F(1,55) = 4.6, p = 0.037), however these relationships did not remain significant after Bonferroni correction at the p ≤ 0.008 level. Similarly, in healthy volunteers, no significant relationships of white matter volume and education on either the left or right were observed (all p-values > 0.15).
3. Discussion
In the current study of first-episode schizophrenia patients, we did not observe any significant reduction in normal MWF in the frontal white matter using T2 myelin water imaging. This result contrasts our previous findings in chronic patients (Flynn et al., 2003); rather, they are consistent with previous data presented by Hirayasu et al. and Ho and colleagues indicating that volumetric frontal white matter reductions are not seen at the first episode, but are progressive over the first 3–5 years of illness (Hirayasu et al., 2001; Ho et al., 2003). In this context, the failure to detect significant frontal white matter MWF abnormality in the present FE sample is congruent with white matter that has not yet undergone measurable physical change. The lack of reductions in frontal white matter volumes in this first-episode cohort provides empirical support for our expectation that 1) MWF indices are unrelated to underlying white matter volume and 2) in the early stages of illness, frontal white matter volumes are preserved in schizophrenia patients. This observation is of interest as frontal areas are thought to have greater vulnerability in schizophrenia (Iwashiro et al., 2012) (Yang et al., 2012) and have been suggested to be affected at very early stages in the disease (Kanahara et al., 2013). The current data suggest a lack of significant reduction in frontal white matter MWF early in the illness, and support the hypothesis of progressive change in later schizophrenia. Interestingly, Yao et al. recently observed decreased posterior internal capsule and frontal white matter volume in untreated first episode patients. These anatomical alterations were related to the clinical symptoms but not the untreated illness duration, suggesting that these deficits are related to aberrations in the myelination process (Hoshi et al., 2006). The myelin-associated fraction index offers a distinct assessment of a physical property of myelin that is independent of white matter volume in our cohort.
In our study, associations between MWF and age in the anterior and posterior internal capsules, the genu, and the splenium in healthy volunteers were observed.
The present results also suggest attenuated associations between years of education and IQ in first-episode patients, particularly in the frontal white matter. These results are consistent with our earlier report in a cohort of chronic schizophrenia patients (Flynn et al., 2003). Flynn and colleagues reported significant relationships between age and frontal white MWF (r = .47, p = .01) and years of education and frontal white MWF (r = .51, p = 0.006) in healthy controls, but not in chronic schizophrenia patients. Such alterations in the relationships between frontal MWF and age, IQ and education suggest that subtle myelin-associated abnormalities already exist during the first-episode. Processes that are expected to drive normal myelination, such as maturation and learning, do not appear to confer the same results in persons with schizophrenia. With respect to age, associations between the anterior and posterior internal capsules, and the genu were observed in healthy volunteers. In contrast, these relationships were not seen in first-episode patients. These findings suggest that aberrant myelination patterns in schizophrenia may result in the emergence of altered white-matter maturation early in illness.
It has been suggested that myelin, an under-appreciated, yet vital component of human brain structure and function, is a possible unifying agent involved in the emergence of many neuropsychiatric disorders (Bartzokis, 2004). Myelination of the human brain is a continuous process in the first 35–40 years of life, undergoing periods of pruning and rapid expansion as part of the normal maturation process (Miller et al., 2012). Genetically mediated alterations, developmental interruptions or externally driven insults may result in perturbations of normal myelination, resulting in different psychiatric sequelae based on the timing of the perturbations. The natural sequencing of functional neurodevelopment would suggest that normally, mid- to late adolescence is a particularly critical phase of expansion in cognitive processing capacity and this increased capacity is supported by molecular and cellular maturation of the frontal circuitry (Catts et al., 2013). Adolescence is a unique period of brain maturation with respect to frontal–striatal myelination, that occurs in parallel with the pubertal to post-pubertal maturational stages (Asato et al., 2010). This timeframe coincides with the typical age at onset for schizophrenia, suggesting that abnormal myelination in the context of post-natal neurodevelopment may be involved in the emergence of psychosis.
Given the accumulating evidence for widespread subtle abnormalities of white matter at illness onset, the role of white matter pathophysiology may be important for the emergence of schizophrenia (Lee et al., 2013). However, white matter pathophysiology in schizophrenia is not yet well defined. Recent electron-microscope evidence has shown ultrastructural alterations in six types of oligodendrocytes. These neuroglial cells form myelin sheaths around axons (Uranova et al., 2011). Additionally, genetic studies of risk-variants of the myelin-associated glycoprotein (MAG) gene have reported that specific genotypes may predispose individuals to alterations of brain morphology, surprisingly in the temporal and parietal gray matter (Felsky et al., 2012). Whether some regions are more likely to be affected early in the course of illness, has not yet been established, although frontal areas appear to have greater vulnerability (Yang et al., 2012). Recent work using phosphorus spectroscopy found reduced brain phospholipid content in people with schizophrenia, which could be interpreted as reduced myelin content considering the high phospholipid content in the myelin bilayer (Vostrikov, 2007). In addition, the efficacy of antipsychotic medication may be partially mediated by its salutary effect on myelin and oligodendrocyte function. This finding provides additional support for a generalized underlying pathophysiology of myelin in psychosis (Ren et al., 2013).
The current application of myelin water imaging in a cohort of minimally treated patients, provides further evidence of abnormalities in frontal myelination patterns with respect to normal aging, IQ and educational attainment. The multiple lines of evidence suggest that aberrant myelination in schizophrenia plays a central role in the emergence of schizophrenia, and in particular, frontal myelin disruptions or deficits may be a key component of the illness.
References
- Andreasen N.C. A unitary model of schizophrenia: Bleuler's “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56(9):781–787. doi: 10.1001/archpsyc.56.9.781. 12884883 [DOI] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cerebral Cortex (New York, N.Y.: 1991) 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. 20051363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol. Aging. 2004;25:49–62. [Google Scholar]
- Benes F.M., Turtle M., Khan Y., Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. 8192550 [DOI] [PubMed] [Google Scholar]
- Blair J.R., Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clinical Neuropsychologist. 1989;3(2):129–136. [Google Scholar]
- Buchsbaum M.S., Schoenknecht P., Torosjan Y., Newmark R., Chu K.W., Mitelman S., Brickman A.M., Shihabuddin L., Haznedar M.M., Hazlett E.A., Ahmed S., Tang C. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Annals of General Psychiatry. 2006;5:19. doi: 10.1186/1744-859X-5-19. 17132158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts V.S., Fung S.J., Long L.E., Joshi D., Vercammen A., Allen K.M., Fillman S.G., Rothmond D.A., Sinclair D., Tiwari Y., Tsai S.Y., Weickert T.W., Shannon Weickert C. Rethinking schizophrenia in the context of normal neurodevelopment. Frontiers in Cellular Neuroscience. 2013;7:60. doi: 10.3389/fncel.2013.00060. 23720610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W.Y., Yang G.L., Chia M.Y., Lau I.Y., Sitoh Y.Y., Nowinski W.L., Sim K. White matter abnormalities in first-episode schizophrenia: a combined structural MRI and DTI study. Schizophrenia Research. 2010;119(1-3):52–60. doi: 10.1016/j.schres.2009.12.012. 20056394 [DOI] [PubMed] [Google Scholar]
- Crow T.J., Paez P., Chance S.A. Callosal misconnectivity and the sex difference in psychosis. International Review of Psychiatry (Abingdon, England) 2007;19(4):449–457. doi: 10.1080/09540260701486282. 17671877 [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Beers S.R., Hall J., Frustaci K., Masalehdan A., Noll J., Boring A.M. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex (New York, N.Y.: 1991) 2001;11(6):552–557. doi: 10.1093/cercor/11.6.552. 11375916 [DOI] [PubMed] [Google Scholar]
- Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:C36666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., Cooper A., Cohen B.M., Renshaw P.F., Ongur D. Water and metabolite transverse T2 relaxation time abnormalities in the white matter of schizophrenia. Schizophrenia Research. 2012;137:241–245. doi: 10.1016/j.schres.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsky D., Voineskos A.N., Lerch J.P., Nazeri A., Shaikh S.A., Rajji T.K., Mulsant B.H., Kennedy J.L. Myelin-associated glycoprotein gene and brain morphology in schizophrenia. Frontiers in Psychiatry. 2012;3:1–6. doi: 10.3389/fpsyt.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn S.W., Lang D.J., Mackay A.L., Goghari V.M., Vavasour I.M., Whittall K.P., Smith G.N., Arango V., Mann J.J., Dwork A.J., Falkai P., Honer W.G. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Molecular Psychiatry. 2003;8(9):811–820. doi: 10.1038/sj.mp.4001337. 12931208 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clinical Neuroscience (New York, N.Y.) 1995;3(2):89–97. 7583624 [PubMed] [Google Scholar]
- Garver D.L., Holcomb J.A., Christensen J.D. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2008;11(1):49–61. doi: 10.1017/S1461145707007730. 17708778 [DOI] [PubMed] [Google Scholar]
- Girard T.A., Christensen B.K., Rizvi S. Visual–spatial episodic memory in schizophrenia: a multiple systems framework. Neuropsychology. 2010;24(3):368–378. doi: 10.1037/a0018313. 20438214 [DOI] [PubMed] [Google Scholar]
- Hao Y., Liu Z., Jiang T., Gong G., Liu H., Tan L., Kuang F., Xu L., Yi Y., Zhang Z. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport. 2006;17(1):23–26. doi: 10.1097/01.wnr.0000195664.15090.46. 16361944 [DOI] [PubMed] [Google Scholar]
- Highley J.R., Walker M.A., Esiri M.M., Crow T.J., Harrison P.J. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cerebral Cortex (New York, N.Y.: 1991) 2002;12(11):1218–1224. doi: 10.1093/cercor/12.11.1218. 12379610 [DOI] [PubMed] [Google Scholar]
- Hirayasu Y., Tanaka S., Shenton M.E., Salisbury D.F., DeSantis M.A., Levitt J.J., Wible C., Yurgelun-Todd D., Kikinis R., Jolesz F.A., McCarley R.W. Prefrontal gray matter volume reduction in first episode schizophrenia. Cerebral Cortex. 2001;11(4):374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- Ho B.C., Andreasen N.C., Nopoulos P., Arndt S., Magnotta V., Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Archives of General Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. 12796222 [DOI] [PubMed] [Google Scholar]
- Hoshi Y., Shinba T., Sato C., Doi N. Resting hypofrontality in schizophrenia: a study using near-infrared time-resolved spectroscopy. Schizophrenia Research. 2006;84(2-3):411–420. doi: 10.1016/j.schres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hunter R., Barry S. Negative symptoms and psychosocial functioning in schizophrenia: neglected but important targets for treatment. European Psychiatry: the Journal of the Association of European Psychiatrists. 2012;27(6):432–436. doi: 10.1016/j.eurpsy.2011.02.015. 21602034 [DOI] [PubMed] [Google Scholar]
- Iwashiro N., Suga M., Takano Y., Inoue H., Natsubori T., Satomura Y., Koike S., Yahata N., Murakami M., Katsura M., Gonoi W., Sasaki H., Takao H., Abe O., Kasai K., Yamasue H. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophrenia Research. 2012;137(1-3):124–131. doi: 10.1016/j.schres.2012.02.024. 22425035 [DOI] [PubMed] [Google Scholar]
- Kanahara N., Sekine Y., Haraguchi T., Uchida Y., Hashimoto K., Shimizu E., Iyo M. Orbitofrontal cortex abnormality and deficit schizophrenia. Schizophrenia Research. 2013;143(2-3):246–252. doi: 10.1016/j.schres.2012.11.015. 23228712 [DOI] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. Kaufman Brief Intelligence Test. American Guidance Service; Circle Pines, MN: 1990. [Google Scholar]
- Kolind S.H., Mädler B., Fischer S., Li D.K., Mackay A.L. Myelin water imaging: implementation and development at 3.0 T and comparison to 1.5 T measurements. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;62(1):106–115. doi: 10.1002/mrm.21966. 19353659 [DOI] [PubMed] [Google Scholar]
- Kong X., Ouyang X., Tao H., Liu H., Li L., Zhao J., Xue Z., Wang F., Jiang S., Shan B., Liu Z. Complementary diffusion tensor imaging study of the corpus callosum in patients with first-episode and chronic schizophrenia. Journal of Psychiatry & Neuroscience. 2011;36(2):120–125. doi: 10.1503/jpn.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C., Kolind S.H., Bjarnason T.A., Li D.K., MacKay A.L. In-vivo multi-echo T2 relaxation measurements using variable TR to decrease scan time. Magnetic Resonance Imaging. 2007;25:834–839. doi: 10.1016/j.mri.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Laule C., Leung E., Lis D.K., Traboulsee A.L., Paty D.W., MacKay A.L., Moore G.R. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Multiple Sclerosis (Houndmills, Basingstoke, England) 2006;12(6):747–753. doi: 10.1177/1352458506070928. 17263002 [DOI] [PubMed] [Google Scholar]
- Laule C., Vavasour I.M., Whittall K.P., Oger J., Paty D.W., Li D.K., MacKay A.L., Arnold D.L. Evolution of focal and diffuse magnetisation transfer abnormalities in multiple sclerosis. Journal of Neurology. 2003;250(8):924–931. doi: 10.1007/s00415-003-1115-z. 12928910 [DOI] [PubMed] [Google Scholar]
- Lee S.H., Kubicki M., Asami T., Seidman L.J., Goldstein J.M., Mesholam-Gately R.I., McCarley R.W., Shenton M.E. Extensive white matter abnormalities in patients with first-episode schizophrenia: a Diffusion Tensor Imaging (DTI) study. Schizophrenia Research. 2013;143(2-3):231–238. doi: 10.1016/j.schres.2012.11.029. 23290268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay A.L., Vavasour I.M., Rauscher A., Kolind S.H., Madler B., Moore G.R.W., Traboulsee A.L., Li D.K., Laule C. MR Relaxation in Multiple Sclerosis Neuroimaging Clinics of North America. 2009:1–26. doi: 10.1016/j.nic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Mackay A.L., Whittall K.P., Adler J., Li D.K., Paty D.W., Graeb D.A. In vivo visualization of myelin water in brain by magnetic resonance. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1994;31(6):673–677. doi: 10.1002/mrm.1910310614. 8057820 [DOI] [PubMed] [Google Scholar]
- Meyers S.M., Laule C., Vavasour I.M., Kolind S.H., Mädler B., Tam R., Traboulsee A.L., Lee J., Li D.K., Mackay A.L. Reproducibility of myelin water fraction analysis: a comparison of region of interest and voxel-based analysis methods. Magnetic Resonance Imaging. 2009;27(8):1096–1130. doi: 10.1016/j.mri.2009.02.001. 19356875 [DOI] [PubMed] [Google Scholar]
- Miller D.J., Duka T., Stimpson C.D., Schapiro S.J., Baze W.B., McArthur M.J., Fobbs A.J., Sousa A.M.M., Sestan N., Wildman D.E., Lipovich L., Kuzawa C.W., Hof P.R., Sherwood C.C. Prolonged myelination in human neocortical evolution. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(41):16480–16485. doi: 10.1073/pnas.1117943109. 23012402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G., Cercignani M., Chu E.M., Barnes T.R., Barker G.J., Joyce E.M., Ron M.A. Brain pathology in first-episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. Neuroimage. 2010;49(1):185–192. doi: 10.1016/j.neuroimage.2009.07.037. 19632338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Wang H., Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2013;16(3):691–700. doi: 10.1017/S1461145712001095. 23164411 [DOI] [PubMed] [Google Scholar]
- Sirrs S.M., Laule C., Mädler B., Brief E.E., Tahir S.A., Bishop C., Mackay A.L. Normal-appearing white matter in patients with phenylketonuria: water content, myelin water fraction, and metabolite concentrations. Radiology. 2007;242(1):236–243. doi: 10.1148/radiol.2421051758. 17185670 [DOI] [PubMed] [Google Scholar]
- Uranova N.A., Vikhreva O.V., Rachmonova V.I., Orlovskaya D. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a post-mortem morphometric study. Schizophrenia Research & Treatment. 2011;2011:13. doi: 10.1155/2011/325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani A.S., Bezchilbnyk-Butler K.Z., Jeffries J.J., Procyshyn R.M. Clinical Handbook of Psychotropic Drugs. nineteenth edition. Hogrefe; Ashland, OH: 2012. [Google Scholar]
- Vostrikov V.M. [Decreased numerical density of pericapillary oligodendrocytes in the cortex in schizophrenia] Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova / Ministerstvo Zdravookhraneniia i Meditsinskoĭ Promyshlennosti Rossiĭskoĭ Federatsii, Vserossiĭskoe Obshchestvo Nevrologov [i] Vserossiĭskoe Obshchestvo Psikhiatrov. 2007;107(12):58–65. 18427462 [PubMed] [Google Scholar]
- Whitford T.J., Ford J.M., Mathalon D.H., Kubicki M., Shenton M.E. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophrenia Bulletin. 2012;38(3):486–494. doi: 10.1093/schbul/sbq105. 20855415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall K.P., Mackay A.L., Graeb D.A., Nugent R.A., Li D.K., Paty D.W. In vivo measurement of T2 distributions and water contents in normal human brain. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1997;37(1):34–43. doi: 10.1002/mrm.1910370107. 8978630 [DOI] [PubMed] [Google Scholar]
- Yakelov P.I., Lecours A.R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Blackwell; Oxford, UK: 1967. [Google Scholar]
- Yang Y., Nuechterlein K.H., Phillips O.R., Gutman B., Kurth F., Dinov I., Thompson P.M., Asarnow R.F. Disease and genetic contributions toward local tissue volume disturbances in schizophrenia: a tensor-based morphometry study. Human Brain Mapping. 2012;33(9):2081–2091. doi: 10.1002/hbm.21349. 22241649 [DOI] [PMC free article] [PubMed] [Google Scholar]


