Abstract
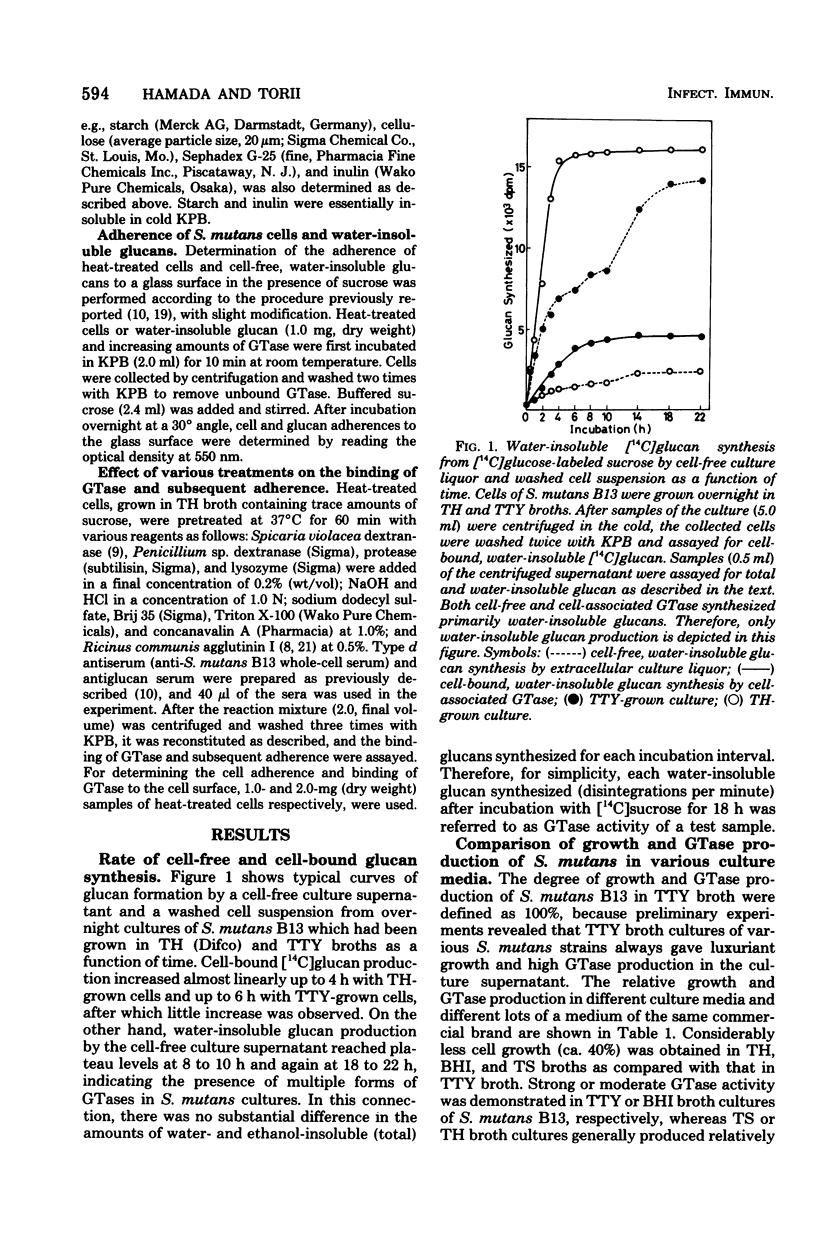
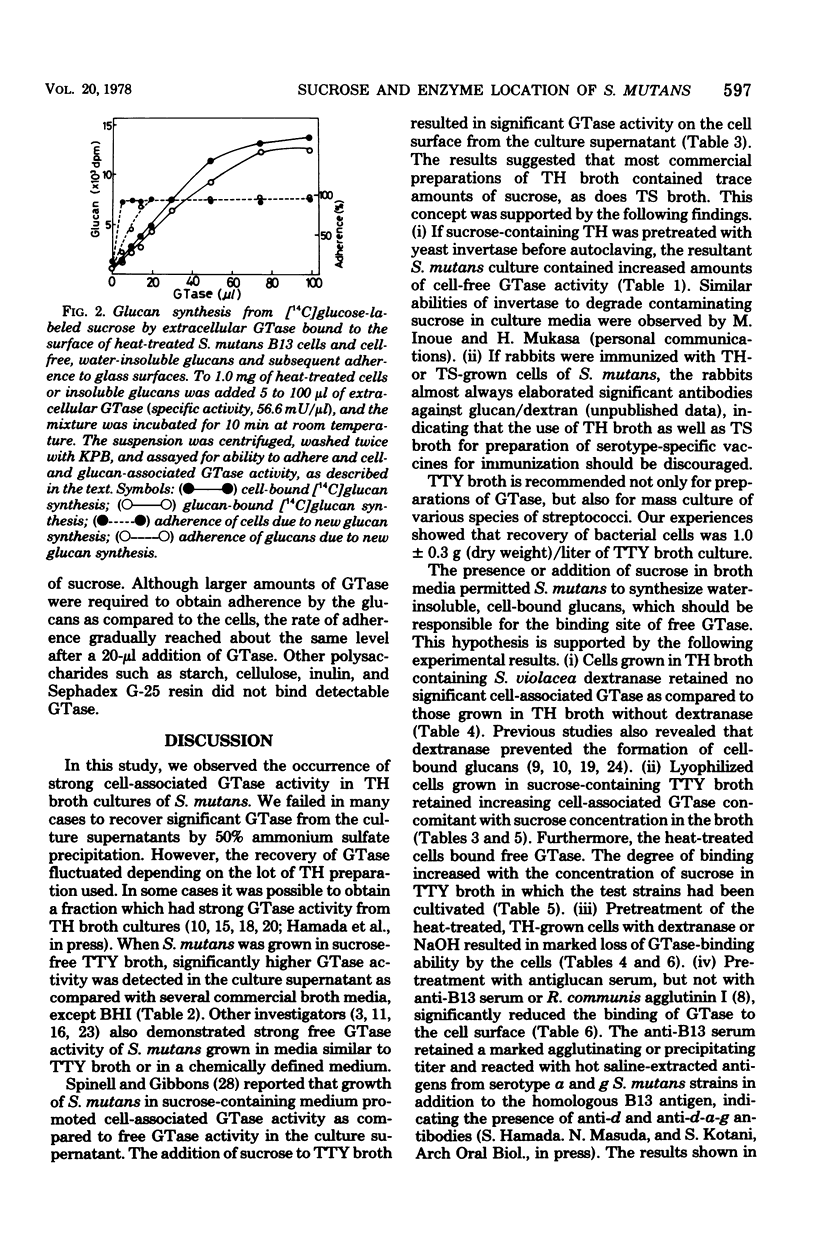
Streptococcus mutans strain B13 (serotype D) almost exclusively produced free glucosyltransferase (GTase) in the culture supernatant when grown in sucrose-free TTY broth medium, which was composed of Trypticase (Baltimore Biological Laboratory [BBL] Cockeysville, Md.), tryptose (Difco Laboratories, Detroit, Mich.), yeast extract (BBL), salts, and 1% glucose. Organisms grown in sucrose-free TTY broth retained very weak cell-associated GTase activity and did not adhere significantly to glass surfaces in the presence of exogenous sucrose. If sucrose was added to TTY broth, however, GTase was found on the cell surface where cell-bound, water-insoluble glucans were synthesized. Most commercially available products of Todd-Hewitt broth were found to contain trace amounts of sucrose, as did Trypticase soy broth (BBL), whereas brain heart infusion broth (Difco and BBL) was found to be essentially free of sucrose. Almost all detectable GTase activity was cell associated when S. mutans B13 was grown in Todd-Hewitt or trypticase soy broth. Heat-treated B13 cells grown in Todd-Hewitt broth and cell-free, water-insoluble glucans bound free GTase and produced marked adherence in the presence of sucrose. Experiments strongly suggest that the binding sites for free GTase are the surface glucans, and cell-associated and extracellular GTases are most likely alternate states of the same enzyme protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozzola J. J., Johnson M. C., Shechmeister I. L. Ultrastructural localization of sucrases in Streptococcus mutans GS-5 and an extracellular polysaccharide mutant: a comparative cytochemical and immunocytochemical study. Infect Immun. 1977 Aug;17(2):447–457. doi: 10.1128/iai.17.2.447-457.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Beaman A. J., Wittenberger C. L. Purification, resolution, and interaction of the glucosyltransferases of Streptococcus mutans 6715. Infect Immun. 1977 Oct;18(1):237–246. doi: 10.1128/iai.18.1.237-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hamada S., Gill K., Slade H. D. Binding of lectins to Streptococcus mutans cells and type-specific polysaccharides, and effect on adherence. Infect Immun. 1977 Dec;18(3):708–716. doi: 10.1128/iai.18.3.708-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Mizuno J., Murayama Y., Ooshima Y., Masuda N. Effect of dextranase on the extracellular polysaccharide synthesis of Streptococcus mutans; chemical and scanning electron microscopy studies. Infect Immun. 1975 Dec;12(6):1415–1425. doi: 10.1128/iai.12.6.1415-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda W. M., Kuramitsu H. K. Regulation and extracellular glucosyltransferase production and the relationship between extracellular and cell-associated activities in Streptococcus mutans. Infect Immun. 1976 Jul;14(1):191–202. doi: 10.1128/iai.14.1.191-202.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Molecular basis for the different sucrose-dependent adherence properties of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1977 Aug;17(2):330–337. doi: 10.1128/iai.17.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Characterization of an anti-glucosyltransferase serum specific for insoluble glucan synthesis by Streptococcus mutans. Infect Immun. 1976 Feb;13(2):494–500. doi: 10.1128/iai.13.2.494-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Purification and characterization of Streptococcus mutans group d cell wall polysaccharide antigen. Infect Immun. 1974 Aug;10(2):361–368. doi: 10.1128/iai.10.2.361-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Specific method for the purification of Streptococcus mutans dextransucrase. Infect Immun. 1977 Jun;16(3):760–765. doi: 10.1128/iai.16.3.760-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Staat R. H., Harlander S. K. Dextranases from oral bacteria: inhibition of water-insoluble glucan production and adherence to smooth surfaces by Streptococcus mutans. Infect Immun. 1975 Aug;12(2):309–317. doi: 10.1128/iai.12.2.309-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIYA H. M., KOEPSELL H. J., CORMAN J., BRYANT G., BOGARD M. O., FEGER V. H., JACKSON R. W. The effect of certain cultural factors on production of dextransucrase by Leuconostoc mesenteroides. J Bacteriol. 1952 Oct;64(4):521–526. doi: 10.1128/jb.64.4.521-526.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]


