Abstract
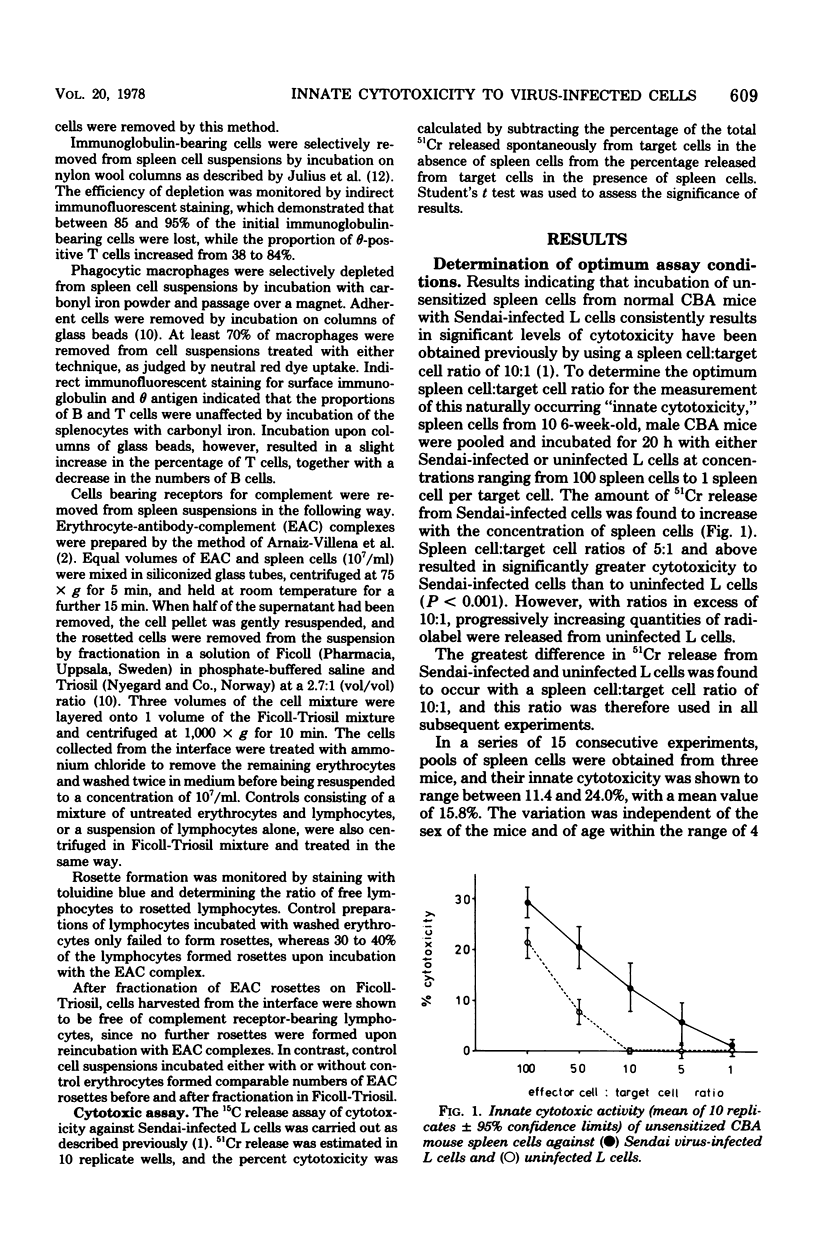
The presence in the spleens of unsensitized CBA mice of cells that are spontaneously cytotoxic for Sendai virus-infected L cells was confirmed. This innate cytotoxic activity to virus-infected cells was shown to exhibit some H-2 restriction. Partial identity of only the D end of the H-2 gene complex between the target and effector cells was required to produce cytolysis. Attempts to characterize the kind of cell active in this system indicated that neither the theta antigen nor the surface immunoglobulin markers were present. Furthermore, the cells appeared to have no adherent or phagocytic properties. The relationship between the effector cells responsible for innate cytotoxicity to virus-infected cells and the natural killer (NK) cells spontaneously cytotoxic for certain tumor cells is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Bainbridge D. R., Pattison J. R., Heath R. B. Cell-mediated immunity to Sendai virus infection in mice. Infect Immun. 1977 Jan;15(1):239–244. doi: 10.1128/iai.15.1.239-244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz-Villena A., Gyöngyössy M. I., Playfair J. H. Rosette formation by mouse lymphocytes. II. T-cell specificities in a CRL subpopulation. Clin Exp Immunol. 1974 Oct;18(2):177–186. [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Gardner I. D., Bowern N. A., Blanden R. V. Cell-medicated cytotoxicity against ectromelia virus-infected target cells. III. Role of the H-2 gene complex. Eur J Immunol. 1975 Feb;5(2):122–127. doi: 10.1002/eji.1830050210. [DOI] [PubMed] [Google Scholar]
- Glimcher L., Shen F. W., Cantor H. Identification of a cell-surface antigen selectively expressed on the natural killer cell. J Exp Med. 1977 Jan 1;145(1):1–9. doi: 10.1084/jem.145.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomard E., Leclerc J. C., Levy J. P. Spontaneous antilymphoma reaction of preleukaemic AKR mice is a non-T-cell killing. Nature. 1974 Aug 23;250(5468):671–673. doi: 10.1038/250671a0. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Shen L., Walker L., Arnaiz-Villena A., Roitt I. M. Characteristics of the effector cells mediating cytotoxicity against antibody-coated target cells. II. The mouse nonadherent K cell. Eur J Immunol. 1976 Jul;5(7):474–480. doi: 10.1002/eji.1830050709. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Kärre K., Jondal M., Tracey D., Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976 Apr 1;143(4):772–780. doi: 10.1084/jem.143.4.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Maclaurin B. P., Clarke G. N. Characterization of the normal lymphocyte population cytolytic to Burkitt's lymphoma cells of the EB2 cell line. Aust J Exp Biol Med Sci. 1976 Oct;53(5):389–398. doi: 10.1038/icb.1975.44. [DOI] [PubMed] [Google Scholar]
- Peter H. H., Eife R. F., Kalden J. R. Spontaneous cytotoxicity (SCMC) of normal human lymphocytes against a human melanoma cell line: a phenomenon due to a lymphotoxin-like mediator. J Immunol. 1976 Feb;116(2):342–348. [PubMed] [Google Scholar]
- Stott E. J., Probert M., Thomas L. H. Cytotoxicity of alveolar macrophages for virus-infected cells. Nature. 1975 Jun 26;255(5511):710–712. doi: 10.1038/255710a0. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Stobo J. D., Paul W. E., Green I. Antibody-dependent lymphoid cell-mediated cytotoxicity: no requirement for thymus-derived lymphocytes. Science. 1972 Jan 14;175(4018):194–196. doi: 10.1126/science.175.4018.194. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Nowinski R. C., Bach F. H. Lysis of leukemia cells by spleen cells of normal mice. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2780–2784. doi: 10.1073/pnas.72.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., Cornain S., Rumke P. Cytotoxity of non-T versus T-lymphocytes from melanoma patients and healthy donors on short- and long-term cultured melanoma cells,. Int J Cancer. 1974 Oct 15;14(4):427–434. doi: 10.1002/ijc.2910140402. [DOI] [PubMed] [Google Scholar]


