Abstract
Little is known about the occurrence and predictors of the psychosis spectrum in large non-clinical community samples of U.S. youths. We aimed to bridge this gap through assessment of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort, a collaborative investigation of clinical and neurobehavioral phenotypes in a prospectively accrued cohort of youths, funded by the National Institute of Mental Health. Youths (age 11-21; N=7,054) and collateral informants (caregiver/legal guardian) were recruited through the Children's Hospital of Philadelphia and administered structured screens of psychosis spectrum symptoms, other major psychopathology domains, and substance use. Youths were also administered a computerized neurocognitive battery assessing five neurobehavioral domains. Predictors of psychosis spectrum status in physically healthy participants (N=4,848) were examined using logistic regression. Among medically healthy youths, 3.7% reported threshold psychotic symptoms (delusions and/or hallucinations). An additional 12.3% reported significant sub-psychotic positive symptoms, with odd/unusual thoughts and auditory perceptions, followed by reality confusion, being the most discriminating and widely endorsed attenuated symptoms. A minority of youths (2.3%) endorsed subclinical negative/disorganized symptoms in the absence of positive symptoms. Caregivers reported lower symptom levels than their children. Male gender, younger age, and non-European American ethnicity were significant predictors of spectrum status. Youths with spectrum symptoms had reduced accuracy across neurocognitive domains, reduced global functioning, and increased odds of depression, anxiety, behavioral disorders, substance use and suicidal ideation. These findings have public health relevance for prevention and early intervention.
Keywords: Psychosis spectrum, U.S. youths, sub-psychotic positive symptoms, neurocognition, functional impairment
Psychotic-like symptoms, including attenuated paranoid delusional thinking and auditory hallucinations, occur in approximately 5-8% of the general adult population (1). In some, these symptoms never cause sufficient distress or functional impairment to necessitate help seeking (2), but in others they can precede the onset of psychotic disorders (3).
A meta-analysis of transition from subthreshold to psychotic experiences in unselected, non-help-seeking population samples reported a 0.6% one-year risk of transition to psychotic disorder (4), a much lower conversion rate than in clinically help-seeking samples (5). This is consistent with a continuum of non-clinical and clinical expressions of psychotic-like experiences in the population (1).
The psychosis spectrum (6) comprises subclinical psychotic-like experiences, threshold psychosis (delusions/hallucinations), and psychosis related symptoms including attenuated negative and disorganized symptoms. Because early attenuated symptoms frequently do not lead to disabling psychosis (7), their utility as a sole means of identifying at-risk individuals is limited. However, through longitudinal evaluation, they provide a window to examine neurobiological risk and protective factors associated with various outcomes (4).
This window may be widened by evaluating the earliest emergence of subclinical spectrum symptoms in younger people from the general population (8). Symptom onset before age 18 is a significant predictor of psychosis conversion in ultra-high risk (9) and birth cohort (7,8,10) samples. Population-based studies in children and adolescents have demonstrated higher rates of psychotic-like experiences in youths than in adults (range=5-35%), with meta-analytically derived medians of 17% in children (9-12 years old), and 7.5% in adolescents (13-18 years old) (11). Although these symptoms are likely transient in most children (8), their evolution into full psychosis may be moderated by symptom severity (4), type (3), and persistence (12,13).
Regardless of eventual psychosis, early subclinical psychotic symptoms are associated with comorbid psychopathology, including depression (5,14-16), anxiety (5), and substance use (5,12,17); impaired global functioning (18) and increased suicidality (18). Consistent with some findings of neurocognitive impairments in individuals at clinical high risk (19,20), a school-based study of children (age 11-13) in Ireland also reported an association of subclinical symptoms with impairment in processing speed and non-verbal working memory (21). Otherwise, little is known about neurocognitive functioning associated with early emerging subclinical spectrum symptoms in the general population.
No prior study has characterized the occurrence of psychosis spectrum features in a large systematic community sample of U.S. youths. Moreover, little is known about demographic and psychopathology predictors of psychosis features in this population. We aimed to bridge this gap through the Philadelphia Neurodevelopmental Cohort, an investigation of clinical and neurobehavioral phenotypes in a prospectively accrued non-clinical sample, funded by the National Institute of Mental Health (NIMH).
METHODS
Participants
Prospective participants (N=50,293) were recruited through the Children's Hospital of Philadelphia pediatric clinics and health care network, which extends to over 30 clinical community sites in the tri-state area of Pennsylvania, New Jersey and Delaware. Participants were not recruited from psychiatric clinics, so the sample is not enriched for those seeking mental health services.
Based on electronic medical review or follow-up phone contact, potential participants from this pool were excluded if they were not proficient in English, had significant developmental delays or other conditions that would interfere with their ability to complete study procedures, or could not be contacted.
From the remaining pool, 13,598 individuals were invited, 2,699 declined, 1,401 were excluded, and 9,498 youths (age 8-21) were enrolled. The total sample for the current analyses included youths aged 11-21 (N=7,054 participants, mean age 15.8±2.7 years; 54% female; 56.3% European American, 32.9% African American, 10.8% other) enrolled between November 2009 and November 2011.
After complete description of the study, written informed consent was obtained for participants aged at least 18, and written assent and parental permission were obtained from children aged less than 18 and their parents/legal guardian. All procedures were approved by the University of Pennsylvania and the Children's Hospital of Philadelphia Institutional Review Boards.
Psychopathology measures
Probands (age 11-21) and collaterals (parent or legal guardian for probands aged 11-17) were administered a computerized structured interview (GOASSESS), including a timeline of life events, demographics and medical history, psychopathology screen, Children's Global Assessment Scale (C-GAS) (22), and interviewer observations.
Psychopathology screen was conducted through an abbreviated computerized version of the NIMH Genetic Epidemiology Research Branch Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) (23,24), that was modified to collect information on symptoms, duration, distress and impairment for lifetime mood, anxiety, behavioral, psychosis spectrum and eating disorders, suicidal thinking and behavior, as well as treatment history.
Computerized algorithms determined screen positive status for each psychopathology domain based on endorsement of symptoms, frequency and duration to approximate DSM-IV disorder or episode criteria, and significant distress or impairment rated at least 5 on an 11-point scale. A self-reported lifetime substance use measure (25) was added later in the study and administered to 4,066 participants. Comparison of the diagnostic algorithms with the full criteria using data from the National Comorbidity Survey – Adolescent (23) yielded fair to excellent area under the receiver operator characteristic curve values for the major classes of disorders.
GOASSESS medical history supplemented the Children's Hospital of Philadelphia electronic medical records, and was used to identify a subgroup of physically healthy participants (no or mild physical illnesses).
Assessors underwent rigorous training, certification and monitoring.
Psychosis spectrum screen
We aimed to perform a brief screen for a broad spectrum of psychosis relevant experiences, ranging from subtle and subclinical (positive, negative and disorganized) symptoms that would not qualify for diagnosable disorders to clinically threshold hallucinations and delusions that could meet criteria for serious psychotic disorders. We thus included three screening tools to assess positive sub-psychosis, positive psychosis, and negative/disorganized symptoms. Individuals evidencing any of those symptoms were classified as “psychosis spectrum”.
Positive sub-psychosis symptoms in the past year were assessed with the 12-item assessor administered PRIME Screen-Revised (26,27). Items were self-rated on a 7-point scale ranging from 0 (“definitely disagree”) to 6 (“definitely agree”). The participant then rated the duration of each endorsed symptom.
Positive psychosis symptoms (lifetime hallucinations and delusions) were assessed using the K-SADS psychosis screen questions, supplemented with structured questions to reduce false positives.
Negative/disorganized symptoms were assessed using six embedded assessor rated items from the Scale of Prodromal Syndromes (SOPS, 28): avolition, expression of emotion, experience of emotions and self, occupational functioning, trouble with focus and attention, disorganized communication.
For positive sub-psychosis, given age effects on PRIME Screen-Revised score, an Age Deviant index was derived identifying children with extreme total scores (z≥2) compared to age mates. In addition, because psychosis risk may not be linearly related to total scores, such that endorsement of even one symptom at a severe level may be indicative of psychosis risk, an Extreme Agreement index was also calculated based on traditional criteria (at least one item rated 6, “definitely agree”, or at least three items rated 5, “somewhat agree”) (27).
Criteria for positive psychosis were hallucinations and/or delusions based on K-SADS screen, with duration of at least one day, occurring outside the context of substance use and physical illness, and accompanied by significant impairment or distress (rating of at least 5).
For negative/disorganized symptoms, an Age Deviant index was generated using SOPS z-scores. Specifically, SOPS total scores were normed within age; z≥2 cutoff reflected extreme ratings of negative and/or disorganized symptoms for age cohort.
Neurocognitive assessment
The 1-hour computerized neurocognitive battery included 14 tests assessing five neurobehavioral domains (29): executive functions (abstraction and mental flexibility, attention, working memory), episodic memory (words, faces, shapes), complex cognition (verbal reasoning, non-verbal reasoning, spatial processing), social cognition (emotion identification, emotion intensity differentiation, age differentiation), sensorimotor speed (motor, sensorimotor).
Except for the tests designed exclusively for measuring speed, each test provides measures of both accuracy and speed. The Reading subtest of the Wide Range Achievement Test, version 4 (WRAT-4) (30) was administered first to determine participants' ability to complete the battery and to provide an estimate of IQ.
Statistical approach
First, we evaluated internal consistency (Cronbach's alpha) and age distributions of psychosis spectrum items in the total sample. Second, to minimize conflation of psychosis spectrum symptoms and experiences occurring in the context of, or attributable to, physical illnesses, we classified psychosis spectrum status, characterizing demographics and screen summary variables as well as neurocognitive function, in the subgroup of physically healthy youths. Third, we evaluated differences between psychosis spectrum and non-spectrum participants using ANOVA's and Cohen's d (quantitative variables) or chi-square (categorical variables). Fourth, logistic regression examined demographic, psychopathology and substance use predictors of spectrum status (Statistical Package for Social Sciences, SPSS, version 20).
Finally, we performed item analysis of positive sub-psychosis items comparing endorsements between groups, summarizing symptom endorsement count, and conducting multivariate analysis of variance (MANOVA) of differences in mean item ratings. Receiver operating characteristics analysis identified positive sub-psychosis items most predictive of psychosis spectrum vs. non-spectrum classification (SPSS, version 20).
RESULTS
Psychosis spectrum screen characteristics
Among the total sample of 7,054 participants, 21.0% (N=1,482) met psychosis spectrum criteria. Positive sub-psychosis Extreme Agreement was 14.6% (N=1,028). PRIME Screen-Revised mean total score was 8.0±10.7 for probands and 2.4±5.9 for collaterals; the proband-collateral pair total difference mean was 8.0±10.0, indicating that probands endorsed higher levels of sub-psychosis than reported by their caregivers.
There was a significant difference in PRIME Screen-Revised total scores across the 11-21 age groups (ANOVA: F=13.69, df=10,7042, p<0.001); pairwise post-hoc tests showed linear and decreasing total scores with age. There was also a significant age effect on SOPS items (ANOVA: F=3.24, df=10,6759, p<0.001), with some younger groups rated lower than older participants.
Internal consistency was high in probands (alpha=0.87) and collaterals (alpha=0.86) for the PRIME-Screen Revised, and was acceptable for the SOPS (alpha=0.70).
Characteristics of young people with psychosis spectrum features
In the subgroup of physically healthy participants (N=4,848), 3.7% reported threshold psychotic symptoms (delusions and/or hallucinations), an additional 12.3% reported significant sub-psychotic symptoms, and 2.3% endorsed subclinical negative/disorganized symptoms in the absence of positive symptoms.
Among youths classified as psychosis spectrum (N=954), 2.0% fulfilled four criteria, 8.5% three criteria, 23.0% two criteria, and 66.4% one criterion. The positive sub-psychosis Extreme Agreement was 67.6%, the positive sub-psychosis Age Deviant was 34.4%, and the negative/disorganized Age Deviant was 23.9%.
Characteristics of psychosis spectrum vs. non-spectrum participants are presented in Table 1. Gender distribution was proportional between the two groups, and although psychosis spectrum were younger, the effect was small. The psychosis spectrum group was disproportionately non-European American, and had lower WRAT-4 Reading standard scores, parental education, and global functioning. Notably, caregivers reported significantly lower positive sub-psychosis ratings than probands.
Table 1.
Characteristics of physically healthy psychosis spectrum and non-spectrum youths
| Psychosis spectrum | Non-spectrum | Test | df | Result | p | Cohen's d | |
|---|---|---|---|---|---|---|---|
| N (%) | 954 (20.0) | 3894 (80.0) | - | - | - | - | - |
| Gender (% male) | 47.9 | 45.0 | Chi-square | 1 | χ2=2.5 | n.s. | - |
| Age (mean±SD) | 15.2±2.7 | 15.8±2.7 | ANOVA | 1,4846 | F=31.9 | 0.001 | −0.22 |
| Ethnicity (% European American) | 37.7 | 58.1 | Chi-square | 1 | χ2=127.5 | 0.001 | - |
| WRAT-4 Reading standard score (mean±SD) | 97.9±17.2 | 103.6±16.9 | ANOVA | 1,4832 | F=86.1 | 0.001 | −0.32 |
| Parental education | |||||||
| Mother (years, mean±SD) | 13.8±2.3 | 14.5±2.4 | ANOVA | 1,4783 | F=70.8 | 0.001 | −0.29 |
| Father (years, mean±SD) | 13.4±2.5 | 14.4±2.7 | ANOVA | 1,4437 | F=93.1 | 0.001 | −0.38 |
| PRIME Screen-Revised | |||||||
| Proband total (mean±SD) | 21.4±12.9 | 4.3±6.1 | ANOVA | 1,4846 | F=3557.2 | 0.001 | 2.16 |
| Proband z (mean±SD) | 1.3±1.2 | −0.4±0.6 | ANOVA | 1,4809 | F=3565.5 | 0.001 | 2.25 |
| Collateral total (mean±SD) | 4.2±8.1 | 1.6±4.7 | ANOVA | 1,3652 | F=128.6 | 0.001 | 0.61 |
| Proband-collateral pair total difference (mean±SD) | 19.1±12.2 | 4.8±6.3 | - | - | - | - | - |
| Threshold psychotic symptoms (%) | 20.2 | - | - | - | - | - | - |
| Hallucinations (%) | 17.9 | - | - | - | - | - | - |
| Delusions (%) | 11.2 | - | - | - | - | - | - |
| Scale of Prodromal Symptoms (SOPS) | |||||||
| Total (mean±SD) | 5.1±4.7 | 1.6±1.8 | ANOVA | 1,4669 | F=1347.4 | 0.001 | 1.33 |
| z (mean±SD) | 0.9±1.5 | −0.3±0.6 | ANOVA | 1,4820 | F=1430.0 | 0.001 | 1.40 |
| Trouble with focus/attention (mean±SD) | 1.69±1.3 | 0.8±1.0 | ANOVA | 1,4757 | F=489.7 | 0.001 | 0.84 |
| Experience of emotions and self (mean±SD) | 0.6±1.1 | 0.1±0.4 | ANOVA | 1,4803 | F=595.4 | 0.001 | 0.83 |
| Expression of emotion (mean±SD) | 1.0±1.2 | 0.3±0.7 | ANOVA | 1,4740 | F=447.0 | 0.001 | 0.85 |
| Avolition (mean±SD) | 0.7±1.2 | 0.1±0.4 | ANOVA | 1,4795 | F=646.1 | 0.001 | 0.94 |
| Disorganized communication (mean±SD) | 0.5±0.9 | 0.1±0.4 | ANOVA | 1,4806 | F=410.7 | 0.001 | 0.75 |
| Occupational functioning (mean±SD) | 0.7±1.2 | 0.1±0.5 | ANOVA | 1,4789 | F=490.9 | 0.001 | 0.86 |
| Children's Global Assessment Scale, current (mean±SD) | 70.8±13.4 | 81.4±10.5 | ANOVA | 1,4807 | F=697.6 | 0.001 | −0.95 |
WRAT-4 – Wide Range Achievement Test, version 4
Among psychosis spectrum youths, the positive sub-psychosis items most frequently endorsed (“definitely agree”) on the PRIME Screen-Revised were odd/unusual thoughts and auditory perceptions, followed by reality confusion (Table 2). The least frequently endorsed was thought control. All PRIME Screen-Revised items yielded higher ratings in psychosis spectrum compared to non-spectrum participants (MANOVA), with effect sizes greater than 0.92 and the largest group differences yielded by reality confusion, auditory perceptions, mind tricks and odd/unusual thoughts.
Table 2.
Item analysis of PRIME Screen-Revised in physically healthy psychosis spectrum vs. non-spectrum youths
| Psychosis spectrum endorsing “Definitely agree” | Psychosis spectrum | Non-spectrum | Pairwise F following significant MANOVA | ROC | |||||
|---|---|---|---|---|---|---|---|---|---|
| PRIME Screen-Revised item | % | Item mean±SD | Item mean±SD | F | p | Cohen's d | AUC | 95% CI lower | 95% CI upper |
| I think that I have felt that there are odd or unusual things going on that I can't explain (Odd/unusual thoughts) | 17.6 | 3.06±2.19 | 0.90±1.45 | 1334.81 | 0.001 | 1.33 | 0.77 | 0.75 | 0.79 |
| I have had the experience of hearing faint or clear sounds of people or a person mumbling or talking when there is no one near me (Auditory perceptions) | 17.5 | 2.19±2.45 | 0.25±0.89 | 1558.80 | 0.001 | 1.44 | 0.72 | 0.70 | 0.74 |
| I think that I may get confused at times whether something I experience or perceive may be real or may be just part of my imagination or dreams (Reality confusion) | 16.4 | 3.08±2.17 | 0.77±1.37 | 1677.88 | 0.001 | 1.48 | 0.79 | 0.78 | 0.81 |
| I think that I may hear my own thoughts being said out loud (Audible thoughts) | 11.1 | 1.85±2.23 | 0.29±0.91 | 1113.47 | 0.001 | 1.22 | 0.69 | 0.67 | 0.71 |
| I believe that I have special natural or supernatural gifts beyond my talents and natural strengths (Grandiosity) | 10.3 | 1.76±2.20 | 0.26±0.89 | 1074.51 | 0.001 | 1.19 | 0.69 | 0.67 | 0.71 |
| I think that I might feel like my mind is “playing tricks” on me (Mind tricks) | 9.7 | 2.14±2.19 | 0.36±0.97 | 1410.89 | 0.001 | 1.37 | 0.73 | 0.71 | 0.75 |
| I have had the experience of doing something differently because of my superstitions (Superstitions) | 8.0 | 1.91±2.12 | 0.44±1.10 | 872.26 | 0.001 | 1.08 | 0.70 | 0.67 | 0.72 |
| I wonder if people may be planning to hurt me or even may be about to hurt me (Persecutory/suspicious) | 6.1 | 1.56±2.01 | 0.27±0.86 | 911.30 | 0.001 | 1.10 | 0.68 | 0.66 | .070 |
| I think that I might be able to predict the future (Predict future) | 5.2 | 1.45±1.97 | 0.33±0.95 | 645.84 | 0.001 | 0.92 | 0.66 | 0.63 | 0.68 |
| I have thought that it might be possible that other people can read my mind, or that I can read other's minds (Mind reading) | 4.9 | 1.29±1.93 | 0.22±0.80 | 692.86 | 0.001 | 0.96 | 0.65 | 0.63 | 0.67 |
| I may have felt that there could possibly be something controlling my thoughts, feelings, or actions (Thought control) | 3.8 | 1.37±1.85 | 0.21±0.73 | 922.45 | 0.001 | 1.11 | 0.67 | 0.65 | 0.70 |
ROC – receiver operating characteristic analysis of PRIME Screen-Revised items, AUC – area under the curve, indicating the ability of the item to discriminate between psychosis spectrum and non-spectrum cases, CI – confidence interval
Receiver operator curve analysis of PRIME Screen-Revised items yielded area under the curve values ranging from 0.65 to 0.79, indicating moderate ability of items to discriminate between psychosis spectrum and non-spectrum. The most discriminating items were again reality confusion, odd/unusual thoughts, mind tricks and auditory perceptions.
Neurocognitive profiles of psychosis spectrum and non-spectrum youths
Neurocognitive profiles are presented in Figure 1. A group (psychosis spectrum, non-spectrum) x domain MANOVA (covariates were age, ethnicity and parental education) on accuracy scores showed a main effect for group (F=92.71, df=1,4550, p<0.0001) and domain (F=4.11, df=11,4540, p<0.0001), as well as a group x domain interaction (F=4.87, df=11,4540, p<0.0001). Psychosis spectrum youths showed a mild but significant decrease in performance accuracy across neurocognitive domains compared to non-spectrum.
Figure 1.
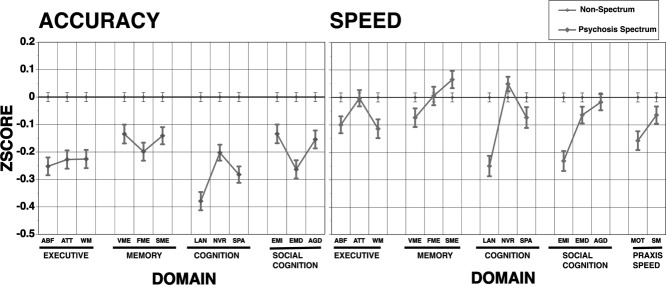
Computerized Neurocognitive Battery profiles of psychosis spectrum and non-spectrum physically healthy youths. ABF – abstraction/flexibility, ATT – attention, WM – working memory, VME – verbal memory, FME – face memory, SME – spatial memory, LAN – language, NVR – non-verbal reasoning, SPA – spatial processing, EMI – emotion identification, EMD – emotion differentiation, AGD – age discrimination, MOT – motor, SM – sensorimotor
The MANOVA on speed scores showed a main effect for group (F=10.21, df=1,4503, p=0.0014) and domain (F=4.75, df=13,4491, p<0.0001), and a group x domain interaction (F=6.97, df=13,4491, p<0.0001). Psychosis spectrum showed slower responding in some but not all domains.
Predicting psychosis spectrum classification from psychopathology and substance use
The prediction success of psychosis spectrum vs. non-spectrum based on demographic and clinical correlates was 84.6% (psychosis spectrum=27.7%, non-spectrum=96.6%; false positive=2.8%, false negative=12.6%) (Table 3). Receiver operator curve analysis revealed a moderate fit of the model.
Table 3.
Bivariate logistic regression predicting psychosis risk status (psychosis spectrum vs. non-spectrum) from psychopathology and substance use
| Psychosis spectrum | Non-spectrum | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|
| % | % | B | Wald chi-square | p | Odds ratio | Lower | Upper | |
| Psychopathology (N=4,665) | ||||||||
| Demographics | ||||||||
| Gender | −0.24 | 6.70 | 0.010 | 0.79 | 0.66 | 0.94 | ||
| Age | −0.06 | 13.88 | 0.001 | 0.94 | 0.91 | 0.97 | ||
| Ethnicity | 0.52 | 27.16 | 0.001 | 1.68 | 1.38 | 2.05 | ||
| Mother education | −0.03 | 1.75 | n.s. | 0.97 | 0.93 | 1.02 | ||
| Father education | −0.03 | 2.47 | n.s. | 0.97 | 0.93 | 1.01 | ||
| WRAT-4 Reading | −0.01 | 3.93 | 0.047 | 0.99 | 0.99 | 1.00 | ||
| Mood | ||||||||
| Depression | 26.9 | 9.6 | 0.28 | 4.55 | 0.033 | 1.32 | 1.02 | 1.71 |
| Mania | 2.1 | 0.3 | 1.08 | 4.22 | 0.040 | 2.94 | 1.05 | 8.24 |
| Anxiety | ||||||||
| Generalized anxiety | 5.2 | 1.6 | 0.07 | 0.07 | n.s. | 1.07 | 0.66 | 1.73 |
| Separation anxiety | 6.9 | 3.8 | −0.06 | 0.10 | n.s. | 0.94 | 0.64 | 1.37 |
| Specific phobia | 43.5 | 28.7 | 0.19 | 3.97 | 0.046 | 1.21 | 1.00 | 1.45 |
| Social phobia | 36.0 | 17.8 | 0.39 | 15.13 | 0.001 | 1.48 | 1.21 | 1.80 |
| Panic | 3.1 | 0.8 | 0.40 | 1.45 | n.s. | 1.49 | 0.78 | 2.86 |
| Agoraphobia | 14.8 | 3.4 | 0.78 | 24.46 | 0.001 | 2.19 | 1.61 | 2.99 |
| Obsessive-compulsive | 7.9 | 1.5 | 0.63 | 8.12 | 0.004 | 1.88 | 1.22 | 2.90 |
| Post-traumatic stress | 22.0 | 8.4 | 0.30 | 5.18 | 0.023 | 1.35 | 1.04 | 1.75 |
| Behavior | ||||||||
| Attention deficit/hyperactivity | 29.8 | 11.9 | 0.50 | 19.20 | 0.001 | 1.64 | 1.32 | 2.05 |
| Oppositional defiant | 44.4 | 25.3 | 0.42 | 17.66 | 0.001 | 1.52 | 1.25 | 1.84 |
| Conduct | 17.5 | 4.3 | 0.12 | 0.58 | n.s. | 1.13 | 0.83 | 1.54 |
| Eating (anorexia or bulimia) | 3.4 | 1.1 | 0.33 | 1.26 | n.s. | 1.39 | 0.78 | 2.49 |
| Morbid thoughts | ||||||||
| Thoughts of death/dying | 32.6 | 12.0 | 0.69 | 36.58 | 0.001 | 2.00 | 1.60 | 2.50 |
| Suicidal ideation | 20.1 | 5.5 | 0.37 | 5.78 | 0.016 | 1.45 | 1.07 | 1.96 |
| Treatment | ||||||||
| Talked with professional | 65.7 | 44.1 | 0.13 | 1.81 | n.s. | 1.14 | 0.94 | 1.39 |
| Psychiatric medications | 18.6 | 7.1 | 0.17 | 1.49 | n.s. | 1.19 | 0.90 | 1.57 |
| Inpatient hospitalization | 6.7 | 2.1 | −0.50 | 4.21 | 0.040 | 0.61 | 0.38 | 0.98 |
| Global Assessment Scale | −0.04 | 104.55 | 0.001 | 0.96 | 0.95 | 0.97 | ||
| Substance use (N=2,733) | ||||||||
| Demographics | ||||||||
| Sex | −0.14 | 1.95 | n.s. | 0.87 | 0.71 | 1.06 | ||
| Age | −0.11 | 19.60 | 0.001 | 0.90 | 0.86 | 0.94 | ||
| Race | 0.68 | 36.25 | 0.001 | 1.98 | 1.59 | 2.47 | ||
| Mother education | −0.06 | 5.10 | 0.024 | 0.94 | 0.89 | 0.99 | ||
| Father education | −0.05 | 4.60 | 0.032 | 0.95 | 0.90 | 1.00 | ||
| WRAT-4 Reading | −0.01 | 7.92 | 0.005 | 0.99 | 0.98 | 1.00 | ||
| Substance (ever used) | ||||||||
| Tobacco | 23.4 | 17.3 | 0.72 | 15.72 | 0.001 | 2.06 | 1.44 | 2.94 |
| Alcohol | 29.5 | 30.4 | −0.17 | 1.17 | n.s. | 0.84 | 0.62 | 1.15 |
| Marijuana | 22.0 | 18.1 | −0.02 | 0.01 | n.s. | 0.98 | 0.67 | 1.43 |
| Stimulants | 4.4 | 2.5 | 0.00 | 0.00 | n.s. | 1.00 | 0.53 | 1.88 |
| Tranquilizers | 2.8 | 1.1 | 0.58 | 1.98 | n.s. | 1.78 | 0.80 | 3.97 |
| Downers | 2.1 | 0.8 | 0.39 | 0.64 | n.s. | 1.48 | 0.56 | 3.90 |
| Inhalants | 6.7 | 3.6 | 0.39 | 2.99 | n.s. | 1.48 | 0.95 | 2.31 |
| Over-the-counter medication | 12.6 | 8.5 | 0.36 | 4.31 | 0.038 | 1.44 | 1.02 | 2.03 |
| Cocaine | 2.5 | 0.8 | 0.51 | 1.27 | n.s. | 1.67 | 0.69 | 4.04 |
| Psychedelics | 2.1 | 0.9 | 0.55 | 1.41 | n.s. | 1.74 | 0.70 | 4.31 |
| Opiates | 2.0 | 1.3 | −0.35 | 0.50 | n.s. | 0.70 | 0.27 | 1.87 |
| Steroids | 1.6 | 1.2 | −0.03 | 0.01 | n.s. | 0.97 | 0.42 | 2.25 |
WRAT-4 – Wide Range Achievement Test, version 4, CI – confidence interval
Significant predictors of psychosis spectrum included male gender, younger age, and non-European American ethnicity. Psychosis spectrum was significantly predicted by depression, mania, specific phobia, social phobia, agoraphobia, obsessive-compulsive, post-traumatic stress, oppositional defiant, and attention deficit/hyperactivity domains. Moreover, a third of psychosis spectrum youths endorsed passive thoughts of death and dying, more than 20% endorsed suicidal ideation, and both were significant predictors of psychosis spectrum group membership.
Ever talking with a professional (school counselor, psychologist, social worker, psychiatrist or other) for “feelings or problems with mood or behavior” was more likely, but not significantly, in psychosis spectrum than non-spectrum youths (65.7% vs. 44.1%). The odds of inpatient hospitalizations and functional impairment were significantly higher in psychosis spectrum (Table 3). On a follow-up item administered only to those who had not received treatment (and therefore not included in the model), 23.1% of non-help-seeking psychosis spectrum (N=84/363) compared to 9.9% of non-help-seeking non-spectrum (N=251/2538) youths reported that others suggested they seek help but they had not done so (Pearson chi-square=62.4, df=1,2901, p<0.001).
The substance use measure was introduced later in the study, and was therefore available on a smaller subsample (N=2,733). To verify that the psychopathology results were comparable in this smaller subset of participants, we re-ran the model including only them, and it yielded similar results (omnibus model chi-square=547.59, p<0.001, df=26; prediction success overall=84.3; area under the curve=0.81, 95% CI: 0.79-0.83), except that depression (Wald chi-square=1.31; odds ratio=1.22), social phobia (Wald chi-square=3.55; odds ratio=1.28), obsessive-compulsive disorder (Wald chi-square=2.81; odds ratio=1.64) and inpatient hospitalizations (Wald chi-square=2.76; odds ratio=0.56) fell short of significance. Finally, including ethnicity in interaction with each psychopathology variable did not improve fit, and only eating disorders (Wald chi-square=4.96; odds ratio=1.20) produced significant interactions with ethnicity.
Logistic regression to predict psychosis spectrum classification from demographics and substance use in the subsample with these data was significant, and prediction success overall was 81.5% (Table 3). However, although specificity was high (non-spectrum=99.5%), sensitivity was low (psychosis spectrum=3%; false positive <1%, false negative =18%). Receiver operator curve analysis revealed that the model classified groups above chance.
Significant demographic predictors were consistent with those observed in the psychopathology model, except that paternal education was a predictor, and gender was not. Among substances, Wald criterion suggested that tobacco and over-the-counter medication were significant contributors to prediction.
Discussion
This is the first large, systematic study of a non-clinical sample of U.S. youths that evaluated psychosis spectrum symptoms, including attenuated and threshold positive psychotic, and negative/disorganized symptoms. Previous community studies of mental disorders in U.S. youths have not included assessment of psychotic symptoms and their correlates (31,32).
The high frequency of psychosis spectrum symptoms, consistent with findings from studies conducted in other countries, and their association with reduced neurocognitive and global functioning, suggests that psychosis spectrum screening should be part of a comprehensive evaluation of psychopathology in youths in general population and pediatric settings. However, the lack of specificity of psychosis spectrum symptoms is evident from the high rates of comorbid mental disorders, that were nearly double than in the U.S. general population (31,32). Follow-up of psychosis spectrum youths will enable us to identify factors associated with transient psychotic experiences from the small minority in whom they presage the subsequent development of psychotic disorders in adulthood.
Among physically healthy young people, 12.3% reported positive sub-psychotic symptoms. The most discriminating and widely endorsed attenuated positive symptoms were unusual thoughts and auditory perceptions, as observed in other populations (11,33). An additional 3.7% of participants (20.2% of psychosis spectrum) reported threshold psychotic symptoms, similar to meta-analytically derived rates of psychotic symptoms in the general adult population (4%) (1). This comparability could reflect our requirement that threshold positive psychosis include significant distress/impairment, which offers a useful link between clinical and non-clinical psychotic experiences (1).
Consistent with prior population samples (11), younger participants endorsed higher levels of sub-psychotic positive symptoms. A minority of youths (2.3%) reported experiencing only negative/disorganized symptoms without positive symptoms, an important finding since prior work suggests that negative/disorganized symptoms in combination with positive symptoms predict poor functioning and help-seeking behavior (3).
Gender differences in psychotic-like experiences have varied in population studies (34). In our sample, being male was significantly predictive of psychosis spectrum, possibly reflecting earlier onset of clinically significant psychotic symptoms in males. Even when controlling for parental education and reading level, the psychosis spectrum group was disproportionately non-European American, and ethnicity was a significant predictor of spectrum status, consistent with prior studies of ethnic minorities (12). However, ethnicity may be confounded with urbanicity, a possibility we can pursue with ethnographic indicators.
Importantly, although caregivers of psychosis spectrum youths reported higher levels of sub-psychotic symptoms than caregivers of non-spectrum youths, they substantially under-reported symptoms compared to their children. Possibly, caregivers can better gauge “normality” and therefore are more accurate or appropriately conservative in their reports. However, several studies suggest that adolescents tend not to confide psychotic-like experiences to their caregivers or clinicians (10,35,36).
Among a comprehensive array of psychopathology domains, the odds of significant symptoms of depression, mania, anxiety (specific and social phobia, agoraphobia, obsessive-compulsive, post-traumatic stress), and behavioral (attention deficit/hyperactivity, oppositional defiant) disturbance were higher in youths with psychosis spectrum. Substance use, including cannabis, has been associated with risk for psychosis (37). In our cohort, only tobacco and over-the-counter medication were predictors of psychosis spectrum membership. The lack of significant effect for other substances may be due to the high rates of “ever use” of those substances in both psychosis spectrum and non-spectrum youths in this U.S. cohort. Alternatively, although the “ever use” criterion is highly heritable and developmentally informative (25), it may not be as sensitive to specific impairing or prolonged patterns of use associated with psychosis risk.
Global functioning was reduced in psychosis spectrum youths, and highly predictive of psychosis spectrum status. Similarly, suicidal ideation was higher, and reported by more than 20% of youths with psychosis spectrum symptoms. Quite concerning is that, despite these distress indicators, help seeking was not predictive of spectrum status, and psychosis spectrum caregivers appear unaware of the symptoms.
The psychosis spectrum state, which is predicted by distressing comorbid psychopathology, substance use, morbid thoughts of death and dying, and functional impairment, will ultimately remit, stabilize, or resolve into a disorder (or disorders) (5). Our results underscore the public health relevance of psychosis spectrum features in a U.S. youth cohort and the potential to intervene at an early stage. By showing associated features, including decreased neurocognitive accuracy, similar to those observed in schizophrenia patients, they also support the concept of a psychosis continuum.
Although we have not yet examined the predictive validity of our psychosis spectrum assessment approach, prior work has shown high sensitivity and specificity of the PRIME screen in young adult clinical (27) and non-clinical (college student) samples (26). Internal consistency of our PRIME Screen-Revised was high as in prior reports (26,38), and our rate of screen positives based on traditional (extreme agreement) criteria (14.6%) is similar to a study of older adolescents (18.4%) (38). The structured administration of selected items from the SOPS by multiple interviewers to accommodate the high participant volume, and the use of only six scale items to screen negative and disorganized symptoms, may have reduced sensitivity to clinically significant symptoms or yielded a high level of false positives in these domains (1). Additionally, the rationally derived psychosis spectrum criteria based on extreme agreement or age deviant responding are only a first step in deriving cut-points for this assessment. A just completed comprehensive diagnostic 18-month follow-up study of 300 psychosis spectrum and 200 typically developing participants indicates acceptable sensitivity and specificity of subsequent clinical high risk status assessed via comprehensive evaluation.
Ongoing follow-up will assist in evaluating the predictive validity of the psychosis spectrum screen and contribute to the limited available information about the use of at-risk criteria in children and adolescents (8). Moreover, as noted by others (1,15,33), incorporating mood, anxiety and other psychopathology dimensions will allow a fuller evaluation of the developmental and predictive significance of observed comorbidity.
Finally, while our results indicate that psychosis spectrum characteristics are common in young people in the U.S., and predicted by comorbid psychopathology, substance use, suicidal ideation and poor global functioning, our evaluation of predictors was limited to demographic and clinical variables. Although the fit of resulting models was adequate, other variables are likely to improve prediction of spectrum status.
The Philadelphia Neurodevelopmental Cohort is a public domain resource for the scientific community that will allow investigation of a wealth of other potential predictors, including phenomenology, brain structure and function, and genomics.
Acknowledgments
The authors thank the participants of this study, and all the members of the recruitment, assessment, and data teams whose individual contributions collectively made this work possible. The study was supported by RC2 grants from the National Institute of Mental Health: MH089983 and MH089924 (Gur and Hakonarson) and K08MH079364 (Calkins).
References
- 1.van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–95. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 2.Collip D, Myin-Germeys I, Van Os J. Does the concept of “sensitization” provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophr Bull. 2008;34:220–5. doi: 10.1093/schbul/sbm163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez MD, Saka MC, Lieb R, et al. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry. 2010;167:1075–82. doi: 10.1176/appi.ajp.2010.09060883. [DOI] [PubMed] [Google Scholar]
- 4.Kaymaz N, Drukker M, Lieb R, et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–53. doi: 10.1017/S0033291711002911. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Yung AR, McGorry P, et al. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol Med. 2014;44:17–24. doi: 10.1017/S0033291713000184. [DOI] [PubMed] [Google Scholar]
- 6.Binbay T, Drukker M, Elbi H, et al. Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr Bull. 2012;38:992–1002. doi: 10.1093/schbul/sbr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulton R, Caspi A, Moffitt TE, et al. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–8. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 8.Schimmelmann BG, Walger P, Schultze-Lutter F. The significance of at-risk symptoms for psychosis in children and adolescents. Can J Psychiatry. 2013;58:32–40. doi: 10.1177/070674371305800107. [DOI] [PubMed] [Google Scholar]
- 9.Amminger GP, Leicester S, Yung AR, et al. Early-onset of symptoms predicts conversion to non-affective psychosis in ultra-high risk individuals. Schizophr Res. 2006;84:67–76. doi: 10.1016/j.schres.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Scott J, Martin G, Welham J, et al. Psychopathology during childhood and adolescence predicts delusional-like experiences in adults: a 21-year birth cohort study. Am J Psychiatry. 2009;166:567–74. doi: 10.1176/appi.ajp.2008.08081182. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher I, Connor D, Clarke MC, et al. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42:1857–63. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 12.Wigman JT, van Winkel R, Raaijmakers QA, et al. Evidence for a persistent, environment-dependent and deteriorating subtype of subclinical psychotic experiences: a 6-year longitudinal general population study. Psychol Med. 2011;41:2317–29. doi: 10.1017/S0033291711000304. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez MD, Wichers M, Lieb R, et al. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. 2011;37:84–93. doi: 10.1093/schbul/sbp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultze-Lutter F, Ruhrmann S, Picker H, et al. Basic symptoms in early psychotic and depressive disorders. Br J Psychiatry. 2007;191(Suppl. 51):s31–7. doi: 10.1192/bjp.191.51.s31. [DOI] [PubMed] [Google Scholar]
- 15.Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–58. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- 16.De Loore E, Gunther N, Drukker M, et al. Persistence and outcome of auditory hallucinations in adolescence: a longitudinal general population study of 1800 individuals. Schizophr Res. 2011;127:252–6. doi: 10.1016/j.schres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–20. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–71. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano AJ, Li H, Mesholam-Gately RI, et al. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- 21.Kelleher I, Murtagh A, Clarke MC, et al. Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cogn Neuropsychiatry. 2013;18:9–25. doi: 10.1080/13546805.2012.682363. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer D, Gould M, Brasic J, et al. A Children's Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–31. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 23.Merikangas K, Avenevoli S, Costello J, et al. National Comorbidity Survey Replication Adolescent Supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 2009;48:367–9. doi: 10.1097/CHI.0b013e31819996f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merikangas KR, Dierker LC, Szatmari P. Psychopathology among offspring of parents with substance abuse and/or anxiety disorders: a high-risk study. J Child Psychol Psychiatry. 1998;39:711–20. [PubMed] [Google Scholar]
- 25.Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–93. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Nemoto T, Koshikawa H, et al. A self-reported instrument for prodromal symptoms of psychosis: testing the clinical validity of the PRIME Screen-Revised (PS-R) in a Japanese population. Schizophr Res. 2008;106:356–62. doi: 10.1016/j.schres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Miller TJ, Cicchetti D, Markovich PJ, et al. The SIPS screen: a brief self-report screen to detect the schizophrenia prodrome. Schizophr Res. 2004;70(Suppl. 1):78. [Google Scholar]
- 28.McGlashan TH, Miller TJ, Woods SW, et al. 2003. Structured Interview for Prodromal Syndromes, Version 4.0. New Haven: Prime Clinic Yale School of Medicine.
- 29.Gur RC, Richard J, Calkins ME, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26:251–65. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson GS, Robertson GJ. 2006. Wide Range Achievement Test, 4th ed. Lutz: Psychological Assessment Resources.
- 31.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication – Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125:75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurens KR, Hobbs MJ, Sunderland M, et al. Psychotic-like experiences in a community sample of 8000 children aged 9 to 11 years: an item response theory analysis. Psychol Med. 2012;42:1495–506. doi: 10.1017/S0033291711002108. [DOI] [PubMed] [Google Scholar]
- 34.Scott J, Welham J, Martin G, et al. Demographic correlates of psychotic-like experiences in young Australian adults. Acta Psychiatr Scand. 2008;118:230–7. doi: 10.1111/j.1600-0447.2008.01214.x. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Yamazawa R, Nemoto T, et al. Correlation between attenuated psychotic experiences and depressive symptoms among Japanese students. Early Interv Psychiatry. 2010;4:200–5. doi: 10.1111/j.1751-7893.2010.00185.x. [DOI] [PubMed] [Google Scholar]
- 36.Varghese D, Scott J, McGrath J. Correlates of delusion-like experiences in a non-psychotic community sample. Aust N Z J Psychiatry. 2008;42:505–8. doi: 10.1080/00048670802050595. [DOI] [PubMed] [Google Scholar]
- 37.Addington J, Case N, Saleem MM, et al. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv Psychiatry. 2014;8:104–12. doi: 10.1111/eip.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fresan A, Apiquian R, Ulloa RE, et al. Reliability study of the translation into Spanish of the PRIME Screen Questionnaire for Prodromic Symptoms. Actas Esp Psiquiatr. 2007;35:368–71. [PubMed] [Google Scholar]


