Abstract
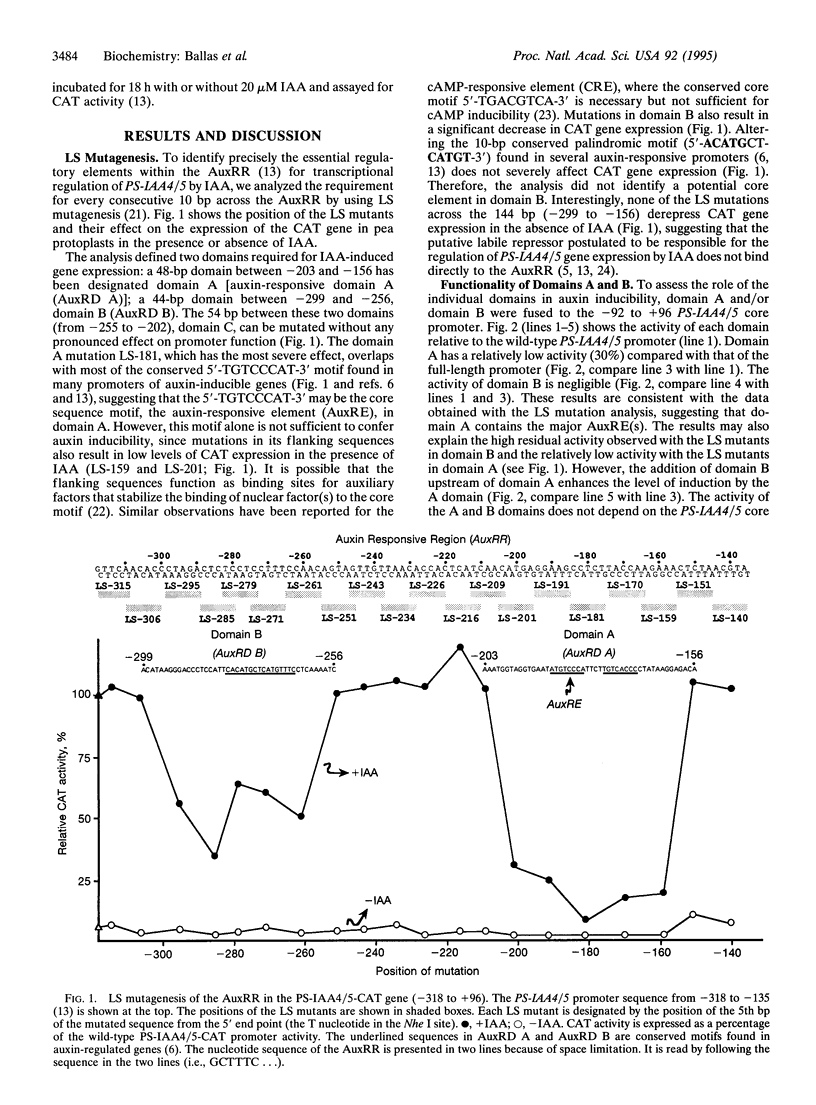
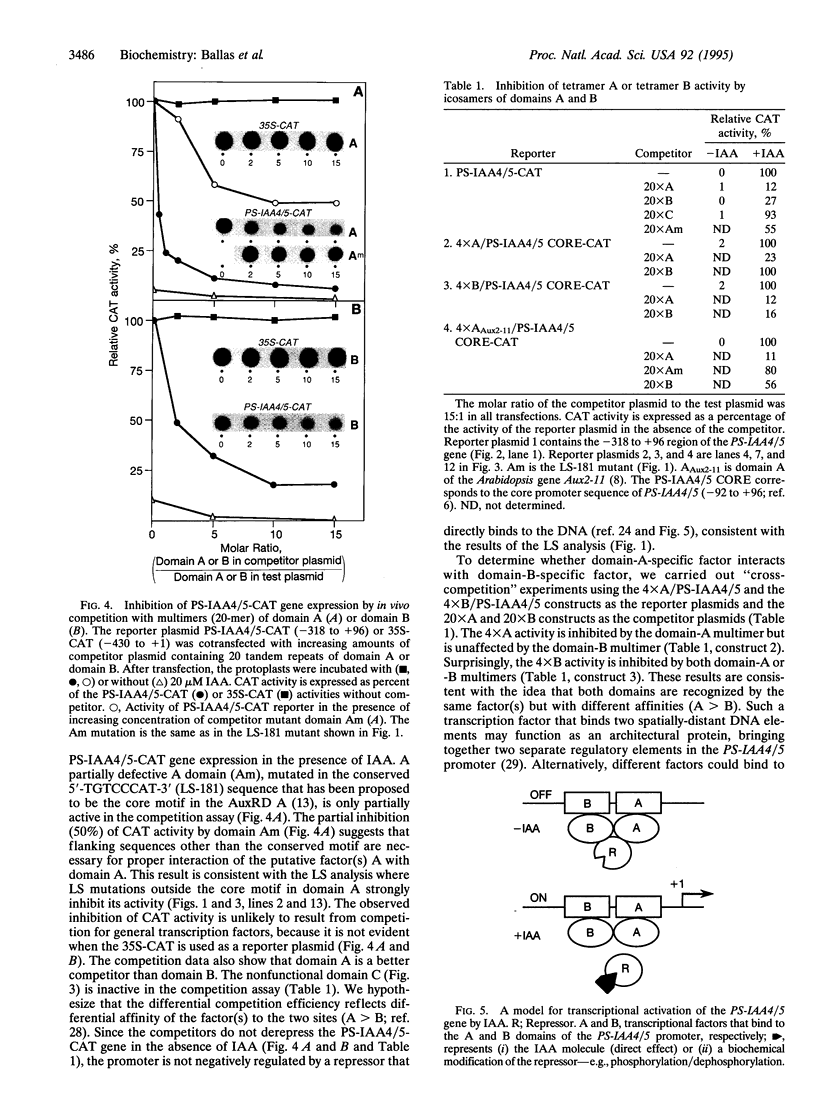
The plant growth hormone indole-3-acetic acid (IAA) transcriptionally activates expression of several genes in plants. We have previously identified a 164-bp promoter region (-318 to -154) in the PS-IAA4/5 gene that confers IAA inducibility. Linker-scanning mutagenesis across the region has identified two positive domains: domain A (48 bp; -203 to -156) and domain B (44 bp; -299 to -256), responsible for transcriptional activation of PS-IAA4/5 by IAA. Domain A contains the highly conserved sequence 5'-TGTCCCAT-3' found among various IAA-inducible genes and behaves as the major auxin-responsive element. Domain B functions as an enhancer element which may also contain a less efficient auxin-responsive element. The two domains act cooperatively to stimulate transcription; however, tetramerization of domain A or B compensates for the loss of A or B function. The two domains can also mediate IAA-induced transcription from the heterologous cauliflower mosaic virus 35S promoter (-73 to +1). In vivo competition experiments with icosamers of domain A or B show that the domains interact specifically and with different affinities to low abundance, positive transcription factor(s). A model for transcriptional activation of PS-IAA4/5 by IAA is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel S., Oeller P. W., Theologis A. Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):326–330. doi: 10.1073/pnas.91.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainley W. M., Walker J. C., Nagao R. T., Key J. L. Sequence and characterization of two auxin-regulated genes from soybean. J Biol Chem. 1988 Aug 5;263(22):10658–10666. [PubMed] [Google Scholar]
- Ballas N., Wong L. M., Theologis A. Identification of the auxin-responsive element, AuxRE, in the primary indoleacetic acid-inducible gene, PS-IAA4/5, of pea (Pisum sativum). J Mol Biol. 1993 Oct 20;233(4):580–596. doi: 10.1006/jmbi.1993.1537. [DOI] [PubMed] [Google Scholar]
- Bazett-Jones D. P., Leblanc B., Herfort M., Moss T. Short-range DNA looping by the Xenopus HMG-box transcription factor, xUBF. Science. 1994 May 20;264(5162):1134–1137. doi: 10.1126/science.8178172. [DOI] [PubMed] [Google Scholar]
- Conner T. W., Goekjian V. H., LaFayette P. R., Key J. L. Structure and expression of two auxin-inducible genes from Arabidopsis. Plant Mol Biol. 1990 Oct;15(4):623–632. doi: 10.1007/BF00017836. [DOI] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Estelle M. The plant hormone auxin: insight in sight. Bioessays. 1992 Jul;14(7):439–444. doi: 10.1002/bies.950140703. [DOI] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. J. Rapid induction of selective transcription by auxins. Mol Cell Biol. 1985 Jun;5(6):1197–1203. doi: 10.1128/mcb.5.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach B. M., Johnson A. D. Transcriptional repression in eukaryotes. Annu Rev Cell Biol. 1993;9:479–509. doi: 10.1146/annurev.cb.09.110193.002403. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P. C., Granner D. K. Hormone response domains in gene transcription. Annu Rev Biochem. 1992;61:1131–1173. doi: 10.1146/annurev.bi.61.070192.005411. [DOI] [PubMed] [Google Scholar]
- Marriott S. J., Brady J. N. Enhancer function in viral and cellular gene regulation. Biochim Biophys Acta. 1989 Dec 17;989(2):97–110. doi: 10.1016/0304-419x(89)90037-1. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller P. W., Keller J. A., Parks J. E., Silbert J. E., Theologis A. Structural characterization of the early indoleacetic acid-inducible genes, PS-IAA4/5 and PS-IAA6, of pea (Pisum sativum L.). J Mol Biol. 1993 Oct 20;233(4):789–798. doi: 10.1006/jmbi.1993.1555. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., O'Malley B. W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at target enhancer elements. Cell. 1989 May 5;57(3):443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. A consensus sequence polymer inhibits in vivo expression of heat shock genes. Mol Cell Biol. 1986 Sep;6(9):3200–3206. doi: 10.1128/mcb.6.9.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski T. I., Theologis A. Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol Biol Cell. 1993 Apr;4(4):363–373. doi: 10.1091/mbc.4.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 1987;154:329–350. doi: 10.1016/0076-6879(87)54083-6. [DOI] [PubMed] [Google Scholar]
- van der Zaal E. J., Droog F. N., Boot C. J., Hensgens L. A., Hoge J. H., Schilperoort R. A., Libbenga K. R. Promoters of auxin-induced genes from tobacco can lead to auxin-inducible and root tip-specific expression. Plant Mol Biol. 1991 Jun;16(6):983–998. doi: 10.1007/BF00016071. [DOI] [PubMed] [Google Scholar]