Abstract
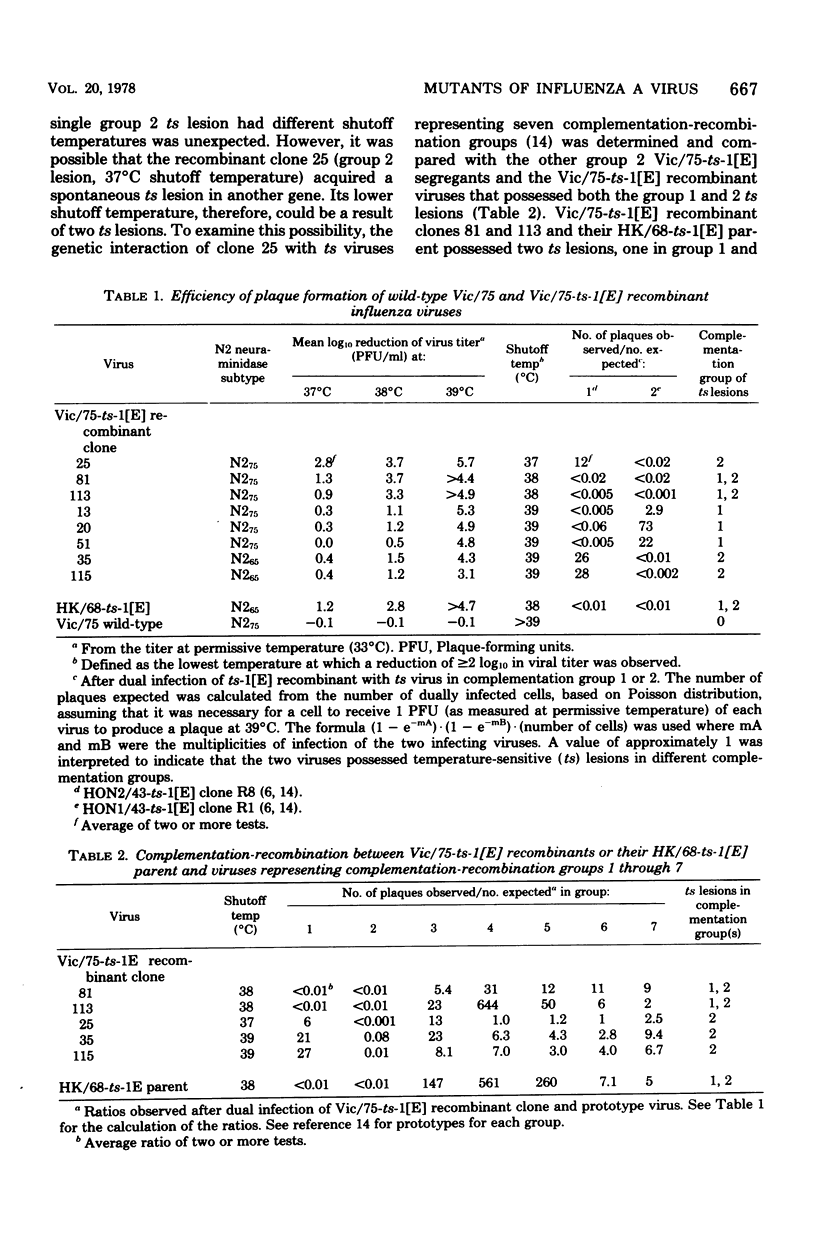
The Hong Kong/68-ts-1[E] virus, which has a 38°C shutoff temperature for plaque formation, has been proposed as a donor of its two ts lesions to new variants of influenza A virus that pose an epidemic threat. To further examine whether the acquisition of the two ts-1[E] lesions will predictably attenuate new influenza A variants, the HK/68-ts-1[E] virus was mated with the A/Vic/3/75 wild-type virus. The Vic/75-ts-[E] recombinants that had the two ts-1[E] lesions also had a 38°C shutoff temperature. Two Vic/75-ts-1[E] recombinants (clones 81 and 113) that had the two ts-1[E] lesions, a 38°C shutoff temperature, and the Vic/75 hemagglutinin and neuraminidase glycoproteins were similar to each other and to their ts-1[E] parent in the pattern of replication and genetic stability in hamsters. These findings support the hypothesis that the acquisition of the two ts-1[E] lesions will predictably attenuate wild-type influenza A virus. Each Vic/75-ts-1[E] recombinant virus that possessed only the group 1 ts-1[E] lesion had a 39°C shutoff temperature. Two of three of the Vic/75-ts-1[E] recombinants that had only the group 2 ts-1[E] lesion had a 39°C shutoff temperature. This suggests that the HK/68-ts-1[E] donor virus contains two ts genes each of which by itself restricts plaque formation at 39°C and above. The HK/68-ts-1[E] parent virus and its Vic/75 recombinant clones 81 and 113 were evaluated in ferret tracheal organ cultures maintained at permissive and restrictive temperatures. The Vic/75-ts-1[E] clone 81 differed from its parent and sister clone 113 in that it replicated readily and caused ciliostasis at 37°C, a temperature restrictive for the replication of other ts-1[E] recombinants with a 38°C shutoff temperature. The genetic basis underlying this difference was not elucidated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Parrott R. H., Murphy B. R., Richman D. D., Chanock R. M. Temperature-sensitive mutants of influenza A virus: response of children to the influenza A/Hong Kong/68-ts-1(E) (H3N2) and influenza A/Udorn/72-ts-1(E) (H3N2) candidate vaccine viruses and significance of immunity to neuraminidase antigen. Pediatr Res. 1976 Apr;10(4):238–242. doi: 10.1203/00006450-197604000-00008. [DOI] [PubMed] [Google Scholar]
- Mostow S. R., Flatauer S., Paler M., Murphy B. R. Temperature-sensitive mutants of influenza virus. XIII. Evaluation of influenza A/Hong Kong/68 and A/Udorn/72 ts and wild-type viruses in tracheal organ culture at permissive and restrictive temperatures. J Infect Dis. 1977 Jul;136(1):1–6. doi: 10.1093/infdis/136.1.1. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Richman D. D., Spring S. B., Chanock R. M. Use of temperature-sensitive mutants of influenza A virus as live virus vaccine strains. Evaluation in laboratory animals, adults and children. Postgrad Med J. 1976 Jun;52(608):381–388. doi: 10.1136/pgmj.52.608.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Spring S. B., Richman D. D., Tierney E. L., Kasel J., Chanock R. M. Temperature-Sensitive mutants of influenza virus. VII. Transfer of the TS-1[E] lesions to a wild-type influenza A virus with the HON1 surface antigens. Virology. 1975 Aug;66(2):533–541. doi: 10.1016/0042-6822(75)90225-1. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B. Live attenuated influenza virus vaccines. Strains with temperature-sensitive defects in P3 protein and nucleoprotein. Virology. 1977 May 1;78(1):183–191. doi: 10.1016/0042-6822(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Belshe R. B., Rusten H. M., Chanock R. M., Blacklow N. R., Parrino T. A., Rose F. B., Levine M. M., Caplan E. Temperature-sensitive mutants of influenza A virus. XIV. Production and evaluation of influenza A/Georgia/74-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1977 Aug;136(2):256–262. doi: 10.1093/infdis/136.2.256. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Chanock R. M., Gwaltney J. M., Jr, Douglas R. G., Betts R. F., Blacklow N. R., Rose F. B., Parrino T. A., Levine M. M. Temperature-sensitive mutants of influenza A virus. XII. Safety, antigenicity, transmissibility, and efficacy of influenza A/Udorn/72-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1976 Dec;134(6):585–594. [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Cline W. L., Alling D. W. Determination of influenzavirus neuraminidase inhibition titres. Bull World Health Organ. 1975;52(2):233–234. [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Spring S. B., Coleman M. T., Chanock R. M. Temperature sensitive mutants of influenza virus. IX. Genetic and biological characterization of TS-1[E] lesions when transferred to a 1972 (H3N2) influenza A virus. Virology. 1975 Aug;66(2):551–562. doi: 10.1016/0042-6822(75)90227-5. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Maassab H. F., Kendal A. P., Murphy B. R., Chanock R. M. Cold adapted variants of influenza A. II. Comparison of the genetic and biological properties of ts mutants and recombinants of the cold adapted A/AA/6/60 strain. Arch Virol. 1977;55(3):233–246. doi: 10.1007/BF01319909. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Nusinoff S. R., Mills J., Richman D. D., Tierney E. L., Murphy B. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. VI. Transfer of TS lesions from the Asian subtype of influenza A virus (H2N2) to the Hong Kong subtype (H3N2). Virology. 1975 Aug;66(2):522–532. doi: 10.1016/0042-6822(75)90224-x. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Nusinoff S. R., Tierney E. L., Richman D. D., Murphy B. R., Chanock R. M. Temperature-sensitive mutants of influenza. VIII. Genetic and biological characterization of TS mutants of influenza virus A (H3N2) and their assignment to complementation groups. Virology. 1975 Aug;66(2):542–550. doi: 10.1016/0042-6822(75)90226-3. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Sell S. H., Shinozaki T., Thompson J., Karzon D. T. Safety and antigenicity of influenza A/Hong Kong/68-ts-1 (E) (H3N2). J Pediatr. 1975 Dec;87(6 Pt 2):1109–1116. doi: 10.1016/s0022-3476(75)80123-5. [DOI] [PubMed] [Google Scholar]


