Abstract
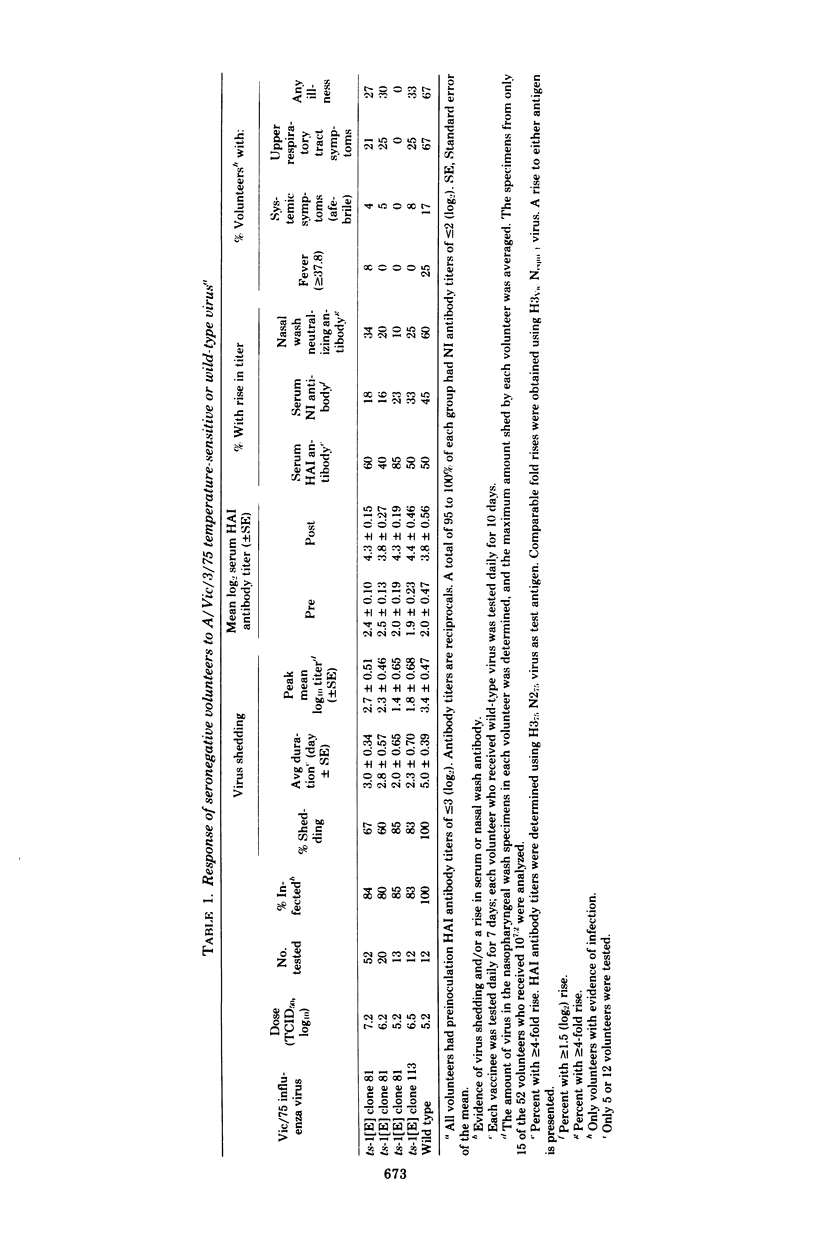
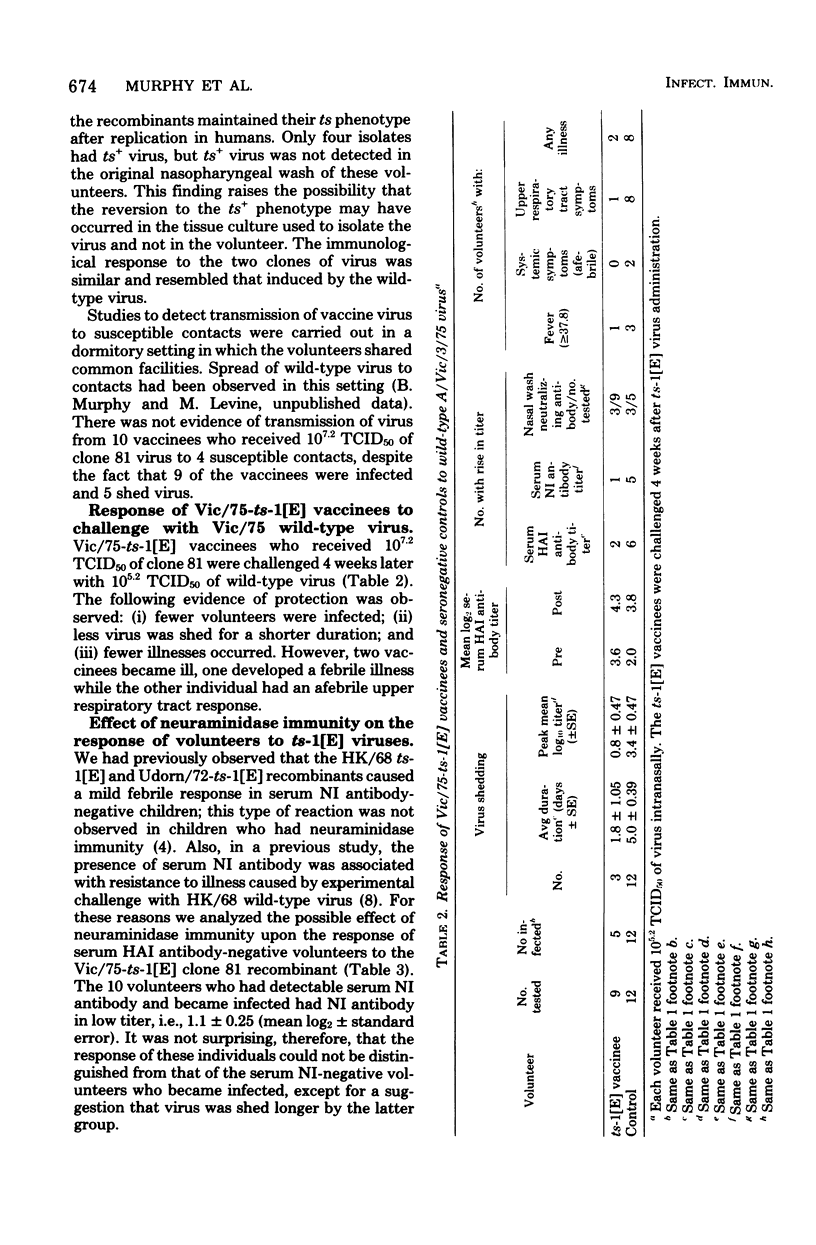
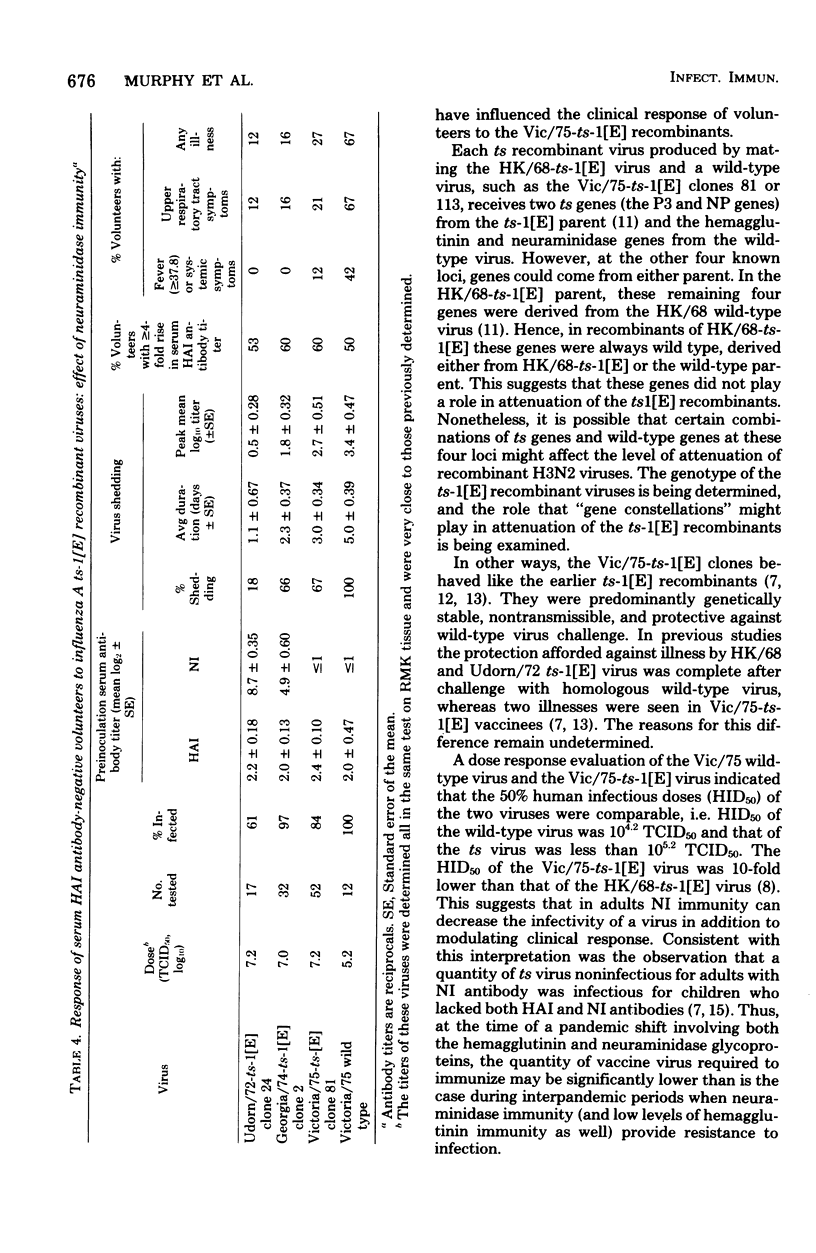
The Hong Kong/68-ts-1[E] virus and its Udorn/72 and Georgia/74 recombinants, which have a 38°C shutoff temperature and a ts lesion(s) on the genes coding for the P3 and NP proteins, were adequately attenuated and immunogenic in adult volunteers who lacked serum hemagglutination-inhibiting antibody (titer, ≤1:8), but who possessed serum neuraminidase-inhibiting antibody. Two Victoria/75-ts-1[E] clones that also had a 38°C shutoff temperature and a ts lesion(s) on the same two genes were administered to adult volunteers who lacked both serum hemagglutination-inhibiting antibody (titer, ≤1:8) and neuraminidase-inhibiting antibody (titer, ≤1:4). In contrast to the behavior of the earlier ts-1[E] recombinants, the Vic/75-ts-1[E] recombinants retained the capacity to cause febrile, systemic illness. However, the recombinants were attenuated compared with wild-type virus. The Vic/75-ts-1[E] virus vaccinees shed a larger amount of virus for a longer time than the previous ts-1[E] vaccinees, but they shed less virus than volunteers infected with wild-type virus. The ts-1[E] virus shed retained its ts phenotype in most instances and failed to spread to susceptible contacts. Vaccinees were partially protected against homologous wild-type virus challenge. The failure of HK/68, Udorn/72, and Georgia/74 ts-1[E] vaccinees to develop systemic reactions may reflect the presence of neuraminidase immunity before infection. In this situation, attenuation probably resulted from the degree of defectiveness of the ts-1[E] recombinant virus and the existence of neuraminidase immunity in the recipients. The 50% human infectious dose of the Vic/75 ts-1[E] virus was less than 105.2 50% tissue culture infective doses. This suggests that at the time of a pandemic shift involving both the hemagglutinin and neuraminidase glycoproteins, a small amount of live virus vaccine might be effective in initiating infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Couch R. B., Douglas R. G., Jr, Fedson D. S., Kasel J. A. Correlated studies of a recombinant influenza-virus vaccine. 3. Protection against experimental influenza in man. J Infect Dis. 1971 Nov;124(5):473–480. doi: 10.1093/infdis/124.5.473. [DOI] [PubMed] [Google Scholar]
- Dolin R., Blacklow N. R., DuPont H., Formal S., Buscho R. F., Kasel J. A., Chames R. P., Hornick R., Chanock R. M. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. J Infect Dis. 1971 Mar;123(3):307–312. doi: 10.1093/infdis/123.3.307. [DOI] [PubMed] [Google Scholar]
- KNIGHT V. THE USE OF VOLUNTEERS IN MEDICAL VIROLOGY. Prog Med Virol. 1964;6:1–26. [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Parrott R. H., Murphy B. R., Richman D. D., Chanock R. M. Temperature-sensitive mutants of influenza A virus: response of children to the influenza A/Hong Kong/68-ts-1(E) (H3N2) and influenza A/Udorn/72-ts-1(E) (H3N2) candidate vaccine viruses and significance of immunity to neuraminidase antigen. Pediatr Res. 1976 Apr;10(4):238–242. doi: 10.1203/00006450-197604000-00008. [DOI] [PubMed] [Google Scholar]
- Mostow S. R., Flatauer S., Paler M., Murphy B. R. Temperature-sensitive mutants of influenza virus. XIII. Evaluation of influenza A/Hong Kong/68 and A/Udorn/72 ts and wild-type viruses in tracheal organ culture at permissive and restrictive temperatures. J Infect Dis. 1977 Jul;136(1):1–6. doi: 10.1093/infdis/136.1.1. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Hosier N. T., Spring S. B., Mostow S. R., Chanock R. M. Temperature-sensitive mutants of influenza A virus: production and characterization of A/Victoria/3/75-ts-1[E] recombinants. Infect Immun. 1978 Jun;20(3):665–670. doi: 10.1128/iai.20.3.665-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Kasel J. A., Chanock R. M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972 Jun 22;286(25):1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B. Live attenuated influenza virus vaccines. Strains with temperature-sensitive defects in P3 protein and nucleoprotein. Virology. 1977 May 1;78(1):183–191. doi: 10.1016/0042-6822(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Belshe R. B., Rusten H. M., Chanock R. M., Blacklow N. R., Parrino T. A., Rose F. B., Levine M. M., Caplan E. Temperature-sensitive mutants of influenza A virus. XIV. Production and evaluation of influenza A/Georgia/74-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1977 Aug;136(2):256–262. doi: 10.1093/infdis/136.2.256. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Chanock R. M., Gwaltney J. M., Jr, Douglas R. G., Betts R. F., Blacklow N. R., Rose F. B., Parrino T. A., Levine M. M. Temperature-sensitive mutants of influenza A virus. XII. Safety, antigenicity, transmissibility, and efficacy of influenza A/Udorn/72-ts-1[E] recombinant viruses in human adults. J Infect Dis. 1976 Dec;134(6):585–594. [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R., Cline W. L., Alling D. W. Determination of influenzavirus neuraminidase inhibition titres. Bull World Health Organ. 1975;52(2):233–234. [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Sell S. H., Shinozaki T., Thompson J., Karzon D. T. Safety and antigenicity of influenza A/Hong Kong/68-ts-1 (E) (H3N2). J Pediatr. 1975 Dec;87(6 Pt 2):1109–1116. doi: 10.1016/s0022-3476(75)80123-5. [DOI] [PubMed] [Google Scholar]


