Abstract
Background
Several studies have examined reversibility of tardive syndromes (TS), primarily in psychotic patients who are maintained on dopamine receptor blocking drugs. The results have varied widely. However, few have assessed remission rates after discontinuing the offending agents. This study evaluated reversibility of TS in patients who permanently withdrew the causative agent(s). We also examined for any possible clinical predictors of reversibility.
Methods
A retrospective cohort of 108 TS patients was studied. Most of the patients were not psychotic; most patients were being treated either for a mood disorder with atypical antipsychotics or for a gastrointestinal disturbance with metoclopramide. Patients were stratified on the basis of reversibility, and statistical tests were used for subgroup comparisons of relevant clinical variables. Logistic regression was undertaken to identify clinical variables predictive of reversibility.
Results
Only 13% of the cohort experienced reversibility of the TS, 2% without medical intervention. When stratified by reversibility, there were no significant differences in any study variables between subgroups. None of the study variables predicted reversibility in the logistic regression.
Discussion
Our study demonstrated a low remission rate for TS in a cohort of psychiatric and non-psychiatric patients seen in a movement disorder clinic after the offending agents were completely withdrawn. Such a finding has significant prognostic implications. It is possible that limitations of the retrospective design may have resulted in an underestimation. There is a clear need for prospective, multicenter, clinical trials in populations that can be safely withdrawn from dopamine receptor blocking agents so that true remission rates can be measured.
Keywords: Tardive syndromes, dopamine receptor blocking agents, reversibility
Introduction
Tardive syndromes (TSs) are iatrogenic movement disorders caused by dopamine receptor blocking agents (DRBAs) and defined by at least 3 months’ exposure (1 month in patients >60 years of age).1 The first cases were reported in the medical literature in 1957: three elderly females developed lip-smacking dyskinetic movements 2–8 weeks after initiating a chlorpromazine derivative.2,3 While the orofacial dyskinesia (“classical tardive dyskinesia”) originally described remains the most common form of TS, other syndromes have been recognized over the following decades, including dystonia, stereotypy, akathisia, tremor, tics, myoclonus, and chorea—generalized, and truncal or respiratory dyskinesias.4–6
With the approval of more than 10 atypical or second-generation antipsychotic agents since the late 1980s, the prevalence rates for TSs have been found in several trials for these drugs dating from 2004 to 2008 to be approximately 13% compared to 32% among subjects treated with first-generation typical antipsychotics.7 This would suggest that atypical antipsychotics may be associated with a lower risk of TSs.7 However, other more recent studies have suggested that there may be no significant changes in the overall incidence of TS since the introduction of second-generation antipsychotics.8,9 Considering that there has been a more widespread use of atypical agents in non-psychotic populations, likely related to their perceived safety in relation to extrapyramidal symptoms, this could ultimately predict an even greater occurrence of TSs.5 Furthermore, metoclopramide and other antiemetics, also DRBAs that are used commonly for gastrointestinal (GI) symptoms, represent important, often unaccounted for, causes of TSs in non-psychiatric patients, with estimated TS frequencies being as high as 30%.10–13
The prevalence rate of TS depends not only on exposure to DRBAs but on reversibility of the disorder. The remission rate of TSs remains unclear. TSs are generally thought to be reversible to some degree; the rate of resolution is often quoted to be approximately 30% overall and possibly lower in elderly people.14–16 While many studies examining reversibility have been conducted in psychotic patients who continue treatment with DRBAs, few studies have examined the remission rate of TSs in patients in whom the offending agents are permanently discontinued.17–21 Remission rates in these studies range from 2% to 33% with follow-up as long as 6.7 years. This is an important question considering the apparent more widespread use of DRBAs.
Our study aims to evaluate the natural history of TSs in a retrospective cohort of 108 mostly non-psychotic patients in whom the offending agents were permanently removed. The primary outcome variable was reversibility of the TS. Additional variables were collected in order to evaluate for any potential clinical predictors of reversibility.
Methods
Approval through an expedited review process from the Emory institutional review board was obtained for development of a patient database and medical record review in the movement disorder clinic. Patients seen between January 1, 2006, and December 31, 2009, with a diagnosis of TSs were captured from this database and records were obtained through our electronic medical record and reviewed. Inclusion criteria were that patients met clinical diagnostic criteria according to the ‘Diagnostic and statistical manual of mental disorders’ 4th ed., text review (DSM-IV TR) of TSs and were diagnosed by a movement disorder specialist; patients were exposed to a known DRBA within 4 weeks prior to symptom onset and the causative agent was specified in the record; the patients had at least one follow-up (primarily by visit but rarely by a phone call [n = 2] that obtained specific data on changes in their condition). Exclusions included those patients who did not meet DSM-IV TR criteria, had dyskinesia that may have been secondary to other causes or had an unknown etiology, had incomplete information regarding the causative agent, and those that did not return for any follow-up. In those with drug-induced parkinsonism, diagnosis was established by history of exposure to block dopamine agents, clinical evidence of at least two of four cardinal features of parkinsonism (bradykinesia, rigidity, rest tremor, and postural instability) and reversibility with removal of the inciting agent.22
Once patients were identified, records were reviewed. All patients had a standard new patient evaluation, including complete medical history, past medical history including drug exposure, family history, and complete neurological examination. Follow-up visits included interim history, therapy review and response, and a neurological examination. Demographic and clinical characteristics were collected through record review, including age, gender, race, history of diabetes mellitus, history of alcohol usage, the primary diagnosis for which the DRBA was prescribed, the specific DRBA(s) prescribed, concomitant extrapyramidal syndromes, the approximate dates the inciting drug was started and stopped, the approximate date of onset of symptoms, the type of TS (for example orofacial, generalized or respiratory dyskinesia; orofacial, lingual, cervical, or generalized dystonia; or akathisia), the date the TS resolved, and the date of last follow-up.
A scale was utilized to designate level of severity of the TS at the initial and last evaluation as follows: none, mild, moderate, and severe. Mild was described as those with mild or intermittent movements that did not disturb the patient. Moderate was described as patients who had minor difficulty with some activities of daily living (ADLs) including eating or who found the movements bothersome or socially embarrassing. Severe was described as those patients who had TS that was disabling or giving trouble with some or all ADLs, such as the disruption of eating to the point of causing weight loss or the loss of the ability to walk or drive, or the presence of respiratory dyskinesia with or without symptomatic shortness of breath.
Reversibility of TS was defined as complete resolution of the movement disorder either spontaneously or with treatment, after which resolution remained when treatment was discontinued.20 For example, patients with tardive dystonia who had complete symptom resolution only while on botulinum toxin treatment but returned after it wore off were not considered as reversed. Those who had treatment, which was then removed, and had no recurrence of symptoms, were considered to be patients who had true resolution or reversibility of TS. We also examined improvement of symptoms through use of the severity scoring scale.
Statistical analysis
The cohort was stratified on the basis of TS reversibility, and Student’s t-tests, chi-squared tests, and Fisher exact tests were used for subgroup comparisons where appropriate. Logistic regression using forward, backward, and stepwise selection strategies was undertaken in order to identify clinical variables predictive of TD reversibility.
Results
We identified 126 patients who met diagnostic criteria for TS and had documented DRBA exposure prior to onset. Sixteen of these patients were lost to follow-up after one visit and two patients did not have an offending agent specified. The remaining 108 patients were the subjects of this analysis. In order to determine if the exclusion of those lost to follow-up may have introduced any selection bias, a separate analysis of baseline characteristics was done for the patients lost to follow-up. This group was not significantly different from the 108 patients included in the analysis with regard to any of the relevant baseline characteristics (median age, gender distribution, primary diagnosis, diabetes, alcohol abuse, offending agent, type of TS, severity at baseline, mean exposure to offending drug, mean latency to onset). For all subjects the DRBAs were stopped as the initial step in treating the movement disorder and follow-up was done with their treating movement disorder neurologist in our clinic, except for two patients with a telephone follow-up.
Baseline characteristics are shown in Table 1. The mean age for the cohort was 58.6±17.7 years (range 10–87 years). Sixty-nine percent were female. Most common ethnicities were 52.8% Caucasians and 12.0% African-American. The three most common primary diagnoses resulting in the prescription of DRBA(s) were mood disorders (38.0%), and GI illnesses (38.0%); only 6.48% had psychotic disorders. Drug-induced parkinsonism was diagnosed in 25.9%. Diabetes was a comorbid illness in 21.3% of the patients and alcohol abuse was documented in 5.6%. Of the offending DRBAs, GI drugs were prescribed in 41.7%, atypical antipsychotics in 36.1%, and first-generation antipsychotics in 16.7%. Of the GI drugs, 86.7% were exposed to metoclopramide. Tardive syndromes were further classified by type of movement disorder: patients who had two or more different types (referred to as mixed) were the most common (45.4%), followed in descending order by orofacial, generalized or respiratory dyskinesias (25.9%), orofacial or generalized dystonias (13%), and akathisia (5. 6%). The mean duration of exposure to the DRBA was 4.81±6.26 years (range 0.02–28 years, median 2.07). The mean latency from drug initiation to onset of the TS was 3.7±5.6 years (range 0–29 years, median 1.66 years). Average follow-up time for the study group was 3.1±3.4 years (range 0–13.52 years, median 1.73 years).
Table 1. Baseline Characteristics of all 108 TS Patients.
| N = 108 | % | ||
|---|---|---|---|
| Age (mean ± SD) | 58.6±17.7 | Range (10–87) | |
| Gender | |||
| Female | 75 | 69.4 | |
| Male | 33 | 30.6 | |
| Ethnicity | |||
| Caucasian | 57 | 52.8 | |
| African-American | 13 | 12.0 | |
| Hispanic | 4 | 3.70 | |
| Asian | 3 | 2.78 | |
| Unknown | 31 | 28.7 | |
| Primary diagnosis | |||
| Mood disorder | 41 | 38.0 | |
| GI illness | 41 | 38.0 | |
| Psychotic disorder | 7 | 6.48 | |
| Neurodegenerative | 1 | 0.930 | |
| Stroke | 1 | 0.930 | |
| Trauma | 2 | 1.85 | |
| Tic disorders | 6 | 5.56 | |
| Developmental d/o | 2 | 1.85 | |
| Anxiety d/o | 4 | 3.70 | |
| Personality d/o | 1 | 0.930 | |
| Unknown | 2 | 1.85 | |
| Diabetes | 23 | 21.3 | |
| Alcohol abuse | 6 | 5.56 | |
| Extrapyramidal symptoms | |||
| EPS (all categories) | 35 | 32.4 | |
| Parkinsonism | 28 | 25.9 | |
| EPS (not Parkinsonism) | 7 | 6.48 | |
| Offending agent | |||
| GI DRBAs | 45 | 41.7 | |
| Atypical antipsychotics | 39 | 36.1 | |
| Mixed | 15 | 13.9 | |
| Typical antipsychotics | 8 | 7.40 | |
| SSRI | 1 | 0.930 | |
| Type of TS | |||
| Mixed | 49 | 45.4 | |
| Orofacial/generalized/respiratory dyskinesia | 28 | 25.9 | |
| Orofacial/generalized dystonia | 14 | 13.0 | |
| Akathisia | 6 | 5.56 | |
| Lingual dystonia | 5 | 4.63 | |
| Cervical dystonia | 4 | 3.70 | |
| Chorea | 1 | 0.930 | |
| Blepharospasm | 1 | 0.930 | |
| Severity at baseline | |||
| Mild | 41 | 37.9 | |
| Moderate | 44 | 40.7 | |
| Severe | 23 | 21.3 | |
| Median duration of exposure to drug in years (range) | 2.07 (0.02–29) (N = 65) | ||
| Median latency to onset of symptoms in years (range) | 1.66 (0–29) (N = 68) | ||
Abbreviations: d/o, disorder; DRBAs, Dopamine Receptor Blocking Agents; EPS, Extrapyramidal Symptoms; GI, Gastrointestinal; SD, Standard Deviation; SSRI, selective serotonin reuptake inhibitors; TS, Tardive Syndromes.
The categories of treatments used for TSs included beta-blockers (N = 35), benzodiazepines (N = 25), anticholinergics (N = 42), antioxidants (N = 14), amantadine (N = 23), vesicular monoamine transporter inhibitors tetrabenazine or reserpine (N = 23), antiepileptics (N = 8), dopamine agonists (N = 8), GABA agonists (N = 15), botulinum toxin (N = 35), typical antipsychotics (N = 5), atypical antipsychotics (N = 25), and deep brain stimulation (N = 7). Some patients were exposed to more than one of these treatment categories. Ten patients had no treatment exposure. Given the methodological limitations, we were unable to assess the effectiveness of the specific TS treatments.
The primary endpoint of reversibility of TS in the 108 patients was 13% (n = 14). A second calculation was done to evaluate reversibility among only those patients who had at least the mean follow-up (since those seen earlier might still be in the process of resolution). Similarly, 13.9% of these patients resolved. Only 2.8% (n = 3) of the patients resolved simply by withdrawing the offending agent. In the 14 patients in whom TSs resolved, the average duration from onset of symptoms to resolution was 4.3±6.99 years (range 0.13–26.86 years, median 1.74 years). The average amount of time it took for TSs to resolve from the end of drug exposure was 2.3±2.3 years (range 0.10–6.34 years, median 1.49 years). When stratified by reversibility, there were no significant differences in any of the study variables (Table 2). None of the study variables predicted reversibility in the logistic regression.
Table 2. Stratified Analysis: TS Resolved Group vs. TS Unresolved Group.
| TS Resolved | TS Unresolved | p-Value | ||
|---|---|---|---|---|
| N = 14 (13.0%) | N = 94 (87.0%) | |||
| Age (mean±SD) | 53.5±20.0 | 59.4±17.3 | 0.25 | |
| Range (18–87) | Range (10–83) | |||
| Gender, n (%) | ||||
| Female | 10 (71.4) | 65 (69.2) | 0.99 | |
| Male | 4 (28.6) | 29 (30.9) | ||
| Ethnicity, n (%) | ||||
| Caucasian | 9 (64.3) | 48 (51.1) | 0.72 | |
| African-American | 1 (7.14) | 12 (12.8) | 0.68 | |
| Hispanic | 0 | 4 (4.26) | 0.99 | |
| Asian | 1 (7.14) | 2 (2.13) | 0.37 | |
| Unknown | 3 (21.4) | 28 (29.8) | ||
| Primary diagnosis, n (%) | ||||
| Mood disorder | 7 (50.0) | 34 (36.2) | 0.35 | |
| Psychotic disorder | 1 (7.14) | 6 (6.38) | 0.99 | |
| GI illness | 3 (21.4) | 38 (40.4) | 0.24 | |
| Neurodeg/stroke/trauma | 0 | 4 (4.25) | 0.99 | |
| Other | 3 (21.4) | 10 (10.6) | 0.37 | |
| Unknown | 0 | 2 (2.13) | ||
| Diabetes, n (%) | 3 (21.4) | 20 (21.3) | 0.99 | |
| Alcohol abuse, n (%) | 0 | 6 (6.38) | 0.99 | |
| Extrapyramidal symptoms, n (%) | ||||
| EPS present | 3 (21.4) | 32 (34.0) | 0.54 | |
| Offending agent, n (%) | ||||
| GI DRBAs | 3 (21.4) | 42 (44.7) | 0.15 | |
| Atypical antipsychotics | 8 (57.1) | 31 (33.0) | 0.13 | |
| Typical antipsychotics | 0 | 8 (8.51) | 0.59 | |
| SSRI | 0 | 1 (1.06) | 0.99 | |
| Mixed | 3 (21.4) | 12 (12.8) | 0.41 | |
| Type of TS, n (%) | ||||
| All dyskinesias | 2 (14.3) | 26 (27.7) | 0.45 | |
| All dystonias | 3 (21.4) | 20 (21.3) | 0.99 | |
| Other | 3 (21.4) | 5 (5.32) | 0.07 | |
| Mixed | 6 (42.9) | 43 (45.7) | 0.99 | |
| Baseline severity | ||||
| Mild | 3 (21.4) | 38 (40.4) | 0.24 | |
| Moderate | 6 (42.9) | 38 (40.4) | 0.99 | |
| Severe | 5 (35.7) | 18 (19.2) | 0.17 | |
| Latency to onset of symptoms (median, range) | (1.3, 0–16.3) | (1.8, 0–29) | 0.99 | |
| Duration of drug exposure (median, range) | (1.9, 0.04–16.4) | (2.3, 0.02–29) | 0.99 | |
| Duration of follow-up (median, range) | (1.9, 0.34–6.1) | (1.7, 0–13.5) | 0.99 | |
| Duration from end of drug exposure to resolved TS (median, range) | (1.5, 0.10–6.3) | — | ||
| Total TS duration (median, range) | (1.7, 0.13–26.9) | — | ||
Abbreviations: DRBAs, Dopamine Receptor Blocking Agents; EPS, Extrapyramidal Symptoms; GI, Gastrointestinal; SD, Standard Deviation; SSRI, selective serotonin reuptake inhibitors; TS, Tardive Syndromes.
p-values calculated by two-sample t-test for continuous variables and Pearson’s χ2 for categorical variables, two-sided p-values calculated at alpha = 0.05. p-value for diabetes and alcohol abuse calculated by Fisher’s exact test, two-sided.
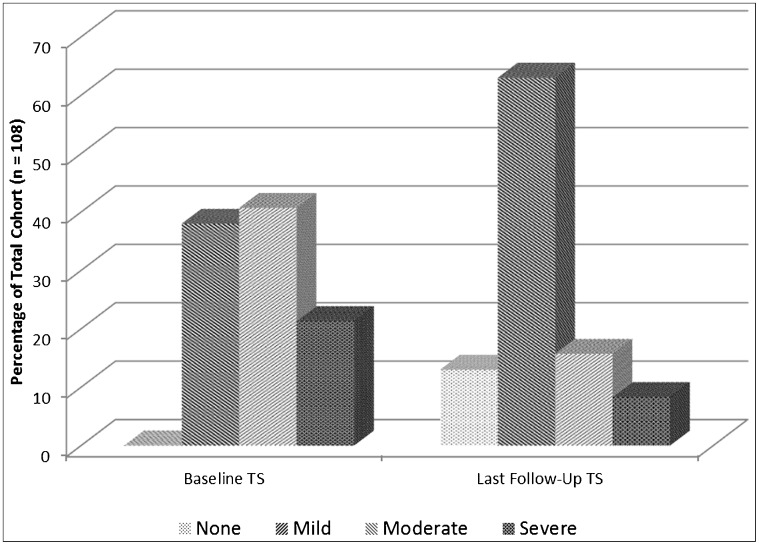
When baseline severity was compared to follow-up severity, a general trend for improvement was seen (Figure 1). The number of patients categorized with mild TSs at baseline examination increased by over 1.6 times at the last follow-up after discontinuation of DRBA regardless of the type of treatment used for TSs. The severe TS cohort decreased by over 2.5 times at the last follow-up visit.
Figure 1. Trend in Tardive Syndrome Severity at Baseline and Last Follow-up.
Discussion
We examined reversibility of TS in patients who were able to permanently stop their inciting drug and found that a low percentage of patients resolved, 13% overall and 2.8% simply with discontinuation of the DRBA. The low percentage of TS reversibility in our cohort is possibly secondary to a selection bias of the more difficult and refractory cases of TS referred for treatment to a tertiary movement disorder clinic. We also excluded 13% of our original cohort from analysis due to loss of follow-up after one visit and these patients may very well have had some form of improvement or even resolution after the offending DRBA was removed perhaps resulting in the lack of need for follow-up. The outcome of these considerations is possible underestimation of the frequency of reversibility reported in this study. Nevertheless, even if we included all of these patients as resolved the frequency remains a quarter or less. There was also a tendency for the severity of TS to improve to some extent over time without full resolution. This may relate to either the treatments provided or just the passage of time after stopping the offending agent. A prospective study would be needed to confirm this finding. In our cohort of patients, 7.4% were exposed to only first generation typical antipsychotics and 36.1% were exposed to only second-generation DRBAs. It would appear that even with atypical antipsychotics, the reversibility rates remain low as only 20.5% of these cases resolved.
Previous studies that examined psychotic patients with TSs who had their offending neuroleptic removed reported complete resolution ranging as high as 92%.23–25 However, most of these studies were conducted before the 1980s, which was the era of first generation antipsychotics, involved small sample sizes and had follow-up periods of 1 year or less.23–25 Our remission rates were more in the range of those reported by Glazer et al.18 and Kang et al.20 Glazer followed 36 patients with a non-schizophrenic diagnosis and an additional 13 patients with schizophrenia or schizoaffective disorder who were weaned off DRBAs for an average of 3.3 years. By the end of the study, week 49, only one patient had “continued improvement” from the baseline AIMS (Abnormal Involuntary Movement Scale) score (2%) and 44 patients had relapses or were lost to follow-up.18 Kang et al.20 retrospectively evaluated 67 patients who primarily had tardive dystonia, though many had mixed TS. Forty-two of them were able to withdraw DRBAs permanently. These patients were followed for a mean of 2.8 years (range 1 month to 5.2 years) and only five patients had remission of their TS (12%).20 These studies suggest that designing future prospective reversibility studies will require adequate follow-up periods with continued visits even after TS has resolved. These study populations were different from ours in that they consisted of primarily psychiatric patients treated with first generation DRBAs while only 6.5% of ours had a psychotic disorder and our patients with psychiatric disorders were treated most commonly with second-generation antipsychotics. In non-psychotic, non-psychiatric patients GI medications such as metoclopramide were the offending agent in 41.7% of the cases used alone or in combination with other DRBAs. This group of patients did not show any statistically significant difference in reversibility when compared to other offending agents implying that TS from GI medications is as chronic and debilitating in a cohort of non-psychiatric patients as TS from antipsychotics are in psychiatric populations.
Prognosticators for good outcomes, i.e., resolution or improvement, have been examined previously and include younger age of onset, psychiatric diagnosis, the course of the psychiatric disorder and duration of therapy.5,26,27 Many of these predictors were reported from studies in which exposure to DRBAs continued or were restarted due to failure of withdrawal.18,28 In the 1990 study by Glazer et al.,18 predictors of improvement or reversibility included age less than 55 years, employment, and having a non-schizophrenic diagnosis. Despite a small sample size and no statistical significance, similar predictors of remission were reported by Kang including young age of onset, shorter duration of exposure to DRBAs, and faster withdrawal of DRBAs.20 We did not corroborate these findings or find any other predictors of remission. This is possibly due in part to the small sample size of patients with complete reversibility (n = 14) and/or the retrospective nature of the study. However, there were a few features with sizable percentage differences worth mentioning. We did find that mood disorder made up a larger percentage of those who resolved than those who did not (50% vs. 36.2%). This would support prior findings noted above. Also, those who resolved were younger 53.5 vs. 59.4 and in a larger percentage of those who resolved the inciting agents were atypical antipsychotics (57%) compared to those who did not (33%). On the other hand, our data show that those who do not resolve are twice as likely to be treated for underlying GI illnesses with GI related dopamine blocking agents such as metoclopramide.
Our study has limitations as alluded to previously. It is a retrospective study, so there was not a standard measure for TSs such as the AIMS, and duration of follow-up varied. A large prospective trial will be needed to confirm our findings. As a movement disorder specialty clinic, there is a likelihood of inclusion bias for the more protracted cases. In addition, our population was mostly non-psychotic so it may not be translatable to schizophrenia. Finally, the number of resolved subjects was small and likely underpowered for examining for prognosticators of good outcomes.
In conclusion, our study demonstrated a low remission rate for TSs in psychiatric and non-psychiatric patients after DRBAs are withdrawn. Despite the fact that our results could have been biased by the retrospective nature of the study and the possible referral bias to the movement disorder clinic, the results were similar to other such examinations in psychiatric populations. The irreversibility of TSs has serious prognostic implications and likely represents an important reason for rising prevalence. The 2013 American Academy of Neurology guideline on the treatment of TSs based on prior reports was unable to provide conclusive guidelines on the benefits of long-term DRBA withdrawal because of inadequate evidence and the lack of longitudinal prospective studies.29 At best, with current treatment options, our data suggest that there are small benefits with gradual partial improvement and a few remissions. At worst it appears that TSs are a permanent complication of DRBAs in most patients. Hence, by the time TSs develop it is too late. There is a clear need for prospective, multicenter, clinical trials in psychiatric and non-psychiatric TS populations who can be safely withdrawn from DRBAs so that true remission rates can be measured. For now, we can conclude that TSs are an iatrogenic problem caused by exposure to DRBAs that are common and increasing in frequency, embarrassing, disabling, and permanent in a majority of those who develop them.5 This should be kept in mind when considering prescribing this class of drugs. It also indicates that some urgency is needed in the development of effective therapies.
Footnotes
Funding: None.
Financial Disclosures: Leslie J. Cloud: Grant from VCU CCTR. Stewart A Factor: Grants from Ceregene, Sangamo, TEVA, Ipsen, Allergan, Medtronics, Auspex, Genzyme, Michael J. Fox Foundation, NIH; Honoraria from Scientiae for CME program, Current Neurology and Neuroscience section editor, Neurotherapeutics guest editor, Merz, Chelsea Therapeutics, ADAMAS, Neurocrine, Lundbeck, Ceregene; Royalties from Demos, Blackwell Futura for textbooks, Uptodate.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.American Psychiatric Association . Washington, DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders. 4th ed., text review (DSM-IV TR) pp. 803–805. p. [Google Scholar]
- 2.Schonecker M. Paroxysmal dyskinesia as the effect of megaphen. Nervenarzt. 1957;28:550–553. German. [PubMed] [Google Scholar]
- 3.Delay J, Deniker P. Trente-huit cas de psychoses traits par la cure prolongee et continue de 4568 R. Annales Medico-Pscyhologiques. 1952;110:364. [Google Scholar]
- 4.Fahn S, Jankovic J, Hallett M. The tardive syndromes: Phenomenology, concepts on pathophysiology and treatment, and other neuroleptic-induced syndromes. In: Fahn S, Jankovic J, Hallett M, editors. Principles and practice of movement disorders. 2nd ed. Philadelphia, PA: Elsevier Sanders; 2011. pp. 415–446. p. [Google Scholar]
- 5.Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: Therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11:166–176. doi: 10.1007/s13311-013-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samie MR, Dannenhoffer MA, Rozek S. Life-threatening tardive dyskinesia caused by metoclopramide. Mov Disord. 1987;2:125–129. doi: 10.1002/mds.870020207. [DOI] [PubMed] [Google Scholar]
- 7.Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21:151–156. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- 8.Woods SW, Morgenstern H, Saksa JR, et al. incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: A prospective cohort study. J Clin Psychiatry. 2010;71:463–474. doi: 10.4088/JCP.07m03890yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DD, Caroff SN, Davis SM, et al. Extrapyramidal side-effects of antipsychotics in a randomized trial. Br J Psychiatry. 2008;193:279–288. doi: 10.1192/bjp.bp.108.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions metoclopramide. BMJ (Clin Res Ed) 1985;291:930–932. doi: 10.1136/bmj.291.6500.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilholm BE, Mortimer O, Boethius G, Häggström JE. Tardive dyskinesia associated with metoclopramide. BMJ (Clinical Research Ed.) 1984;288:545–547. doi: 10.1136/bmj.288.6416.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sewell DD, Kodsi AB, Caligiuri MP, Jeste DV. Metoclopramide and tardive dyskinesia. Biol Psychiatry. 1994 Nov 1;36:630–632. doi: 10.1016/0006-3223(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 13.Ganzini L, Casey DE, Hoffman WF, McCall AL. The prevalence of metroclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders. Arch Intern Med. 1993;153:1469–1475. doi: 10.1001/archinte.1993.00410120051007. [DOI] [PubMed] [Google Scholar]
- 14.Hyde TM, Apud JA, Fisher WC, Egan MF. Tardive dyskinesia. In: Factor SA, Lang AE, Weiner WJ, editors. Drug induced movement disorders. 2nd ed. Oxford: Blackwell Publishing; 2005. pp. 213–256. p. [Google Scholar]
- 15.Smith JM, Baldessarini RJ. Changes in prevalence, severity and recovery in tardive dyskinesia with age. Arch Gen Psychiatry. 1980;37:1368–1373. doi: 10.1001/archpsyc.1980.01780250054006. [DOI] [PubMed] [Google Scholar]
- 16.Burke RE. Tardive dyskinesia: Current clinical issues. Neurology. 1984;34:1348–1353. doi: 10.1212/WNL.34.10.1348. [DOI] [PubMed] [Google Scholar]
- 17.Glazer WM, Moore DC, Schooler NR, Brenner LM, Morgenstern H. Tardive dyskinesia. A discontinuation study. Arch Gen Psychiatry. 1984;41:623–627. doi: 10.1001/archpsyc.1984.01790170097011. [DOI] [PubMed] [Google Scholar]
- 18.Glazer WM, Morgenstern H, Schooler N, Berkman CS, Moore DC. Predictors of improvement in tardive dyskinesia following discontinuation of neuroleptic medication. Br J Psychiatry. 1990;157:585–592. doi: 10.1192/bjp.157.4.585. [DOI] [PubMed] [Google Scholar]
- 19.Soares KV, McGrath JJ. The treatment of tardive dyskinesia—a systematic review and meta-analysis. Schizophr Res. 1999;39:1–16. doi: 10.1016/S0920-9964(99)00021-3. discussion 17–18. [DOI] [PubMed] [Google Scholar]
- 20.Kang UJ, Burke RE, Fahn S. Natural history and treatment of tardive dystonia. Mov Disord. 1986;1:193–208. doi: 10.1002/mds.870010305. [DOI] [PubMed] [Google Scholar]
- 21.Kane JM, Woerner M, Borenstein M, Wegner J, Lieberman J. Integrating incidence and prevalence of tardive dyskinesia. Psychopharmacol Bull. 1986;22:254–258. [PubMed] [Google Scholar]
- 22.Esper CD, Factor SA. Failure of recognition of drug-induced parkinsonism in the elderly. Mov Disord. 2008;23:401–404. doi: 10.1002/mds.21854. [DOI] [PubMed] [Google Scholar]
- 23.Paulson GW. An evaluation of the permanence of the “tardive dyskinesias.”. Dis Nerv Syst. 1968;29:692–694. [PubMed] [Google Scholar]
- 24.Quitkin F, Rifkin A, Gochfeld L, Klein DF. Tardive dyskinesia: Are first signs reversible? Am J Psychiatry. 1977;134:84–87. doi: 10.1176/ajp.134.1.84. [DOI] [PubMed] [Google Scholar]
- 25.Jeste DV, Potkin SG, Sinha S, Feder S, Wyatt RJ. Tardive dyskinesia—reversible and persistent. Arch Gen Psychiatry. 1979 May;36:585–590. doi: 10.1001/archpsyc.1979.01780050095012. [DOI] [PubMed] [Google Scholar]
- 26.Tarsy D, Baldessarini RJ. Epidemiology of tardive dyskinesia: Is risk declining with modern antipsychotics? Mov Disord. 2006;21:589–598. doi: 10.1002/mds.20823. [DOI] [PubMed] [Google Scholar]
- 27.Waln O, Jankovic J. An update on tardive dyskinesia: From phenomenology to treatment. Tremor Other Hyperkinet Mov. 2013;3 doi: 10.7916/D88P5Z71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez HH, Krupp B, Friedman JH. The course of tardive dyskinesia and parkinsonism in psychiatric inpatients: 14-year follow-up. Neurology. 2001;56:805–807. doi: 10.1212/WNL.56.6.805. [DOI] [PubMed] [Google Scholar]
- 29.Bhidayasiri R, Fahn S, Weiner WJ, Gronseth GS, Sullivan KL, Zesiewicz TA. Evidence-based guideline: Treatment of tardive syndromes: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81:463–469. doi: 10.1212/WNL.0b013e31829d86b6. [DOI] [PubMed] [Google Scholar]