Abstract
Background
PsbS is a 22-kDa Photosystem (PS) II protein involved in non-photochemical quenching (NPQ) of chlorophyll fluorescence. Rice (Oryza sativa L.) has two PsbS genes, PsbS1 and PsbS2. However, only inactivation of PsbS1, through a knockout (PsbS1-KO) or in RNAi transgenic plants, results in plants deficient in qE, the energy-dependent component of NPQ.
Results
In studies presented here, under fluctuating high light, growth of young seedlings lacking PsbS is retarded, and PSII in detached leaves of the mutants is more sensitive to photoinhibitory illumination compared with the wild type. Using both histochemical and fluorescent probes, we determined the levels of reactive oxygen species, including singlet oxygen, superoxide, and hydrogen peroxide, in leaves and thylakoids. The PsbS-deficient plants generated more superoxide and hydrogen peroxide in their chloroplasts. PSII complexes isolated from them produced more superoxide compared with the wild type, and PSII-driven superoxide production was higher in the mutants. However, we could not observe such differences either in isolated PSI complexes or through PSI-driven electron transport. Time-course experiments using isolated thylakoids showed that superoxide production was the initial event, and that production of hydrogen peroxide proceeded from that.
Conclusion
These results indicate that at least some of the photoprotection provided by PsbS and qE is mediated by preventing production of superoxide released from PSII under conditions of excess excitation energy.
Electronic supplementary material
The online version of this article (doi:10.1186/s12870-014-0242-2) contains supplementary material, which is available to authorized users.
Keywords: Photoprotection, PsbS, ROS, Superoxide, Photosynthesis, NPQ, Rice
Background
Light energy is converted to chemical energy during photosynthesis. However, because excess light is harmful, plants engage several protective mechanisms, including non-photochemical quenching (NPQ) of chlorophyll (Chl) fluorescence. NPQ is subdivided into three components that involve relaxation kinetics under darkness followed by a period of illumination. The first component, qE, relaxes quickly (within seconds to minutes) and is triggered by an increase in the trans-thylakoid proton gradient, or ΔpH. The second component, qT, relaxes more slowly and is a state transition phenomenon. The last component, qI, with the slowest relaxation, is a rather ill-defined component which traditionally includes a non-relaxing component related to irreversible damage, such as the inactivation of D1 protein in the Photosystem (PS) II reaction center [1,2]. Recently, the third very slow component, qZ was proposed, which depends on zeaxanthin [3]. Zeaxanthin directly or indirectly contributes to all NPQ mechanisms except qT [2].
The major component, qE, is dependent on three factors: the ΔpH [4], pigments in the xanthophyll cycle [5], and a 22-kDa PSII protein called PsbS [6]. These control qE in an integrated manner. Although the signal largely disappears when one factor is absent, qE can still be induced in the absence of PsbS, albeit much more slowly [7]. The qE signal is characterized by several activities, e.g., light-induced absorbance changes at 535 nm [8], shortening of a specific Chl fluorescence lifetime component from ~2.0 to ~0.4 ns [9], formation of carotenoid cation radicals [10], or changes in the configuration of neoxanthin molecules in the light-harvesting complex (LHC) II [11]. Alterations in absorbance and the Chl fluorescence lifetime often reflect structural changes in pigment-protein complexes of the thylakoid membranes.
The role of the PsbS protein in qE was first described in npq4-1 mutants of Arabidopsis thaliana that lack PsbS [6]. Although this protein is evidently necessary for qE, Arabidopsis mutants completely lacking PsbS show normal photochemistry without any visible phenotype under controlled-environment conditions of non-fluctuating light [6,12]. However, when grown in the field or under rapidly fluctuating moderate light in a laboratory, those mutants produce fewer seeds than wild-type plants [13] and also show retarded growth [14]. The function of PsbS in qE development remains unclear, and the role of protonation of its glutamate residues in Chl fluorescence quenching is still debated [15,16]. Two thylakoid lumen-exposed glutamate residues of PsbS sense shifts in pH [17] and induce conformational changes that control qE [18]. PsbS does not seem to bind pigments [19] but may either interact with CP29 [20] or induce conformational modifications in it that modulate the energy of the Chl/zeaxanthin charge-transfer state [21]. Recent data have provided information on how PsbS controls the conformation and organization of PSII supercomplexes [22-25]. Recently have been shown that PsbS controls over photosynthesis in fluctuating light which optimize the photoprotective processes [26].
When NPQ is inhibited, one might expect more reactive oxygen species (ROS) to be produced in the chloroplasts. Powerful ROS include the highly reactive singlet oxygen [27], the superoxide anion radical, and hydrogen peroxide [28]. Biotic- and abiotic-stress conditions lead to an imbalance between ROS generation and scavenging; those accumulated ROS can cause damage to cells near the sites where they are generated [29]. Even though ROS are scavenged by diverse antioxidative defense substances (e.g., antioxidant enzymes and antioxidants such as ascorbate, tocopherol, and glutathione; [30,31]), ROS levels may rise rapidly following environmental changes [32]. Due to their highly reactive nature, ROS react with a wide range of molecules in biological organisms and can damage these molecules with consequences that may be fatal to the cell or even the plant [33].
The main source of ROS in chloroplasts is the electron transport chain; the generation site for each ROS depends upon the stress applied [30,34,35]. Singlet oxygen is a byproduct of photosynthesis, mainly formed at PSII [36] but also in other locations where triplet Chl molecules are produced. Generally, three different sites within the photosynthetic apparatus are associated with singlet oxygen production: i) the PSII reaction center; ii) the antennae of the LHC, and iii) the PSI acceptor site [37]. The destructive effect of singlet oxygen on D1 protein within the PSII reaction center is well understood [38,39]. However, little is known about how singlet oxygen influences other components of the thylakoid membrane. The singlet oxygen produced in flu mutants of Arabidopsis strongly affects ATP synthase activity and causes changes in NPQ, although its production site differs from those mentioned above [40]. The water–water cycle is considered the main source for superoxide production on the reducing side of PSI, helping plants to dissipate excess light energy by increasing the rate of electron transport and lowering the luminal pH [41-43]. Generation of superoxide within PSII has also been reported [44-46]. The next site for superoxide production is the plastoquinone pool [47]. There, superoxide is rapidly dismutated to the more stable hydrogen peroxide by superoxide dismutase (SOD) [28]. If superoxide is produced within the thylakoid membrane [48] where SOD is absent, hydrogen peroxide can be produced by the reduction of superoxide by plastohydroquinone PQH2 [47,49], and the same pathway also occurs within mitochondrial membrane [50].
To elucidate the role of PsbS protein in the photoprotective mechanism of NPQ, we investigated the consequences of eliminating this protein, especially on the generation of ROS. We found that superoxide produced at PSII was greater in PsbS-knockout rice leaves than in the wild type leaves. However, the levels of superoxide produced at PSI did not differ between the mutants and wild-type plants. Therefore, we suggest that PsbS protects against superoxide production at PSII when excess energy is absorbed by the PSII antennae.
Results
Isolation of a rice PsbS knockout plant and generation of PsbS RNAi transgenic plants
The rice genome has two PsbS genes -- OsPsbS1 (LOC_Os01g64960) on Chromosome 1 and OsPsbS2 (LOC_Os04g59440) on Chromosome 4 [51]. OsPsbS1 and OsPsbS2 encode proteins of 268 and 254 amino acids, respectively. Arabidopsis PsbS (AtPsbS) shares 68% sequence similarity with OsPsbS1 and 71% with OsPsbS2; the two rice proteins show 72% overall amino acid identity with each other.
We selected PsbS-KO, a putative knockout mutant for OsPsbS1, from a pool of T-DNA insertion rice lines. It had been generated by transformation with a T-DNA vector (pGA2707) containing a promoterless GUS gene next to the left border (LB) of the T-DNA [52]. Sequencing via inverse-PCR of the region flanking that insertion [53] revealed that the T-DNA was inserted in the 3rd exon of OsPsbS1 (Figure 1a). By genotyping multiple segregating lines in the T2 generation, we selected four plants homozygous for the T-DNA insertion. The result of the genotyping of PsbS1 line is shown in Additional file 1: Figure S1. Western blotting with a PsbS-specific antibody from AgriSera indicated that all four of the homozygous mutant plants lacked PsbS (Figure 1b). In those plants, the NPQ value that developed within 5 min was approximately 0.4 (Figure 1f), which is similar to that reported for the Arabidopsis npq4-1 mutant [54]. A more detailed analysis of NPQ relaxation (or dark recovery of developed NPQ) indicated that qE was completely lacking in PsbS-KO leaves.
Figure 1.
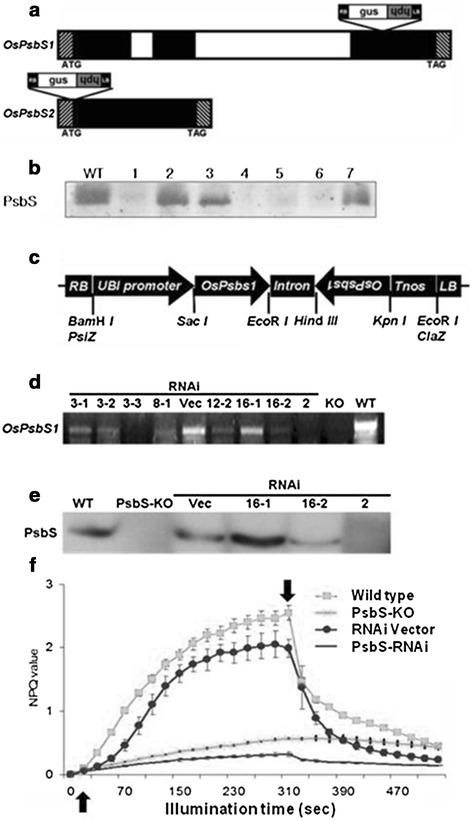
Characterization of PsbS-KO and PsbS-RNAi rice plants. (a) Schematic diagrams of the rice PsbS genes and the T-DNA insertion positions. The exons are shaded and introns are indicated with open boxes. For OsPsbS1, T-DNA was inserted into 3rd exon; for OsPsbS2, T-DNA was inserted into the beginning of the exon. (b) Western blot analysis of putative homozygous and heterozygous plants. PsbS protein was detected with a PsbS-specific polyclonal antibody. Lines 1 to 7 were segregated in the T2 generation of OsPsbS1 plants. (c) Schematic diagram of rice PsbS-RNAi vector. RB, right border; UBI promoter, ubiquitin I promoter; OsPsbS1, inverted repeat of a unique 102-bp fragment of coding region for PsbS gene; Intron, 204-bp portion of the 3rd intron of OsEMF1 gene (AF326768); Tnos, nopaline synthase terminator; LB, left border. (d) Transcript levels for PsbS gene in wild-type, PsbS-KO, PsbS-RNAi, and vector-only rice. Numbers indicate Lines of the PsbS-RNAi transformants. (e) Western blot analysis of PsbS-RNAi transformants and vector-only rice. Wild-type (WT) and PsbS-KO plants were used as positive and negative controls. (f) Light-induced NPQ generation in leaves (intensity of actinic light: 700 μmol photons m−2 s −1). Up arrow, light switched on; down arrow, light switched off. Each point represents mean of at least 4 experiments (SD indicated by bar). NPQ was calculated as described in Methods.
A KO mutant plant for OsPsbS2 was also chosen from the pool of T-DNA insertion lines. Its flanking sequence revealed that the T-DNA was inserted in the sole exon near its start codon (Figure 1a). This rice gene product shares very high sequence similarity with AtPsbS, and exposure of etiolated seedlings to red and blue light induces a several-fold increase in the steady-state level of OsPsbS2 transcripts [55]. Nevertheless, this rice mutant exhibited no visible phenotypic deviations with respect to the wild type, and their NPQ levels were also very similar. No OsPsbS2 product was found in OsPsbS1 knockout mutant plants when two different PsbS-antibodies were used, suggesting that such a product could not be detected by these antibodies. This was probably because either the immunological characteristics of OsPsbS and the Arabidopsis protein differ from that of the OsPsbS2 gene product or else the protein encoded by OsPsbS2 does not accumulate in the chloroplasts. These data are also consistent with previous genetic evidence that OsPsbS1, but not OsPsbS2, is co-localized with a QTL for NPQ [56]. Thus, our subsequent characterization focused solely on OsPsbS1, which we refer to as OsPsbS hereafter.
To confirm that the reduced NPQ of PsbS-KO leaves was caused by the insertion in OsPsbS1 and not by either the insertion of multiple T-DNAs or other genetic differences, we used RNA interference (RNAi) technology to generate transgenic rice with significantly reduced PsbS protein levels. Plants were transformed with an RNAi construct that contained an inverted repeat of a unique 102-bp region of OsPsbS1, with a portion of the pBSIIKS vector serving as a linker and driven by the ubiquitin I promoter (Figure 1c). Transformants were screened by RT-PCR for OsPsbS1 transcripts (Figure 1d) and confirmed by western blotting (Figure 1e). From these, we identified three RNAi lines with varying OsPsbS1 transcript and PsbS protein levels. Among them, Line #2 produced little or no PsbS. Its NPQ development level (Figure 1f) and its corresponding light curve (Additional file 1: Figure S2) were comparable to those found from the PsbS-KO line. However, electron transport rates were similar between all investigated samples and their wild type counterparts (Additional file 1: Figure S3).
Lack of PsbS protein in rice plants results in increased sensitivity to photoinhibitory illumination
At the whole-plant level, an Arabidopsis mutant (npq4-1) lacking the PSII protein PsbS has no visible phenotype except for reduced fitness when grown under either oscillating light or in the field [13,14]. We also observed that the growth rates of PsbS-KO and PsbS-RNAi rice plants under fluctuating light were significantly reduced (Additional file 1: Figure S4), and that grain yield from PsbS-KO plants was only about 30% of that reported from the wild type [57]. Under strong illumination (1,200 μmol photons m−2 s−1, white light), npq4-1 becomes more susceptible to photoinhibition than wild-type plants [58]. When we exposed leaf segments to photoinhibitory illumination (2,000 μmol photons m−2 s−1 for 2 h), values calculated for Fv/Fm in the rice PsbS-KO mutant and RNAi plants dropped very rapidly, to about 40% of the level for dark-adapted controls (Figure 2a). By contrast, the Fv/Fm in the wild type was reduced to about 55% of the dark-adapted control. In all plant types, this decline was largely completed within 1 h of treatment, and no further decrease was observed thereafter (Figure 2a,b - closed symbols). The initial decline in Fv/Fm probably resulted because photodamage to PSII occurred more rapidly than it could be repaired. Moreover, the significant reduction in this rate of decline after 1 h was due to activation of the PSII recovery process [59,60]. The initial rates of photodamage were apparently higher in both PsbS-KO and RNAi leaves than in the wild type; however, recovery seemed to be activated in a similar manner regardless of genotype (Figure 2a,b).
Figure 2.
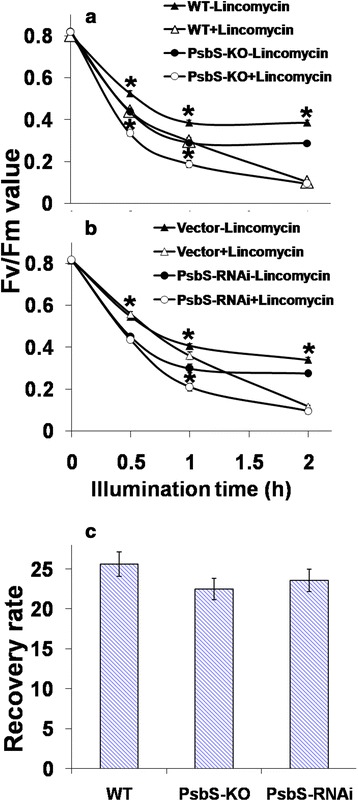
Photoinhibition of PSII defined as decrease in Fv/Fm during photoinhibitory illumination. (a) wild-type and PsbS-KO plants. (b) PsbS-RNAi and vector-only plants. Leaves were illuminated at 2,000 μmol photons m−2 s−1 for photoinhibition in absence (closed symbols) or presence (open symbols) of 2 mM lincomycin. (c) Recovery of damaged PSII for 2 h in absence of lincomycin under dim light (50 μmol photons m−2 s−1). Recovery rate was calculated as % increase in Fv/Fm after 2-h recovery period relative to decreased value before recovery began. Each point represents mean of at least 4 experiments (SE indicated by bar) and the asterisks denote the results that were significantly different from those in the wild type (*P < 0.05). The statistical significance was evaluated using the Student’s t-test.
Photoinhibition is a complex process entailing photodamage, repair of D1 protein of PSII, and re-assembly of active PSII [61]. Leaf infiltration with lincomycin, an inhibitor of protein synthesis in the chloroplasts, allows one to assess this process in isolation. Here, when lincomycin was applied, the rates of photodamage in both PsbS-KO and PsbS-RNAi leaves were higher than in the treated wild type. Blockage of the recovery process meant that the Fv/Fm for all three plant types continued to decrease until it reached ~10% of the dark-adapted value. This demonstrated that PsbS-KO and PsbS-RNAi leaves are more susceptible to PSII photodamage.
To monitor how leaf segments recovered in the absence of lincomycin after photoinhibition, we measured changes in Fv/Fm after 2 h of exposure to dim light (50 μmol photons m−2 s−1) at room temperature. The illumination used in this experiment resulted in ~40% and ~50% reductions in the Fv/Fm values of PsbS-KO and wild-type leaves, respectively. After a 2-h recovery period, Fv/Fm of the PsbS-KO, PsbS-RNAi, and wild type reached 82%, 84% and 90% of their dark-adapted values, respectively (Figure 2c). This indicated that the recovery process, including repair and re-assembly, is normal in PsbS-KO and PsbS-RNAi leaves.
Superoxide and hydrogen peroxide production is higher in PsbS-deficient rice leaves
Singlet oxygen is a photosynthesis byproduct that is mainly formed at PSII under high-light conditions [36]. Because our data indicated that PsbS-KO and PsbS-RNAi rice has increased susceptibility to photoinhibition, we measured singlet oxygen production in wild type, PsbS-KO, and PsbS-RNAi leaves using singlet oxygen sensor green (SOSG) which specifically detects singlet oxygen [62,63]. Here, its fluorescence emission in wild-type plants increased almost four times by photoinhibitory illumination, and the increase of the SOSG fluorescence emission in PsbS-KO or PsbS-RNAi leaves was not significantly different from that in wild type (Figure 3a). We could get very similar results using dansyl-2, 2, 5, 5,-tetramethyl-2, 5-dihydro1H-pyrrole (DanePy) (data not shown).
Figure 3.
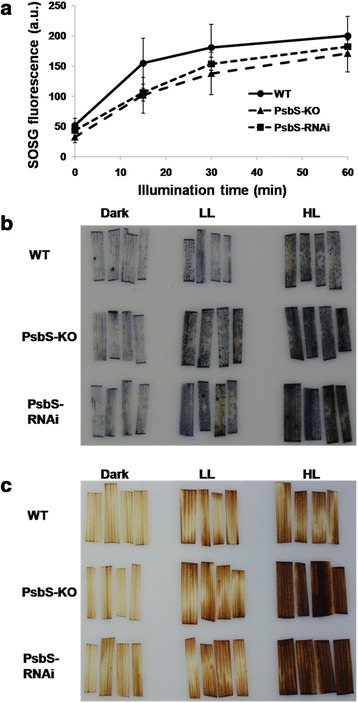
ROS production in rice. (a) Detection of singlet oxygen in leaves, as monitored by increase in SOSG fluorescence emission at 530 nm. Leaf segments were vacuum infiltrated with 200 μM SOSG solution before being illuminated at 2,000 μmol photons m−2 s−1. (b) Production of superoxide anion radicals. Histochemical staining with NBT in wild-type (WT), PsbS-KO and PsbS-RNAi leaves incubated under darkness for 2 h (Dark), under moderate light at 200 μmol photons m−2 s−1 (LL), or under photoinhibitory illumination at 2,000 μmol photons m−2 s−1 (HL). (c) Production of hydrogen peroxide. Histochemical staining with DAB in wild-type (WT), PsbS-KO and PsbS-RNAi leaves under control conditions (Dark), under moderate light at 200 μmol photons m−2 s−1 (LL), or after 2 h of photoinhibitory illumination at 2,000 μmol photons m−2 s−1 (HL). Experiments were repeated 4–6 times and representative images shown.
Because high light-induced production of singlet oxygen was no higher in plants lacking PsbS than in the wild type, we measured the levels of other ROS, including superoxide and hydrogen peroxide. We visualized generation of the former by histochemically staining of rice leaves with nitroblue tetrazolium (NBT) (Figure 3b). In dark-adapted samples, no difference was observed among all genotypes, but both PsbS-KO and PsbS-RNAi leaves were stained dark-blue at 2,000 μmol photons m−2 s−1. Even under moderate light intensity (200 μmol photons m−2 s−1), more superoxide was accumulated in both PsbS-KO and PsbS-RNAi leaves than in the wild type.
Superoxide is rapidly dismutated to more stable hydrogen peroxide by SOD [28]. Therefore, we measured hydrogen peroxide production in wild type, PsbS-KO and PsbS-RNAi leaves by histochemically staining with 3, 3′-diaminobenzidine (DAB) (Figure 3c). Under photoinhibitory illumination at 2,000 μmol photons m−2 s−1 for 2 h, more hydrogen peroxide was detected in both PsbS-KO and PsbS-RNAi leaves than in the wild type.
To confirm this result, we visualized the production of singlet oxygen, superoxide, and hydrogen peroxide at high resolution, using a confocal laser scanning microscope with DanePy, dihydroethidium (DHE), and 2′,7′-dichlorofluorescein diacetate (DCFDA), respectively (Additional file 1: Figures S5-7), and the results were virtually the same as those observed by histochemical staining.
Superoxide production is the initial event
To confirm the results obtained using leaf segments, we then determined the levels of three ROS in isolated thylakoids before and after illumination with 700 μmol photons m−2 s−1 for 10 min. Although SOSG fluorescence emission increased by illumination, no significant differences in singlet oxygen generation were found between PsbS-KO and wild-type plants (Figure 4a). For more accurate detection of superoxide, we monitored increases in the fluorescence of DHE because it has been proven to detect superoxide in both intact cells and isolated subcellular fractions [64-66]. The suitability of DHE for assaying superoxide has also been verified by demonstrating that its fluorescence increases dose-dependently [64]. In the case of superoxide, the fluorescence emission at 615 nm rose linearly for 10 min, and the rate of increase was almost 40% as high in PsbS-KO thylakoids as in the wild type (Figure 4b). For hydrogen peroxide, the DCFDA fluorescence in thylakoids increased more rapidly in the PsbS-KO thylakoids (Figure 4c). However, production of hydrogen peroxide began 2 to 3 min after the start of superoxide generation. This suggested that most of the hydrogen peroxide resulted from the conversion of superoxide, thereby indicating that the main ROS overproduced in PsbS-KO plants is superoxide rather than hydrogen peroxide. In addition, the results obtained by using fluorescence sensors were confirmed by measuring the levels of superoxide and hydrogen peroxide based on NBT absorbance at 560 nm and DAB absorbance at 450 nm, respectively (Additional file 1: Figure S8).
Figure 4.
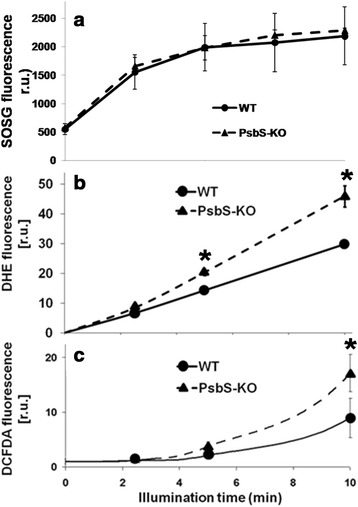
Time course for generation of individual ROS in thylakoids of PsbS-KO and wild-type (WT) rice under photoinhibitory illumination at room temperature. (a) Singlet oxygen production was monitored as relative increasing of SOSG (10 μM) fluorescence at 530 nm. (b) Fluorescence emission of dihydroethidium (25 μM) at 590 nm was used to detect superoxide production. (c) Fluorescence emission of DCFDA (10 μM) at 525 nm was used to detect hydrogen peroxide. Thylakoid suspensions were illuminated at 700 μmol photons m−2 s−1. Samples contained 10 μg chlorophyll per mL. Each point represents mean of at least 4 experiments (SD indicated by bar; in some cases, SD is less than marker size) and the asterisks denote the results that were significantly different from those in the wild type (*P < 0.05). The statistical significance was evaluated using the Student’s t -test.
Superoxide produced at PSII is more in PsbS-KO rice leaves than in wild-type leaves
Under stress, superoxide is believed to be produced mostly in PSI [41,43]. In that case, PSI in PsbS-KO rice plants should be more damaged during photoinhibitory illumination where superoxide generated more than in wild-type plants. Upon photoinhibitory illumination, the decrease in P700+ formation in PsbS-KO was no greater than in the wild type [67], even though more superoxide was generated in the former. The increase in DHE fluorescence from PSII particles illuminated for 10 min was higher in PsbS-KO leaves (Figure 5a).
Figure 5.
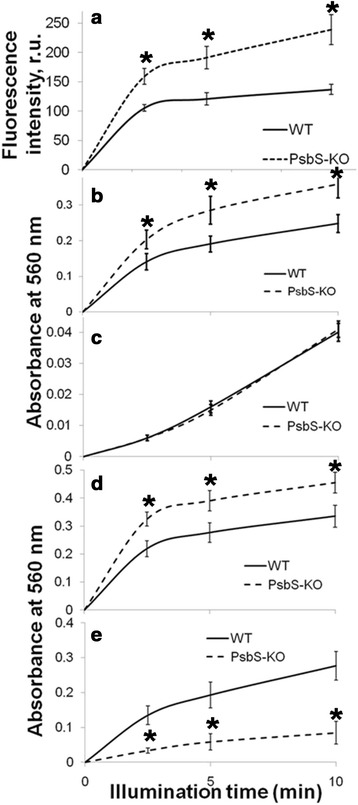
Superoxide generated by photosystems of PsbS-KO and wild-type rice. (a) PSII (BBY) particles. (b) Photosystem II complex isolated along sucrose gradient. (c) Photosystem I complex isolated along sucrose gradient. (d) Thylakoids with PSII-driven superoxide production. (e) Thylakoids with PSI-driven superoxide production. In (a), fluorescence emission of dihydroethidium (25 μM) at 590 nm was used to detect production. In (b-e), absorbance of NBT (15 μM) at 560 nm was used to detect production. Samples were illuminated at 700 μmol photons m−2 s−1 for photoinhibition at room temperature. Each sample contained 10 μg chlorophyll per mL. Each point represents mean of at least 4 experiments (SD indicated by bar) and the asterisks denote the results that were significantly different from those in the wild type (*P < 0.05). The statistical significance was evaluated using the Student’s t -test.
These results were again confirmed by measuring changes in NBT absorbance at 560 nm, using PSI and PSII particles separated along a sucrose gradient [68]. As shown in Figures 5b,c, superoxide production in PSII particles was higher in PsbS-KO than in wild-type plants, whereas production in PSI complexes was similar for both genotypes. Because superoxide production was greater in PSII particles, we have compared protein composition of the PSI and PSII proteins using Western blotting (Additional file 1: Figure S9). Although BBY particles show contamination with PSI proteins, their amount do not differ significantly to affect superoxide production. We also measured PSI- and PSII-driven superoxide production in thylakoids using corresponding donor-acceptor pairs. As expected, PSII-driven production was higher in PsbS-KO (Figure 5d). Surprisingly, PSI-driven production was lower in PsbS-KO thylakoids (Figure 5e). These results were verified by measuring changes in NBT absorbance at 560 nm. When we instead used NADP+ as an electron acceptor, whole chain-driven superoxide production was again higher in PsbS-KO thylakoids (Figure 6a). However, PSI-driven production was lower in those thylakoids (Figure 6b). Presumably, this decrease was a consequence of the activation of cyclic electron flow around PSI in the PsbS-KO plants [67].
Figure 6.
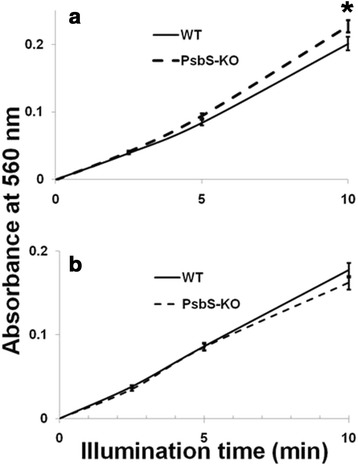
Superoxide generated by whole electron transport chain and PSI of PsbS-KO and wild-type rice. (a) Thylakoids with whole chain-driven production. (b) Thylakoids with PSI-driven production. NADP+ was used as final electron acceptor. Absorbance of NBT (15 μM) at 560 nm was used to detect production. Samples were illuminated at 700 μmol photons m−2 s−1 for photoinhibition at room temperature. Each sample contained 10 μg chlorophyll per mL. Each point represents mean of at least 4 experiments (SD indicated by bar) and the asterisks denote the results that were significantly different from those in the wild type (*P < 0.05). The statistical significance was evaluated using the Student’s t -test.
To make sure that the differences in superoxide production are not due the differences in electron transport rates, we measured electron transport rates in all samples by a Clark-type electrode (Table 1). As expected, we observed no striking differences in rates between wild-type and PsbS-KO thylakoids. Moreover, isolated PSI and PSII samples showed similar rates. Despite the differences noted in Fv/Fm between wild type and PsbS-KO leaves after photoinhibitory illumination for 2 h (Figure 2a), we could not observe such differences between the electron transport rates of two plants during illumination (Additional file 1: Figure S3). In fact, we could also not observe such differences between the two plants during illumination of isolated thylakoids with 700 μmol photons m−2 s−1 for 10 min. In both thylakoids, the decrease in Fv/Fm was 40% of the initial value. Taken together, our data suggested that, in the absence of qE, excess energy is released to molecular oxygen via an electron transport reaction. The observed phenotype in PsbS-KO leaves was probably a consequence of increased superoxide generation in PSII.
Table 1.
Photosynthetic electron transport rate (ETR) of rice thylakoids and isolated photosystems
| Sample | Whole chain ETR | PSII-driven ETR | PSI-driven ETR | ETR of isolated PSII | ETR of isolated PSI |
|---|---|---|---|---|---|
| WT | 158 ± 12 | 275 ± 8 | 215 ± 45 | 334 ± 35 | 185 ± 65 |
| PsbS-KO | 147 ± 14 | 269 ± 10 | 245 ± 55 | 341 ± 18 | 180 ± 70 |
PSII-driven ETR and ETR of isolated PSII were measured by oxygen evolution (H2O to phenyl-p-benzoquinone), and whole chain ETR (H2O to methyl viologen), PSI-driven ETR and ETR of isolated PSI (sodium ascorbate and 2,6-dichlorophenol-indophenol to methyl viologen) were measured by oxygen consumption. Unit: μmol O2 (mg Chl)−1 h−1.
Superoxide can be produced at different sites within PSII, such as through cyclic electron flow with the participation of cytochrome b559 [46] or at the QA site [45]. Therefore, we measured the redox state of cytochrome b559 in Mn-depleted PSII complexes as well as the re-oxidation of QA− in wild-type and PsbS-KO leaves. No significant differences between genotypes were found in the redox difference spectra for the high-potential form of cytochrome b559 in Mn-depleted PSII complexes (Additional file 1: Figure S10). However, we observed a difference in QA− re-oxidation kinetics, measured as Chl fluorescence decay, after a single turnover flash in the wild-type and PsbS-KO thylakoids (Figure 7, Additional file 1: Table S1).
Figure 7.
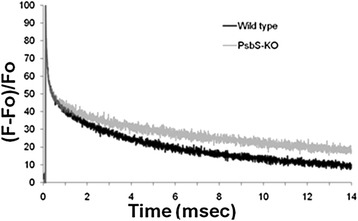
Chlorophyll fluorescence decay after a single turnover flash in wild-type and PsbS-KO thylakoids. For each experiment at least 12–15 repetitive flashes were given every 15 s; 24 traces from two independent experiments were averaged and plotted after normalization to the maximum fluorescence yield in the averaged trace.
Discussion
In this study, we used biophysical, biochemical, physiological, and molecular biological approaches to characterize rice plants lacking the PsbS protein at PSII. Our objective was to elucidate the role of PsbS in the photoprotective mechanism of the qE component of NPQ. We confirmed previous conclusions that PsbS-deficient plants lack energy-dependent quenching [57,69]. Furthermore, we demonstrated that i) under high-light stress, PsbS-deficient plants produce more superoxide, followed by greater generation of hydrogen peroxide but not singlet oxygen; ii) their PSII (but not PSI) centers are more sensitive to photooxidative stress under constant illumination; and iii) more superoxide is produced by PSII in PsbS-KO plants compared with the wild type, probably occurring at the QA site. Because the functions of PsbS are likely to be similar in rice and Arabidopsis, we believe that our data offer new insights into the role of PsbS and the qE type of NPQ.
Although qE is a major photoprotective mechanism of the photosynthetic apparatus in higher plants; its absence can affect the photochemical efficiency of PSII. However, this effect seems to be negligible under constant light conditions in rice, as is true in Arabidopsis [6]. Nevertheless, under fluctuating light, PsbS-deficient rice plants show growth retardation at the seedling stage (Additional file 1: Figure S4) and reduced fitness at the reproductive stage [57], which is similar to that reported with Arabidopsis [13]. These reductions are likely caused by an increase in oxidative stress in plants lacking PsbS, even though the ROS species that may underlie this effect have remained unknown.
The triple knock-out mutant of the moss, Physcomitrella patens, (psbs lhcsr1 lhcsr2) lacking NPQ has a far higher triplet chlorophyll steady-state level than wild type [70] suggesting that the level of the singlet oxygen also should be higher in the absence of NPQ. However, in the early stage of photoinhibition, when singly reduced QA is reversibly stabilized, the triplet chlorophyll is rapidly quenched by the interaction with QA−, preventing formation of harmful singlet oxygen [71]. We assume that our experimental condition is similar to this case. In our experiment, in the early stage of photoinhibition we do not expect more damage to the mutants (Figures 2a,b) and consequently, our result is acceptable showing that the level of singlet oxygen was not significantly more in the PsbS mutant lines compared with wild type.
Singlet oxygen, which has been implied to inhibit D1 protein synthesis [72-74], does not accumulate in plants lacking qE. In contrast, hydrogen peroxide, which also can influence the D1 repair system [72,75], damages cells under photoinhibitory illumination and may cause oxidative bursts leading to cell death. Here, hydrogen peroxide as well as superoxide produced more in rice that lacked qE. To determine accurate levels of ROS in leaf tissues both in vivo and in vitro, one should apply a variety of methods and take multiple measurements [76,77]. Here, we used assay systems based on several fluorescent dyes and other ROS sensors. In the case of superoxide, all data obtained using NBT were confirmed using DHE because NBT acts as an electron acceptor for PSII ([78], Krieger-Liszkay, Cedex, France; (unpublished data)). From our perspective, it was acceptable to employ NBT for in vitro assays because electron transfer to NBT will be minimal in the presence of artificial electron acceptors. Because superoxide can be converted to hydrogen peroxide even without SOD enzymes, we might explain the increase in hydrogen peroxide by an initial rise in the production of superoxide. Although we cannot rule out the possibility that synthesis of hydrogen peroxide is also elevated in rice leaves lacking qE, our data (most importantly those from time-course experiments performed on isolated thylakoids) are much better explained if superoxide production is the initial event that eventually leads to the formation of hydrogen peroxide. Similar data were reported in a parallel study with Arabidopsis [79]. There, fluorescence sensors were used in the thylakoids, and EPR spin-trapping with 4-pyridyl-1-oxide-N-tert-butylnitrone was tested in intact leaves, in order to detect the hydroxyl radicals that are produced from hydrogen peroxide/superoxide. Furthermore, in the presence of 20 μM nigericin (which eliminates the proton gradient over the thylakoid and, hence, qE), the signals were similar between the wild type and npq4, indicating that increased ROS production was due to a lack of qE.
The main site of superoxide generation in thylakoids under high-light conditions is thought to be PSI [43,80]. However, several researchers have also demonstrated light-induced generation of superoxide in PSII [44-46]. Instead, we showed that, when PsbS-KO and wild-type plants were analyzed, leaves from the former produced more superoxide. In our comparison between the two plant sources, we found that more superoxide was produced only in PSII, not in PSI. Moreover, we found that PSI does not seem to incur more photodamage in treated plants, and we also observed superoxide production in PSII particles. In fact, the proof of the Mehler reaction mainly referred with data from algae, and it remains much more controversial for higher plants because of the deficiency of proof of the Mehler reaction in higher plants. Badger et al. [81] reviewed a number of studies with higher plants, algae and cyanobacteria that have attempted to quantify O2 fluxes under various conditions and their contributions to the energy dissipation. The authors conclude that the Mehler reaction is unlikely to support a significant flow of electron transport in C3 and Crassulacean acid metabolism plants (probably less than 10%). Thus they questioned the Mehler reaction as a significant source of ROS in higher plants [81]. This uncertainty was fully justified by Driever and Baker [82] who could not detect any evidence for significant light driven Mehler reaction at ambient CO2 levels in two plant species. In addition, the data presented in this study on the lack of increase in ROS production from PSI centres would be consistent with this view that not much Mehler reaction occurs in higher plants in vivo. Cytochrome b559 may also be involved in superoxide generation in PSII, based on reports that superoxide can be detected by EPR spectroscopic analysis of PSII particles isolated from a cytochrome b559 mutant of tobacco [46]. The detection of superoxide in isolated thylakoids by a voltammetric method suggests that when the photosynthetic electron transport chain becomes over-reduced, superoxide may be generated at the QA site of PSII [45]. Altering the QA− re-oxidation kinetics (Figure 7, Additional file 1: Table S1) suggests that the QA site of PSII, rather than cyclic electron flow involving cytochrome b559, is the superoxide generation site in PSII. Another reason for the involvement of QA in PSII in higher superoxide production in PsbS mutant is probably the shift of redox-potential of QA to a more negative value due to the lack of PsbS, similar to the A249S mutant of Thermosynechococcus elongatus [83], which can make QA− to be able to reduce the molecular oxygen. Because in normal conditions the redox-potential of QA/QA− is −80 mV [84] while the redox-potential of O2/superoxide is −160 mV [41]. Because PsbS controls the conformation and organization of PSII supercomplexes [22-25], it is reasonable to predict more superoxide production at PSII in PsbS-KO leaves than in the wild type. It is likely that superoxide production at PSII has received little attention because it is so rapidly converted to hydrogen peroxide and because PsbS-dependent light dissipation provides such an efficient system of protection. In PsbS-deficient plants this protection, which may be an important component of the role of qE in vivo, is compromised, facilitating detection of superoxide release.
Conclusions
This study demonstrate that the PsbS-deficient rice plants to compensate for their lack of qE appear to develop other mechanisms for releasing excess energy to molecular oxygen; those protective systems may be initially triggered by superoxide production in PSII. Studies in Arabidopsis have shown that these systems may also provide broader protection against other sources of stress. When grown in the field, plants lacking PsbS induce a metabolic and transcriptomic shift that activates defense response pathways [85], resulting in an increase in resistance against biotic stress [79,85]. Whether this response influences the susceptibility of rice mutants lacking PsbS, as it does in Arabidopsis, remains to be established. It is an open question whether one can enhance crop yields by manipulating NPQ levels [86]. However, the intricate interplay between costs and benefits for NPQ has apparently not resulted in the selection of plants with “maximal” levels of NPQ. Potential positive effects of photooxidative processes [85] may be one factor favoring plants with less capacity for NPQ and could help to achieve food security for the growing human population using less available resources [87]. We hope that future studies will provide a deeper understanding of why regulation of photosynthetic light harvesting has been so elegantly organized.
Methods
Plants and growth conditions
One-month-old wild-type and PsbS-KO mutant seedlings of rice (Oryza sativa L.) were grown in rice soil (pH 4.5-5.5; Nonghyup, SamhwaGreentech, Seoul, Republic of Korea) in a greenhouse under natural sunlight. Growth conditions included a 16-h photoperiod and temperatures of 28/22 ± 2°C (day/night). For some experiments, rice seeds were germinated and cultured for one week on a solid agar Murashige and Skoog nutrient medium (Duchefa Biochemie, The Netherlands). Unless otherwise stated, plants were dark-adapted for at least 4 h before measurements were taken.
Isolation of OsPsbS-KO transgenic rice
Putative PsbS-knockout mutant plants were selected from T-DNA insertional knockout mutant lines. These were generated by transformation with a T-DNA vector (pGA2707) containing the promoter-less GUS gene next to the LB of the T-DNA [52]. Seeds segregating in the T2 generation or their amplified progenies were used for experiments. T-DNA flanking sequences were determined as previously described [53].
Genomic DNA was isolated from the leaves of three-week-old plants by the CTAB method [88]. Leaf samples were extracted with an Rtech® MM301 Mixer Mill (Rtech GmbH and Co., Germany). For genotyping, 2 μg of total DNA was used in PCR reactions with genomic DNA-specific primers: for OsPsbS1, 5′- ATCACCGGGAAGGGAATC –3′ (left) and 5′- GTCGTCGCTGACGAA –3′ (right); and for OsPsbS2, 5′-AGCGTGAAGAGGATGAAGA–3′ (left) and 5′-CCAAGAGAGCAAGCCAAGAT–3′ (right). A T-DNA-specific primer set was also used: 5′-TTGGGGTTTCTACAGGAC–3′ and 5′-AGAAGATCAAGGTGGGGACG–3′. PCR conditions for amplification were an initial 95°C for 5 min, followed by 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min; plus a final extension at 72°C for 10 min. The products were electrophoretically separated on a 0.8% (w/v) agarose gel containing ethidium bromide. DNA bands were visualized with an imaging system (Vilber Lourmat, France).
Generation of OsPsbS RNAi transgenic rice
To generate an RNA interference vector for OsPsbS1, we amplified a 102-bp gene fragment by PCR, using forward primer 5′-ATAGGATCCCTCGAGCGCGCGGTGTCCGTCAAGAC-3′ and reverse primer 5′-GCGGAATTCAAGCTTGTCCTCGGTCTTGAACTTTG-3′. Afterward, the fragment was cloned into the XhoI-HindIII and BamHI-EcoRI sites of pFGL727 (pBSIIKS-Intron). A SacI-KpnI fragment of pBSIIKS-Intron-OsPsbS1 was transferred into the SacI-KpnI sites of pGA1611. The OsPsbS RNAi plasmid was then transformed into rice using Agrobacterium strain LBA4404 as previously described [89].
Analysis of transcript levels and immunoblots
Total RNA was isolated as described in [90]. The gene-specific primers for OsPsbS1 were amplified using forward primer 5′-CTGTTCGGCAGGTCCAAGAC-3′ and reverse primer 5′-TTCAGCTGCGCCAGGATTC-3′. PCR products were separated by electrophoresis on a 1.2% agarose gel. Immunoblots were conducted as previously described [91].
Measurements of fluorescence and electron transport
After dark-adaptation for 10 min (in addition to the 4 hours), Chl fluorescence was measured at room temperature from detached leaves with a PAM2000 pulse-amplitude-modulated fluorometer (Walz, Effeltrich, Germany) in a room where influences from air-conditioning system are negligible. Actinic light was provided by a halogen lamp (Schott KL1500, Mainz, Germany). Fluorescence parameters Fm and Fm’ were induced by a saturating pulse of white light (0.8 s, 5,000 μmol photons m−2 s−1). Fm and Fo are defined as the maximal and minimal fluorescence yields of a “dark-adapted” sample when all PSII reaction centers are fully closed or opened, respectively. Fm' is the maximal fluorescence yield attained with a pulse of saturating light while leaves are illuminated by actinic light. We used Fv/Fm [or (Fm – Fo)/Fm] to monitor the potential efficiency of PSII photochemistry. Parameters for photochemical quenching (qP) and NPQ were calculated by the equations described in [92]. Electron transport rates were computed as previously described as: ETR = (ΔF/Fm’) x PAR x 0.5 × 0.84 [ΔF = (Fm’ - Ft)] assuming equal distribution of excitation between PSI and PSII [93]. For kinetic analysis of QA− re-oxidation, fluorescence decay in wild-type and PsbS-KO thylakoids was recorded after a single turnover flash [94]. The data were fitted to a three-component exponential decay equation after normalization.
The extent of P700 photooxidation, P700+, was assessed from differential changes in absorbance (810 nm minus 860 nm) using a PAM101/102/103 fluorometer (Walz, Germany) in the reflectance mode [95]. After 5 min of pre-illumination (120 μmol m−2 s−1, actinic white light), values for P700+ were calculated as (Imax – Imin) ⁄ Imax, where Imax is the signal intensity after applying saturating far-red light, and Imin is the signal intensity [93,96]. This pre-illumination was done to overcome any possible acceptor-side limitation of PSI that might have prevented full P700 oxidation.
Photoinhibitory treatment
To inflict photodamage to PSII with and without lincomycin, in vivo photoinhibition was induced by illuminating leaf segments with 2,000 μmol photons m−2 s−1 of white light from metal halide lamps at 27°C of leaf surface. For lincomycin treatment, leaf segments were infiltrated with 2 mM lincomycin by submergence [97] for 12 h under darkness, and the leaf segments were kept floated on the same treatment solution during photoinhibitory illumination. For recovery, samples were kept under 50 μmol photons m−2 s−1 of white light from a fluorescence lamp to induce the repair of damaged PSII. Following photoinhibitory illumination, the tissues were dark-adapted for 30 min prior to fluorescence measurements, unless otherwise stated.
Histochemical staining of superoxide and hydrogen peroxide
Histochemical staining for ROS production was conducted as previously described [98-100], with some modifications. For superoxide determinations, leaf samples were immersed in 6 mM NBT solution containing 50 mM sodium phosphate (pH 7.5) for 12 h in the dark. To detect hydrogen peroxide, detached leaves from wild-type and mutant plants were immersed in 5 mM DAB solution containing 10 mM MES (pH 3.8) for 12 h under darkness. Both reactions were stopped by soaking the leaves with lacto-glycerol-ethanol (1:1:4 by vol) and boiling in water for 5 min. The cleared leaves were preserved in 50% ethanol and photographed.
Determination of ROS levels in isolated thylakoids and photosystems by fluorescence emission analysis and absorbance of ROS sensors
The fluorescence emission spectra of the ROS sensors were acquired with an F-4500 fluorescence spectrophotometer (Hitachi, Japan). Singlet oxygen was detected both in leaves and in thylakoids as described in [62,63]. To detect superoxide, we examined the fluorescence of dihydroethidium according to the method described in [64]. Fluorescence of DCFDA was used to detect hydrogen peroxide [101]. Leaf segments from one-month-old seedlings were submerged for 12 to 14 h (25°C under darkness) in a fluorescent sensor solution for infiltration.
The absorbance of each ROS sensor was measured by an UV-1650PC UV-Visible spectrophotometer (Shimadzu, Japan). To detect superoxide, NBT absorbance was measured at 560 nm as described in [102]. The absorbance of DAB at 450 nm was used to detect hydrogen peroxide [103].
Superoxide production by PSI- and/or PSII-driven electron transport in the thylakoids was monitored with corresponding donor-acceptor pairs. To detect levels of individual ROS generated by PSII-driven electron transport, we used a reaction buffer containing 0.1 M sucrose, 10 mM NaCl, 10 mM KCl, 5 mM MgCl2, 10 mM Tricine, 1 mM KH2PO4, and 0.2% BSA (pH 8.0). The following ingredients were added to this buffer immediately prior to the experiments: 30 mM sodium ascorbate to mediate de-epoxidation of violaxanthin to zeaxanthin; 3 mM phenyl-p-benzoquinone to mediate PSII-driven electron transport; and 0.3 mM ATP to fuel ATP hydrolysis. To detect levels of individual ROS generated by PSI-driven electron transport, we again used the buffer described above. The following ingredients were added immediately before those experiments began: 5 mM NH4Cl as an uncoupler of the oxygen-evolving complex; 20 μM 3-(4,4-dichlorophenyl)-1,1-dimethylurea (DCMU) as a PSII electron transport inhibitor; 30 mM sodium ascorbate and 0.1 mM 2,6-dichlorophenol-indophenol as an electron donor for PSI; 50 μM methyl viologen only or 1 mM NADP+ supplemented with 10 μM ferredoxin to mediate linear electron transport; and 0.3 mM ATP to fuel ATP hydrolysis. ROS sensors were also added: 15 μM NBT to detect superoxide and 15 μM DAB for hydrogen peroxide. In all experiments the Chl concentration was 10 μg mL−1. Samples were illuminated at 700 μmol photons m−2 s−1 for photoinhibition.
Isolation of thylakoids and PSI and PSII complexes and detection of cytochrome b559
Thylakoids were isolated from PsbS-KO and wild-type plants according to the method described in [104]. PSII complexes (BBY particles) were prepared by Triton X-100 purification, as described in [105]. Those particles were stored at −80°C in a re-suspension medium containing 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2, and 40 mM Mes (pH 6.5). To confirm that there are no large differences in protein composition of thylakoids and BBY particles (except PsbS protein) in wild-type and PsbS-KO plants, we have conducted SDS-PAGE with Coomassie staining (Additional file 1: Figure S11), and the result showed that there are no large differences in the protein compositions between wild-type and PsbS-KO plants. Light-induced redox changes in the spectra of high-potential cytochrome b559 were determined as described in [106]. Mn-depleted PSII complexes were prepared by incubating the BBY particles in 0.8 M Tris (pH 8.0) for 20 min [46]. Afterward, the isolated PSII complexes were washed several times in a re-suspension medium and stored at −80°C. The PSII and PSI complexes were also isolated by sucrose-gradient centrifugation after solubilizing the thylakoids through the addition of octyl glucoside (0.88% (w/v)) and sodium dodecyl sulfate (0.22% (w/v)) [68]. Superoxide production was monitored in those isolated complexes by using corresponding donor-acceptor pairs for PSI and PSII. The concentration of Chl was determined in 80% acetone extracts as described in [107].
Acknowledgments
We are grateful to Prof. Jong-Seong Jeon, Prof. Ki-Hong Jung, and Dr. Jung-il Cho (Kyung Hee University, Yongin, Korea) and Prof. Anja Krieger-Liszkay (CEA, iBiTecS, Cedex, France) for critical reading of the manuscript and insightful comments. This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant No. 2009-0071776, 2010-0015946, 2011-0003040) and the Ministry of Science, ICT, and future Planning (Grant No. NRF-2014R1A2A2A01005741). This study was also financially supported by the ‘2012 Post-doc. Development Program’ of Pusan National University to Ismayil S. Zulfugarov.
Additional file
Figure S1. Genotyping of the PsbS-KO mutant line. Figure S2. Light-response curves for NPQ. Figure S3. Light-response curves for electron transport rates. Figure S4. Relative growth rates of 1-week-old seedlings. Figure S5. Production of singlet oxygen. Figure S6. Detection of superoxide anion radical production. Figure S7. Detection of hydrogen peroxide production. Figure S8. Time course for ROS production in thylakoids. Figure S9. Immunoblotting of thylakoids and BBY particles. Figure S10. Light-induced redox changes in high-potential cyt b559. Figure S11. Protein composition of thylakoids and BBY particles. Table S1. Analysis of decay time constant (τi, μs) and amplitude (Ai, arbitrary units) of QA − reoxidation kinetics after a single-turnover flash.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ISZ and CHL designed the study and wrote the manuscript; ISZ, AT, RSP, KN performed the experiments and analyzed the data; YJE, BD, MH, MB, UCY, YHM, GA, SJ contributed reagents/materials/analysis tools. All authors read and approved the final manuscript.
Contributor Information
Ismayil S Zulfugarov, Email: iszulfugarov@pusan.ac.kr.
Altanzaya Tovuu, Email: zaya_erdem@yahoo.com.
Young-Jae Eu, Email: eu2jee@gmail.com.
Bolormaa Dogsom, Email: bdogsom@yahoo.com.
Roshan Sharma Poudyal, Email: roshansharmap@yahoo.com.
Krishna Nath, Email: krishnanath125@hotmail.com.
Michael Hall, Email: Michael.Hall@chem.umu.se.
Mainak Banerjee, Email: mainak669@yahoo.co.in.
Ung Chan Yoon, Email: ucyoon@pusan.ac.kr.
Yong-Hwan Moon, Email: moonyh@pusan.ac.kr.
Gynheung An, Email: genean@khu.ac.kr.
Stefan Jansson, Email: stefan.jansson@plantphys.umu.se.
Choon-Hwan Lee, Email: chlee@pusan.ac.kr.
References
- 1.Horton P, Ruban AV, Walter RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 2.Jahns P, Holzwarth AR. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta. 1817;2012:182–193. doi: 10.1016/j.bbabio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Nilkens M, Kress E, Lambrev PH, Miloslavina Y, Müller M, Holzwarth AR, Jahns P. Identification of a slowly inducible zeaxanthin-dependent component of nonphotochemical quenching of chlorophyll fluorescence generated under steady state conditions in Arabidopsis. Biochim Biophys Acta. 2010;1797:466–475. doi: 10.1016/j.bbabio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Müller P, Li X-P, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demmig-Adams B, Winter K, Krüger A, Czygan FC. Light response of carbon dioxide assimilation dissipation of excess excitation energy and zeaxanthin content of sun and shade leaves. Plant Physiol. 1989;90:881–886. doi: 10.1104/pp.90.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403:391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MP, Ruban AV. Arabidopsis plants lacking PsbS protein possess photoprotective energy dissipation. Plant J. 2010;61:283–289. doi: 10.1111/j.1365-313X.2009.04051.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruban AV, Young AJ, Horton P. Induction of non-photochemical energy dissipation and absorbance changes in leaves. Evidence for changes in the state of the light harvesting system of Photosystem II in vivo. Plant Physiol. 1993;102:741–750. doi: 10.1104/pp.102.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore AM, Hazlett TL, Govindjee Xanthophyll cycle-dependent quenching of photosystem II chlorophyll fluorescence: Formation of a quenching complex with a short fluorescence lifetime. Proc Natl Acad Sci U S A. 1995;92:2273–2277. doi: 10.1073/pnas.92.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307:433–436. doi: 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]
- 11.Ruban V, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- 12.Peterson RB, Havir EA. A nonphotochemical-quenching-deficient mutant of Arabidopsis thaliana possessing normal pigment composition and xanthophyll-cycle activity. Planta. 2000;210:205–214. doi: 10.1007/PL00008127. [DOI] [PubMed] [Google Scholar]
- 13.Külheim C, Ågren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2002;297:91–93. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- 14.Krah NM, Logan BA. Loss of psbS expression reduces vegetative growth, reproductive output, and light-limited, but not light-saturated, photosynthesis in Arabidopsis thaliana (Brassicaceae) grown in temperate light environments. Am J Bot. 2010;97:644–649. doi: 10.3732/ajb.0900163. [DOI] [PubMed] [Google Scholar]
- 15.Holt NE, Fleming GR, Niyogi KK. Towards an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry. 2004;43:8281–8289. doi: 10.1021/bi0494020. [DOI] [PubMed] [Google Scholar]
- 16.Niyogi KK, Li X-P, Rosenberg V, Jung H-S. Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot. 2004;56:375–382. doi: 10.1093/jxb/eri056. [DOI] [PubMed] [Google Scholar]
- 17.Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem. 2004;279:22866–22874. doi: 10.1074/jbc.M402461200. [DOI] [PubMed] [Google Scholar]
- 18.Jeong MS, Hwang EY, Jin G-E, Park SY, Zulfugarov IS, Moon Y-H, Lee C-H, Jang SB. Expression and pH-dependence of the Photosystem II Subunit S from Arabidopsis thaliana. Bull Korean Chem Soc. 2010;31:1479–1484. doi: 10.5012/bkcs.2010.31.6.1479. [DOI] [Google Scholar]
- 19.Bonente G, Howes BD, Caffarri S, Smulevich G, Bassi R. Interactions between the photosystem II subunit PsbS and xanthophylls studied in vivo and in vitro. J Biol Chem. 2008;283:8434–8445. doi: 10.1074/jbc.M708291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teardo E, de Laureto PP, Bergantino E, Vecchia FD, Rigoni F, Szabò I, Giacometti GM. Evidences for interaction of PsbS with photosynthetic complexes in maize thylakoids. Biochim Biophys Acta. 2007;1767:703–711. doi: 10.1016/j.bbabio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Ahn TK, Avenson TJ, Ballottari M, Cheng Y-C, Niyogi KK, Bassi R, Fleming GR. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science. 2008;320:794–797. doi: 10.1126/science.1154800. [DOI] [PubMed] [Google Scholar]
- 22.Kiss AZ, Ruban AV, Horton P. The PsbS protein controls the organization of the photosystem II antenna in higher plant thylakoid membranes. J Biol Chem. 2008;283:3972–3978. doi: 10.1074/jbc.M707410200. [DOI] [PubMed] [Google Scholar]
- 23.Kereïche S, Kiss AZ, Kouřil R, Boekema EJ, Horton P. The PsbS protein controls the macro-organisation of photosystem II complexes in the grana membranes of higher plant chloroplasts. FEBS Lett. 2010;584:759–764. doi: 10.1016/j.febslet.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Lambrev PH, Nilkens M, Miloslavina Y, Jahns P, Holzwarth AR. Kinetic and spectral resolution of multiple non-photochemical quenching components in Arabidopsis leaves. Plant Physiol. 2010;152:1611–1624. doi: 10.1104/pp.109.148213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zulfugarov IS, Tovuu A, Dogsom B, Lee CY, Lee C-H. PsbS-specific zeaxanthin-independent changes in fluorescence emission spectrum as a signature of energy-dependent non-photochemical quenching in higher plants. Photochem Photobiol Sci. 2010;9:697–703. doi: 10.1039/b9pp00132h. [DOI] [PubMed] [Google Scholar]
- 26.Hubbart S, Ajigboye OO, Horton P, Murchie EH. The photoprotective protein PsbS exerts control over CO2 assimilation rate in fluctuating light in rice. Plant J. 2012;71:402–412. doi: 10.1111/j.1365-313X.2012.04995.x. [DOI] [PubMed] [Google Scholar]
- 27.Kearns DR. Physical and chemical properties of singlet molecular oxygen. Chem Rev. 1971;71:395–427. doi: 10.1021/cr60272a004. [DOI] [Google Scholar]
- 28.Fridovich I: Superoxide Anion Radical (O-2), superoxide dismutases, and related matters.J Biol Chem 1997, 272:18515–18517. [DOI] [PubMed]
- 29.Hideg É, Barta C, Kálai T, Vass I, Hideg K, Asada K. Detection of singlet oxygen and superoxide with fluorescent sensors in leaves under stress by photoinhibition or UV radiation. Plant Cell Physiol. 2002;43:1154–1164. doi: 10.1093/pcp/pcf145. [DOI] [PubMed] [Google Scholar]
- 30.Foyer CH, Noctor G. Leaves in the dark see the light. Science. 1999;284:599–601. doi: 10.1126/science.284.5414.599. [DOI] [PubMed] [Google Scholar]
- 31.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 32.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/S0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 34.Laloi C, Apel K, Danon A. Reactive oxygen signaling: the latest news. Curr Opin Plant Biol. 2004;7:323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Mittler R, Vanderauwera S, Gollery M, Breusegem V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Telfer A, Bishop SM, Phillips D, Barber J. Isolated photosynthetic reaction-center of photosystem-II as a sensitizer for the formation of singlet oxygen – detection and quantum yield determination using a chemical trapping technique. J Biol Chem. 1994;269:13244–13253. [PubMed] [Google Scholar]
- 37.Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 38.Keren N, Berg A, van Kann PJM, Levanon H, Ohad I. Mechanism of photosystem II inactivation and D1 protein degradation at low light: The role of back electron flow. Proc Natl Acad Sci U S A. 1997;94:1579–1584. doi: 10.1073/pnas.94.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trebst A. Function of β–carotene and tocopherol in photosystem II. Z Naturforsch C. 2003;58c:609–620. doi: 10.1515/znc-2003-9-1001. [DOI] [PubMed] [Google Scholar]
- 40.Mahler H, Wuennenberg P, Linder M, Przybyla D, Zoerb C, Langraf F, Forreiter C. Singlet oxygen affects the activity of the thylakoid ATP synthase and has a strong impact on its γ subunit. Planta. 2007;225:1073–1083. doi: 10.1007/s00425-006-0416-8. [DOI] [PubMed] [Google Scholar]
- 41.Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 42.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonoike K. Photoinhibition and protection of photosystem I. In: Golbeck GH, editor. Photosystem I: The Plastocyanin:Ferredoxin Oxidoreductase in Photosynthesis. Dordrecht: Springer; 2006. pp. 657–668. [Google Scholar]
- 44.Ananyev GM, Renger G, Wacker U, Klimov VV. Photoreduction of superoxide radicals and the superoxide dismutase activity of Photosystem II. The possible involvement of cytochrome b559. Photosynth Res. 1994;41:327–338. doi: 10.1007/BF00019410. [DOI] [PubMed] [Google Scholar]
- 45.Cleland RE, Grace SC. Voltammetric detection of superoxide production by photosystem II. FEBS Lett. 1999;457:348–352. doi: 10.1016/S0014-5793(99)01067-4. [DOI] [PubMed] [Google Scholar]
- 46.Pospíšil P, Šnyrychova I, Kruk J, Strzałka K, Nauš J: Evidence that cytochrome b559is involved in superoxide production in photosystem II: effect of synthetic short-chain plastoquinone in a cytochrome b559tobacco mutant.Biochem J 2006, 397:321–327. [DOI] [PMC free article] [PubMed]
- 47.Mubarakshina MM, Ivanov BN. The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol Plant. 2010;140:103–110. doi: 10.1111/j.1399-3054.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 48.Kozuleva M, Klenina I, Proskuryakov I, Kirilyuk I, Ivanov B. Production of superoxide in chloroplast thylakoid membranes. ESR study with cyclic hydroxylamines of different lipophilicity. FEBS Lett. 2011;585:1067–1071. doi: 10.1016/j.febslet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Kruk J, Jemiola-Rzeminska M, Burda K, Schmid G, Strzalka K. Scavenging of superoxide generated in photosystem I by plastoquinol and other prenyllipids in thylakoid membranes. Biochemistry. 2003;42:8501–8505. doi: 10.1021/bi034036q. [DOI] [PubMed] [Google Scholar]
- 50.Maroz A, Anderson RF, Smith RAJ, Murphy MP. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radical Bio Med. 2009;46:105–109. doi: 10.1016/j.freeradbiomed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Umate P. Genome-wide analysis of the family of light-harvesting chlorophyll a/b-binding proteins in arabidopsis and rice. Plant Signal Behav. 2010;5:1537–1542. doi: 10.4161/psb.5.12.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong D-H, An S, Kang H-G, Moon S, Han J-J, Park S, Lee HS, An K, An G. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol. 2002;130:1636–1644. doi: 10.1104/pp.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.An S, Park S, Jeong D-H, Lee D-Y, Kang H-G, Yu J-H, Hur J, Kim S-R, Kim Y-H, Lee M, Han S, Kim S-J, Yang J, Kim E, Wi SJ, Chung HS, Hong J-P, Choe V, Lee H-K, Choi J-H, Nam J, Kim S-R, Park P-B, Park KY, Kim WT, Choe S, Lee C-B, An G. Generation and analysis of end sequence database for T-DNA tagging lines in rice. Plant Physiol. 2003;133:2040–2047. doi: 10.1104/pp.103.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X-P, Gilmore AM, Niyogi KK. Molecular and global time-resolved analysis of a psbS gene dosage effect on pH- and xanthophyll cycle-dependent nonphotochemical quenching in photosystem II. J Biol Chem. 2002;277:33590–33597. doi: 10.1074/jbc.M204797200. [DOI] [PubMed] [Google Scholar]
- 55.Iwasaki T, Saito Y, Harada E, Kasai M, Shoji K, Miyao M, Yamamoto N. Cloning of cDNA encoding the rice 22 kDa protein of Photosystem II (PSII-S) and analysis of light-induced expression of the gene. Gene. 1997;185:223–229. doi: 10.1016/S0378-1119(96)00646-4. [DOI] [PubMed] [Google Scholar]
- 56.Kasajima I, Ebana K, Yamamoto T, Takahara K, Yaho M, Kawai-Yamada M, Uchimiya H. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc Natl Acad Sci U S A. 2011;108:13835–13840. doi: 10.1073/pnas.1104809108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koo HY, Zulfugarov IS, Oh M-H, Moon Y-H, Jansson S, An G, Lee C-H. The function of the PsbS protein in relation to nonphotochemical energy dependent quenching in rice plants. In: van der Est A, Bruce D, editors. Photosynthesis: Fundamental Aspects to Global Perspectives: 30 August – 03 September 2004. Montreal: Allen Press, Incorporated; 2005. pp. 527–530. [Google Scholar]
- 58.Li X-P, Müller-Moulé P, Gilmore AM, Niyogi KK. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci U S A. 2002;99:15222–15227. doi: 10.1073/pnas.232447699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyystjärvi A, Kettunen R, Aro E-M. The rate constant of photoinhibition in vitro is independent of the antenna size of photosystem II but depends on temperature. Biochim Biophys Acta. 1994;1186:177–185. doi: 10.1016/0005-2728(94)90177-5. [DOI] [Google Scholar]
- 60.Tyystjärvi E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev. 2007;252:361–376. doi: 10.1016/j.ccr.2007.08.021. [DOI] [Google Scholar]
- 61.Goh C-H, Ko S-M, Koh S, Kim Y-J, Bae H-J. Photosynthesis and Environments: Photoinhibition and Repair Mechanisms in Plants. J Plant Biol. 2012;55:93–101. doi: 10.1007/s12374-011-9195-2. [DOI] [Google Scholar]
- 62.Dall’Osto L, Cazzaniga S, Havaux M, Bassi R. Enhanced Photoprotection by Protein-Bound vs Free Xanthophyll Pools: A Comparative Analysis of Chlorophyll b and Xanthophyll Biosynthesis Mutants. Mol Plant. 2010;3:576–593. doi: 10.1093/mp/ssp117. [DOI] [PubMed] [Google Scholar]
- 63.de Bianchi S, Betterle N, Kouril R, Cazzaniga S, Boekema E, Bassi R, Dall’Ostoa L. Arabidopsis Mutants Deleted in the Light-Harvesting Protein Lhcb4 Have a Disrupted Photosystem II Macrostructure and Are Defective in Photoprotection. Plant Cell. 2011;23:2659–2679. doi: 10.1105/tpc.111.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georgiou AD, Papapostolou I, Patsoukis N, Tsegenidis T, Sideris T. An ultrasensitive fluorescent assay for the in vivo quantification of superoxide radical in organisms. Anal Biochem. 2005;347:144–151. doi: 10.1016/j.ab.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Robinson KM, Janes MS, Pehaf M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peshavariya HM, Dusting GG, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 67.Zulfugarov IS, Tovuu A, Lee C-H. Acceleration of cyclic electron flow in rice plants (Oryza sativa L.) deficient in the PsbS protein of Photosystem II. Plant Physiol Biochem. 2014;84:233–239. doi: 10.1016/j.plaphy.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Dunahay TG, Staehelin LA. Isolation of photosystem I complexes from octyl glucoside/sodium dodecyl sulfate solubilized spinach thylakoids. Characterization and reconstitution into liposomes. Plant Physiol. 1985;78:606–613. doi: 10.1104/pp.78.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishida S, Morita K, Kishine M, Takabayashi A, Murakami R, Takeda S, Shimamoto K, Sato F, Endo T. Allocation of absorbed light energy in PSII to thermal dissipations in the presence or absence of PsbS subunits of rice. Plant Cell Physiol. 2011;52:1822–1831. doi: 10.1093/pcp/pcr119. [DOI] [PubMed] [Google Scholar]
- 70.Carbonera D, Gerotto C, Posocco B, Giacometti GM, Morosinotto T. NPQ activation reduces chlorophyll triplet state formation in the moss Physcomitrella patens. Biochim Biophys Acta. 1817;2012:1608–1615. doi: 10.1016/j.bbabio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Noguchi T. Dual role of triplet localization on the accessory chlorophyll in the photosystem II reaction center: Photoprotection and photodamage of the D1 protein. Plant Cell Physiol. 2002;43:1112–1116. doi: 10.1093/pcp/pcf137. [DOI] [PubMed] [Google Scholar]
- 72.Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001;20:5587–5594. doi: 10.1093/emboj/20.20.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N. Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp PCC 6803. Biochemistry. 2004;43:11321–11330. doi: 10.1021/bi036178q. [DOI] [PubMed] [Google Scholar]
- 74.Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13:178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species. Nitric oxide, superoxide, hydrogen peroxide, and proxynitrite. Circ Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 77.Zulfugarov IS, Tovuu A, Kim J-H, Lee C-H. Detection of reactive oxygen species in higher plants. J Plant Biol. 2011;54:351–357. doi: 10.1007/s12374-011-9177-4. [DOI] [Google Scholar]
- 78.Halliwell B, Gutterdge JMC. Free radicals in biology and medicine. Oxford: Clarendon; 1989. [Google Scholar]
- 79.Johansson JH, Frenkel M, Zulfugarov I, Reichelt M, Krieger-Liszkay A, Gershenzon J, Moen J, Lee C-H, Jansson S. Non-photochemical quenching capacity in Arabidopsis thaliana affects herbivore behaviour. PLoS One. 2013;8(1):art. no. e53232. doi: 10.1371/journal.pone.0053232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheller HV, Haldrup A. Photoinhibition of photosystem I. Planta. 2005;221:5–8. doi: 10.1007/s00425-005-1507-7. [DOI] [PubMed] [Google Scholar]
- 81.Badger MR, von Caemmerer S, Ruuska S, Nakano H. Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos Trans R Soc Lond B Biol Sci. 2000;355:1433–1446. doi: 10.1098/rstb.2000.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Driever SM, Baker NR. The water–water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 2011;34:837–846. doi: 10.1111/j.1365-3040.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 83.Fufezan C, Gross CM, Sjödin M, Rutherford AW, Krieger-Liszkay A, Kirilovsky D. Influence of the Redox Potential of the Primary Quinone Electron Acceptor on Photoinhibition in Photosystem II. J Biol Chem. 2007;282:12492–12502. doi: 10.1074/jbc.M610951200. [DOI] [PubMed] [Google Scholar]
- 84.Krieger A, Rutherford AW, Johnson GN: On the determination of redox midpoint potential of the primary quinone electron acceptor, QA, in Photosystem II.Biochim Biophys Acta 1995, 1229:202–207.
- 85.Frenkel M, Külheim C, Jänkänpää HJ, Skogström O, Dall’Osto L, Ågren J, Bassi R, Moritz T, Moen J, Jansson S. Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol. 2009;9:12. doi: 10.1186/1471-2229-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murchie EH, Niyogi KK. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011;155:86–92. doi: 10.1104/pp.110.168831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gruissem W, Lee C-H, Oliver M, Pogson B. The Global Plant Council: Increasing the Impact of Plant Research to Meet Global Challenges. J Plant Biol. 2012;55:343–348. doi: 10.1007/s12374-012-0901-5. [DOI] [Google Scholar]
- 88.Chen D-H, Ronald PC. A rapid DNA minipreparation method suitable for AFLP and other PCR applications. J Plant Biol. 1999;17:53–57. [Google Scholar]
- 89.Dai S, Zheng P, Marmey P, Zhang S, Tian W, Chen S, Beachy RN, Fauquet C. Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breed. 2001;7:25–33. doi: 10.1023/A:1009687511633. [DOI] [Google Scholar]
- 90.Lee S, Kim J-H, Yoo ES, Lee C-H, Hirochika H, An G. Different regulation of chlorophyll a oxygenase genes in rice. Plant Mol Biol. 2005;57:805–818. doi: 10.1007/s11103-005-2066-9. [DOI] [PubMed] [Google Scholar]
- 91.Zulfugarov IS, Ham O-K, Mishra SR, Kim J-Y, Nath K, Koo H-Y, Moon Y-H, Kim H-S, An G, Lee C-H. Dependence of reaction center-type of energy-dependent quenching on antenna size of photosystem II. Biochim Biophys Acta. 2007;1767:773–780. doi: 10.1016/j.bbabio.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 92.van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- 93.Kim J-H, Kim S-J, Cho SH, Chow WS, Lee C-H. Photosystem I acceptor side limitation is a prerequisite for the reversible decrease in the maximum extent of P700 oxidation after short-term chilling in the light in four plant species with different chilling sensitivities. Physiol Plant. 2005;123:100–107. doi: 10.1111/j.1399-3054.2005.00443.x. [DOI] [Google Scholar]
- 94.Kim EH, Razeghifard R, Anderson JM, Chow WS. Multiple sites of retardation of electron transfer in Photosystem II after hydrolysis of phosphatidylglycerol. Photosynth Res. 2007;93:149–158. doi: 10.1007/s11120-006-9126-0. [DOI] [PubMed] [Google Scholar]
- 95.Klughammer C, Schreiber U. Measuring P700 absorbance changes in the near infrared spectral region with a dual wavelength pulse modulation system. In: Dordrecht GG, editor. Photosynthesis: Mechanism and Effects: 17–22 August 1998; Budapest. Budapest: Kluwer Academic Publishers; 1998. pp. 4357–4360. [Google Scholar]
- 96.Harbinson J, Woodward FI. The use of light induced absorbance changes at 820 nm to monitor the oxidation stage in leaves. Plant Cell Environ. 1987;12:357–369. doi: 10.1111/j.1365-3040.1989.tb01952.x. [DOI] [Google Scholar]
- 97.Bachmann KM, Ebbert V, Adams WW, III, Verhoeven AS, Logan BA, Demmig-Adams B. Effects of lincomycin on PSII efficiency, non-photochemical quenching, D1 protein and xanthophyll cycle during photoinhibition and recovery. Funct Plant Biol. 2004;31:803–813. doi: 10.1071/FP04022. [DOI] [PubMed] [Google Scholar]
- 98.Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. J Exp Bot. 2002;53:1249–1254. doi: 10.1093/jexbot/53.372.1249. [DOI] [PubMed] [Google Scholar]
- 99.Kariola T, Brader G, Li J, Palva ET. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell. 2005;17:282–294. doi: 10.1105/tpc.104.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahalingam R, Jambunathan N, Gunjan SK, Faustin E, Weng H, Ayoubi P. Analysis of oxidative signaling induced by ozone in Arabidopsis thaliana. Plant Cell Environ. 2006;29:1357–1371. doi: 10.1111/j.1365-3040.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- 101.Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2’7’-dichlorodihydrofluorescein diacetate, 5 (and 6)-carboxy-2’7’-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Med. 1999;270:146–159. doi: 10.1016/S0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 102.Auclair C, Voisin E. Nitro blue tetrazolium reduction. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press Inc.; 1985. pp. 123–132. [Google Scholar]
- 103.Geerts A, Roels F. Quantitation of catalase activity by microspectrophotometry after diaminobenzidine staining. Histochem Cell Biol. 1981;72:357–367. doi: 10.1007/BF00501778. [DOI] [PubMed] [Google Scholar]
- 104.Gilmore AM, Shinkarev VP, Hazlett TL, Govindjee Quantitative analysis of the effects of intrathylakoid pH and xanthophyll cycle pigments on chlorophyll a fluorescence lifetime distribution and intensity in thylakoids. Biochemistry. 1998;37:13582–13593. doi: 10.1021/bi981384x. [DOI] [PubMed] [Google Scholar]
- 105.Ford RC, Evans MCW. Isolation of a photosystem 2 preparation from higher plants with highly enriched oxygen evolution activity. FEBS Lett. 1983;160:159–164. doi: 10.1016/0014-5793(83)80957-0. [DOI] [Google Scholar]
- 106.Tiwari A, Pospíšil P: Superoxide oxidase and reductases activity of cytochrome b559in photosystem II.Biochim Biophys Acta 2009, 1787:985–994. [DOI] [PubMed]
- 107.Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b with four different solvents: verification of the concentration of chlorophyll by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]


