Abstract
This study was carried out to investigate the prevalence and risk factors of Giardia infection among indigenous people in rural Malaysia. Faecal samples were collected from 1,330 participants from seven states of Malaysia and examined by wet mount and formalin-ether sedimentation methods while demographic, socioeconomic and environmental information was collected using a pre-tested questionnaire. The overall prevalence of Giardia infection was 11.6% and was significantly higher among those aged ≤ 12 years compared to their older counterparts. Multivariate logistic regression identified age of ≤12 years, lacking of toilet at household, not washing hands before eating, not washing hands after playing with animals, not boiling water before consumption, bathing in the river, and not wearing shoes when outside as the significant risk factors of Giardia infection among these communities. Based on a multilocus genotyping approach (including tpi, gdh and bg gene sequences), 69 isolates were identified as assemblage A, and 69 as assemblage B. No association between the assemblages and presence of symptoms was found. Providing proper sanitation, as well as provision of clean drinking water and proper health education regarding good personal hygiene practices will help significantly in reducing the prevalence and burden of Giardia infection in these communities.
Giardia duodenalis (syn. G. intestinalis; G. lamblia), a flagellate enteric protozoan, is the most frequently reported intestinal parasite in the world, with about 280 million people suffering from symptomatic Giardia infection every year1,2. It is a major cause of acute and chronic diarrhoea, particularly among children in underprivileged communities, with a prevalence range of between 10% and 50% in developing countries3,4. Moreover, it is the most common human intestinal parasite in many developed countries, with a prevalence range of between 2% and 5%5,6. The ingestion of Giardia cysts through contaminated food or water is the most common mode of transmission. In addition to which, person-to-person transmissions may occur through direct faecal-oral contact among family members7, children in day care centres8, and by sexual practices (oral-anal contact)9,10. The cysts are instantly infectious once they are passed out through faeces, with the potential to remain infectious for several months as they can withstand unfavourable environmental conditions8.
The clinical presentation of Giardia infection varies from an asymptomatic carrier state to a severe disease which is associated with fat malabsorption and lactose intolerance due to disaccharidase deficiency11,12. Furthermore, Giardia infection contributes substantially to the 2.5 million annual deaths from diarrheal disease13. Several studies have revealed that a chronic infection of Giardia during childhood contributes to protein-energy malnutrition, vitamin A deficiency, iron deficiency anaemia, zinc deficiency and poor cognitive and educational performance14,15,16,17,18. Socioeconomic factors such as poverty, lack of adequate sanitation and water treatment systems, illiteracy and poor hygienic practices have been identified as significant risk factors associated with Giardia infection in different communities19,20.
In Malaysia, data on the risk factors of Giardia infection is limited. However, several studies on various parasitic infections concerning the indigenous people living in Peninsular Malaysia (West Malaysia) have been enthusiastically carried out since the 1970s, these revealed that the prevalence of Giardia infection among Orang Asli (Aboriginal) communities could be as high as 29.2%20,21,22,23,24. In addition to which, Giardia infection has been associated with protein-energy malnutrition and vitamin A deficiency among the children in these communities15,16. Unfortunately, the majority of existing studies were conducted across a small sample size and limited geographic areas, most of which were exclusively limited to the peninsular Malaysia Orang Asli population. This makes it difficult to apply the results to a larger population, or to make strong conclusions about risk factors or control intervention. To make matters more difficult, data on Giardia infections in East Malaysia is not available.
It is known that G. duodenalis consists of eight morphologically identical but genetically distinct genotypes or assemblages, designated A–H25. Assemblages A and B have been identified to infect humans and many other mammalian hosts, including domestic animals and wildlife26, while other assemblages are host restricted; assemblages C and D infect dogs, assemblage F infects cats, assemblage E infects hoofed livestock, assemblage G infects rats, and assemblage H infects marine animals25,27. With regards to clinical manifestation, studies on the correlation between the assemblages and clinical symptoms have reported controversial results. Some studies have pointed out that symptoms are more associated with assemblage A28, while others have found that assemblage B infections are more likely to be symptomatic29. Data on the genotypes of Giardia in Malaysia are scarcely available30,31. Within this context, this community-based study was conducted to provide a comprehensive dataset regarding the prevalence and potential factors associated with Giardia infection, as well as the genotypes of Giardia among different indigenous groups in Malaysia, with a preference towards evaluating the connection of Giardia infection in indigenous groups with socioeconomic status in rural Malaysia.
Results
Study cohort and socioeconomic profile
A total of 1330 participants (50% males, 50% females) with a mean age of 15 years from seven states of Malaysia (986 from Peninsular Malaysia and 344 from Sabah, East Malaysia) were enrolled in the study. Among the cohort from Sabah, 175 (50.9%) were from the Dusun tribe, 97 (28.2%) from the Murut tribe, and 72 (20.9%) from the Bajau tribe. Among the cohort from Peninsular Malaysia, 484 (49.1%) were from the Semai tribe, 268 (27.2%) from the Temuan tribe, 99 (10.0%) from the Temiar tribe, 68 (6.9%) from the Jahut tribe, and 67 (6.8%) from the Kensiu tribe. Overall poverty prevails in these communities, with almost half (50.9%) of all families having a low monthly income (under RM500, the poverty income threshold in Malaysia). The greatest number of families with household incomes of less than RM500 is located in Peninsular Malaysia (56.6%), with Sabah also struggling with a spread low income households (34.0%).
With regard to educational status, 62.9% of the participants had at least primary levels of education, with a higher percentage of subjects in Sabah being educated when compared to Peninsular Malaysia (75.7% vs 58.5%). Almost half of the adult participants are not working (48.6%), with almost similar frequencies of unemployment in both Peninsular Malaysia and Sabah (49.7% vs 46.8%). Those working were mainly farmers (39.5%) or otherwise engaged in agriculture (25.6%) (rubber and oil palm plantations), forestry, fishing and related occupations. General characteristics of the participants are presented in Table 1.
Table 1. General characteristics of the indigenous communities that participated in this study.
| Characteristics | Peninsular Malaysia (n = 986) | Sabah (n = 344) | Overall (n = 1330) |
|---|---|---|---|
| Age group | |||
| ≤12 years (1 month–12 years) | 723 (73.3) | 207 (60.2) | 930 (69.9) |
| >12 years (13–84 years) | 263 (26.7) | 137 (39.8) | 400 (30.1) |
| Gender | |||
| Male | 500 (50.7) | 164 (47.7) | 664 (49.9) |
| Female | 486 (49.3) | 180 (52.3) | 666 (50.1) |
| Socioeconomic status | |||
| Low household income (<RM500) | 558 (56.6) | 119 (34.6) | 677 (50.9) |
| >7 members-large | 353 (35.8) | 79 (23.0) | 432 (32.5) |
| Not working | 100 (50.5) | 60 (47.6) | 160 (49.4) |
| Educational level | |||
| Secondary education | 66 (6.7) | 63 (18.3) | 129 (9.7) |
| Primary education | 512 (51.9) | 196 (57.0) | 708 (53.2) |
| Non educated | 408 (41.4) | 85 (24.7) | 493 (37.1) |
| Supplied with piped water | 471 (47.8) | 325 (94.5) | 796 (59.8) |
| Presence of toilet at household | 706 (71.6) | 296 (86.0) | 1002 (75.3) |
| Presence of animals at household | 698 (70.8) | 245 (71.8) | 943 (71.1) |
Prevalence of Giardia infection
The overall prevalence rate of Giardia infection was 11.6% (154/1330), with a significantly higher infection rate in Peninsular Malaysia when compared to Sabah (13.6%; 95% CI = 11.6, 15.9 vs 5.8%; 95% CI = 3.8, 8.8; P < 0.001). The prevalence of infection was also significantly higher among participants aged under 12 years of age (14.2%; 95% CI = 12.1, 16.6 vs 5.5%; 95% CI = 3.7, 8.2; P < 0.001). There was a similar prevalence of infections reported among both males and females (12.1%; 95% CI = 9.8, 14.8 vs 11.1%; 95% CI = 8.9, 13.8; P = 0.593). With regards to the tribes in Peninsular Malaysia, the prevalence of Giardia infection was significantly higher among participants from the Semai tribe (17.8%), followed by the Kensiu (13.4%) and Temuan (10.8%) tribes. Similarly, the amount of infections were higher among the Dusun tribe of Sabah (8.6%) compared to the Murut and Bajau tribes. At the level of states, the highest prevalence of infections were reported in Pahang (15.9%) followed by Negeri Sembilan (14.9%) and Kedah (13.4%), while the lowest reported levels of infections were in Malacca (4.6%).
Faecal specimens were also screened for the presence of other intestinal parasitic infections, with participants being found to be infected with Trichuris trichiura (54.0%), Ascaris lumbricoides (28.7%), Entamoeba histolytica/dispar/moshkovskii (16.5%), and hookworm (10.5%). Overall, the prevalence of all detected infections was significantly higher among the participants from Peninsular Malaysia compared to those from Sabah (P < 0.001). About two-thirds of Giardia cases (104/154) were mixed infections with one or more parasite species, while one third were Giardia single infection (50/154). Regarding co-infections, Giardia and Trichuris was the most common co-infection, followed by Giardia with Ascaris and Giardia with Entamoeba species. The prevalence and distribution of infections according to location and tribes are shown in Table 2.
Table 2. Prevalence and distribution of intestinal parasitic infections among the indigenous communities that participated in this study.
| Parasite | Giardia | Trichuris | Ascaris | Entamoeba | Hookworm |
|---|---|---|---|---|---|
| States | |||||
| Selangor | 6.1 | 83.7 | 57.1 | 18.4 | 0.0 |
| Malacca | 4.6 | 53.8 | 27.7 | 9.2 | 0.0 |
| Negeri Sembilan | 14.9 | 61.7 | 34.4 | 5.2 | 11.0 |
| Kedah | 13.4 | 62.7 | 61.2 | 32.8 | 14.9 |
| Pahang | 15.9 | 69.9 | 40.6 | 27.0 | 17.6 |
| Kelantan | 8.1 | 9.1 | 3.0 | 15.2 | 1.0 |
| Sabah | 5.8 | 1.2 | 4.4 | 2.9 | 4.1 |
| Tribes | |||||
| Semai | 17.8 | 71.7 | 37.4 | 29.8 | 17.6 |
| Kensiu | 13.4 | 62.7 | 61.2 | 32.8 | 14.9 |
| Jahut | 2.9 | 57.4 | 63.2 | 7.4 | 17.6 |
| Temiar | 8.1 | 9.1 | 3.0 | 15.2 | 1.0 |
| Temuan | 10.8 | 63.8 | 36.9 | 8.6 | 6.3 |
| Dusun | 8.6 | 0.0 | 0.0 | 2.9 | 4.0 |
| Murut | 3.1 | 4.1 | 15.5 | 3.1 | 7.2 |
| Bajau | 2.8 | 0.0 | 0.0 | 2.8 | 0.0 |
| Overall prevalence | 11.6 | 46.0 | 28.7 | 16.5 | 10.5 |
Associated factors with Giardia infection
The associations of Giardia infection with demographic, socioeconomic and environmental factors are illustrated in Table 3. Besides location (Peninsular Malaysia) and age (≤12 years), participants from large households (with family sizes numbering more than 7 members living together) experienced a significantly higher prevalence of Giardia infection than those from smaller families (14.8% 95% CI = 12.1, 18.5 vs 10.0%; 95% CI = 8.2, 12.0).
Table 3. Univariate analysis of factors associated with Giardia infection among the indigenous communities that participated in this study.
| Variables | No. Examined | No. Infected | % Infected | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Location | ||||||
| Peninsular Malaysia | 986 | 134 | 13.6 | 2.5 | 1.6, 4.1 | <0.001 |
| Sabah (East Malaysia) | 344 | 20 | 5.8 | 1 | ||
| Age group | ||||||
| < = 12 Years | 930 | 132 | 14.2 | 2.8 | 1.8, 4.5 | <0.001 |
| >12 Years | 400 | 22 | 5.5 | 1 | ||
| Gender | ||||||
| Male | 664 | 80 | 12.0 | 1.1 | 0.8, 1.5 | 0.593 |
| Female | 666 | 74 | 11.1 | 1 | ||
| Size of household | ||||||
| >7 members | 432 | 64 | 14.8 | 1.6 | 1.1, 2.2 | 0.011 |
| < = 7 members | 898 | 90 | 10.0 | 1 | ||
| Household income | ||||||
| <RM500 | 677 | 88 | 13.0 | 1.3 | 0.9, 1.9 | 0.099 |
| > = RM500 | 653 | 66 | 10.1 | 1 | ||
| Educational level | ||||||
| Secondary education | 129 | 6 | 4.7 | 1 | ||
| Primary education/non educated | 1201 | 148 | 12.3 | 2.9 | 1.2–6.7 | 0.010 |
| Employment status | ||||||
| Not working | 160 | 5 | 3.1 | 0.6 | 0.2, 2.0 | 0.421 |
| Working | 164 | 8 | 4.9 | 1 | ||
| Source of drinking water | ||||||
| Unsafe water (river, well, rain) | 534 | 62 | 11.6 | 1.0 | 0.7, 1.4 | 0.977 |
| Piped water | 796 | 92 | 11.6 | 1 | ||
| Presence of toilet at household | ||||||
| No | 328 | 57 | 17.4 | 2.0 | 1.4, 2.8 | <0.001 |
| Yes | 1002 | 97 | 9.7 | 1 | ||
| Boiling water before consumption | ||||||
| No | 192 | 40 | 20.8 | 2.4 | 1.6, 3.5 | <0.001 |
| Yes | 1138 | 114 | 10.0 | 1 | ||
| Bathing place | ||||||
| River | 279 | 52 | 18.6 | 2.1 | 1.5, 3.1 | <0.001 |
| Bathroom | 1049 | 102 | 9.7 | 1 | ||
| Indiscriminate defecation | ||||||
| Yes | 437 | 77 | 17.6 | 2.3 | 1.6, 3.2 | <0.001 |
| No | 890 | 77 | 8.7 | 1 | ||
| Washing hands before eating | ||||||
| No | 327 | 49 | 15.0 | 1.5 | 1.0, 2.2 | 0.028 |
| Yes | 1003 | 105 | 10.5 | 1 | ||
| Washing hands after defecation | ||||||
| No | 269 | 35 | 13.0 | 1.2 | 0.8, 1.8 | 0.420 |
| Yes | 1058 | 119 | 11.2 | 1 | ||
| Consumption of raw vegetables | ||||||
| Yes | 453 | 45 | 9.9 | 0.8 | 0.5, 1.1 | 0.171 |
| No | 874 | 109 | 12.5 | 1 | ||
| Washing vegetables/fruits before consumption | ||||||
| No | 335 | 59 | 17.6 | 1.9 | 1.3, 2.9 | <0.001 |
| Yes | 993 | 95 | 9.6 | 1 | ||
| Wearing shoes when outside | ||||||
| No | 316 | 58 | 18.4 | 2.2 | 1.5, 3.1 | <0.001 |
| Yes | 1014 | 96 | 9.5 | 1 | ||
| Garbage disposal | ||||||
| Indiscriminate | 452 | 75 | 16.6 | 2.0 | 1.4, 2.8 | <0.001 |
| Proper disposal | 875 | 79 | 9.0 | 1 | ||
| Presence of domestic animals | ||||||
| Yes | 943 | 117 | 12.4 | 1.3 | 0.9, 2.0 | 0.153 |
| No | 384 | 37 | 9.6 | 1 | ||
| Washing hands after playing with animals | ||||||
| No | 266 | 50 | 18.8 | 2.1 | 1.5, 3.1 | <0.001 |
| Yes | 1061 | 104 | 9.8 | 1 |
There was also a significant association between Giardia infection and subjects educational level, with a higher prevalence among those who were either non educated or only had primary education when compared to those who had a secondary education (12.3% 95% CI = 10.6, 14.3 vs 4.7%; 95% CI = 2.2, 9.8). However, prevalence of Giardia was not significantly different between those who had a primary education (12.7%; 95% CI = 10.5, 15.4) and those who were non educated at all (11.8%; 95% CI = 9.8, 15.0).
Furthermore, the prevalence of Giardia infection was significantly higher among those who live in houses without toilets when compared to those living in houses with functioning toilets (17.4%; 95% CI = 13.7, 21.9 vs 9.7%; 95% CI = 8.0, 11.7). With regards to hygienic practices, the results of univariate analyses revealed that the prevalence of Giardia infection is significantly associated with not washing hands before eating, not boiling water before consumption, bathing in the river, indiscriminate defecation, not washing vegetables/fruits before consumption, not wearing shoes when outside, not washing hands after playing with animals and indiscriminate garbage disposal.
Interestingly, when we stratified the univariate analyses according to location, a significantly higher prevalence of Giardia infection was found among those drinking pipe water when compared to those who collect drinking water from unsafe sources (15.9% vs 11.5%; χ2 = 4.181; P = 0.041). It was found that participants from East Malaysia who still collect their drinking water from rivers, wells and rain have a higher prevalence of infection when compared to their counterparts; however the difference was not statistically significant (15.8% vs 5.2%; χ2 = 3.655; P = 0.056).
Overall, all the significant associations were retained by the Peninsular Malaysia group, while only not washing vegetables/fruits before consumption (OR = 3.4; 95% CI = 1.1, 11.1) was retained as a significant variable in regards to the Sabah group.
Risk factors of Giardia infection
Table 4 shows that a multivariable logistic regression model retained 7 variables as being significant risk factors in terms of inducing Giardia infection among the studied indigenous people. The results confirmed that those aged ≤ 12 years (OR = 2.1) and living in a house without a functioning toilet (OR = 1.5) were at higher odds of having a Giardia infection when compared with their counterparts. Moreover, poor personal hygiene practices, including not washing hands after playing with animals, not boiling water before consumption, bathing in the river, not wearing shoes when outside and not washing hands before eating were also retained as significant risk factors of Giardia infection among these people.
Table 4. Multivariate analysis of risk factors associated with Giardia infection among the indigenous communities that participated in this study.
| Variables | Adjusted OR | 95% CI | P-value |
|---|---|---|---|
| Location (Peninsular Malaysia) | 1.5 | 0.9, 2.6 | 0.115 |
| Being aged ≤ 12 years | 2.1 | 1.3, 3.4 | 0.003 |
| Gender (males) | 1.1 | 0.7, 1.5 | 0.799 |
| Large household members (>7 members) | 1.4 | 0.9, 2.0 | 0.065 |
| Low educational level | 1.0 | 0.4, 2.7 | 0.968 |
| No toilet at household | 1.5 | 1.0, 2.2 | 0.049 |
| Not boiling water before consumption | 2.1 | 1.4, 3.3 | 0.001 |
| Bathing in the river | 1.7 | 1.2, 2.6 | 0.007 |
| Indiscriminate defecation | 1.2 | 0.7, 2.0 | 0.608 |
| Not washing hands before eating | 1.5 | 1.1, 2.2 | 0.029 |
| Not washing hands after playing with animals | 2.1 | 1.4, 3.1 | <0.001 |
| Not washing vegetables/fruits before consumption | 1.1 | 0.8, 1.7 | 0.561 |
| Not wearing shoes when outside | 1.6 | 1.1, 2.4 | 0.012 |
| Indiscriminate garbage disposal | 1.1 | 0.7, 1.7 | 0.673 |
Association of Giardia infection with symptoms
The majority of participants, 1252 (94.1%), had no complaints about gastrointestinal signs or symptoms. Of 78 symptomatic cases, 34.6% (27/78) had diarrhea, 46.2% (36/78) had diarrhea and abdominal pain, 6.4% (5/78) had abdominal pain, 10.3% (8/78) had vomiting, and 2.6% (2/78) had dysentery. Most Giardia-positive cases in this study were asymptomatic (129 out of 154 cases), and about one-third (25/78) of the symptomatic individuals were infected with Giardia. The prevalence of infection was significantly higher among the 63 participants who had diarrhea when compared to their asymptomatic counterparts (36.5% vs 10.3%; χ2 = 40.142; P < 0.001). Similarly, the prevalence of infection was higher among those who had abdominal pain compared to the asymptomatic individuals, however the difference was not statistically significant (19.5% vs 11.3%; χ2 = 2.601; P = 0.107). It was noted that 57 (90.5%) of the diarrhea cases were from Peninsular Malaysia, compared to only 6 (9.5%) cases from Sabah (χ2 = 9.209; P = 0.002).
Molecular characterization of G. duodenalis
Analysis using multilocus genotyping approach on the positive isolates had successfully amplified 138 samples using at least one of the following markers: triose phosphate isomerase (tpi), glutamate dehydrogenase (gdh), and beta-giardin (bg) genes. When sequences generated from the amplicons were analyzed using multiple alignment with previously published reference sequences, 69/138 (50.0%) were genotyped as assemblage A and 69/138 (50.0%) were genotyped as assemblage B. The distribution of both assemblages was almost similar between the West and East Malaysia (P = 0.613).
Gastrointestinal symptoms were reported in 18.8% (13/69) of the individuals infected with assemblage A; 12 of the cases had diarrhea. Likewise, 14.5% (10/69) of the individuals infected with assemblage B were symptomatic and had diarrhea. That said, among Giardia-positive and symptomatic individuals (i.e. 23 individuals), the prevalence of assemblages A and B was 56.5% and 43.5% respectively, and the difference was not significant (OR = 1.37; 95% CI = 0.56, 3.38).
Discussion
Despite intensive efforts to improve the quality of life in rural Malaysian communities, intestinal parasitic infections including giardiasis, amoebiasis and soil-transmitted helminthiasis are still highly prevalent, especially among aboriginal and rural populations. The present study provides information on the status of Giardia infection among different indigenous people in rural Malaysia, including the Orang Asli (aboriginal) population in West Malaysia and other indigenous groups in East Malaysia. Our findings revealed that the overall prevalence of Giardia infection was 11.6%, with a significantly higher prevalence among the participants from West Malaysia when compared to those from East Malaysia (13.6% vs 5.8%). This is in agreement with a previous study among 716 rural individuals from five states of West Malaysia32. Furthermore, several small-scale studies have been conducted among the aboriginal population in West Malaysia which showed that the prevalence of Giardia ranges between 4.0% and 29.2%24,30,31,32,33,34. In addition to which, a recent study among three aboriginal tribes in three different states of West Malaysia revealed a high prevalence (20.0%) of Giardia infections20.
In West Malaysia, our findings showed that the prevalence of Giardia infection was highest among participants from Pahang state (15.9%) followed by Negeri Sembilan (14.9%) and Kedah (13.4%), while the lowest levels of prevalence were found among the participants from Malacca 4.6% and Selangor 6.1%. This could be attributed to the differences in the culture, environment and population of Orang Asli communities in these areas. Orang Asli villages in Pahang, Kedah and Negeri Sembilan are located deep in the jungle, with inadequate sanitary facilities and away from healthcare facilities. In contrast, Selangor and Malacca are in peripheral areas with better sanitation and environmental factors, as well as being located near to the region's main health facilities. Our findings further revealed that the Senoi group (Semai, Jahut and Temiar tribes) had the lowest prevalence of infection amongst its population (9.6%), while the prevalence among the Negrito group (Kensiu) was higher than the Proto-Malay group (Temuan) (13.4% vs 10.8%).
These findings are consistent with previous studies conducted among the different ethnic groups of Orang Asli in West Malaysia, which reported a higher prevalence of Entamoba species and STH among Negrito groups, followed by Senoi and Proto-Malay groups35,36. Similarly, an earlier study among 1,273 individuals from different ethnic groups showed that the Negritos harboured more intestinal parasites species when compared with other ethnic tribes37. Orang Asli belonging to the Negrito group live in remote areas, with their villages being made of wood or bamboo, which means they suffer from poor housing conditions, a lack of proper sanitation and no provisions for a clean water supply when compared to the Senoi or Proto-Malay groups who live in suburban areas under better conditions.
With regards to East Malaysia (Sabah and Sarawak states), supporting information on the prevalence of intestinal parasitic infections is lacking. Our findings showed that the prevalence of Giardia infection in Sabah was much lower (5.8%) than in West Malaysia, with a significantly higher prevalence among participants from the Dusun tribe compared to those from Murut and Bajau tribes. Unfortunately, the only previous report from East Malaysia was conducted solely in Sarawak, revealing a very low prevalence of Giardia infection (2.0%) among 355 individuals from five interior communities38. The government has made intensive efforts to improve the quality of life of indigenous people throughout the country, with their main strategy being to the reallocate those living in remote areas to new settlements at the periphery of towns. New houses were provided to the settlers which ensured access to basic amenities, which has helped greatly reduce the prevalence of many parasitic infections amongst the indigenous communities in Sabah. On the other hand, the adherence of the Orang Asli people in West Malaysia to their jungle habitats has constrained the overall effectiveness of this strategy. Therefore, providing new houses within the same locality will not help reduce the prevalence of intestinal parasitic infections in this area due to the heavily contaminated environment.
The present study investigated the possible risk factors associated with Giardia infection among the participants, revealing that children under 13 years old (1 month to 12 years old) were significantly associated with higher Giardia infection rates when compared to adults and children over 12 years old (13 to 84 years old), and this is in agreement with previous studies22,24,31. This could be attributed to the higher exposure of young children to the source of a wide range of infections, which could be due to having lower standards of personal hygiene when compared to the adults and older children.
In addition, the research revealed that not boiling drinking water before consumption was reported as a significant predictor of Giardia infection. It is well documented that Giardia and Cryptosporidium have been the most common causes of waterborne diseases outbreaks worldwide39. Our findings further showed that living in houses without functioning toilets increased the odds of the observed population acquiring Giardia infections, and this is consistent with many previous studies in Malaysia and elsewhere32,40,41. It was further found that defecating in indiscriminate places, such as in rivers and bushes was a common practice in communities with inadequate toilet facilities.
In general, Orang Asli have a habit of building their villages beside rivers where water can be conveniently collected for multiple purposes, including drinking and cooking, whilst also conducting daily activities such as bathing and washing clothes in the river water. Besides which some inhabitants, especially children, prefer defecating at the site of the stream40. As a result, it is likely that the environment of the local rivers are heavily contaminated, becoming a source of infection for Giardia and other intestinal parasites/bacteria/viruses23,40. This presumption is supported by a finding from the present study, in which bathing in the rivers appeared as one of the significant risk factors. In the same vein, an interesting finding was uncovered when we stratified the univariate analyses according to location, in which a significantly higher prevalence of Giardia infection was found among Orang Asli who drink piped water when compared to those collected drinking water from unsafe sources (i.e. rivers, wells and rain) (15.9% vs 11.5%; χ2 = 4.181; P = 0.041). In contrast, participants from East Malaysia who use piped water for drinking had a lower prevalence of infections when compared to their counterparts who used unsafe water (5.2% vs 15.8%; χ2 = 3.655; P = 0.056). Drinking piped water has been identified as a significant risk factor among the aboriginal population in Pahang, Malaysia32. However, results from previous studies conducted in these communities showed that treated water is free from faecal coliforms, Giardia and Cryptosporidium contamination42,43. Hence, we believe that the contamination of the piped water is occurring after the treatment process has taken place, and could be attributed to the usage of containers and utensils that may have been previously soiled with Giardia cysts when handling and storing drinking water. Furthermore, it was observed that the Orang Asli people in West Malaysia who used tanks or other containers for drinking water collection left these containers uncovered, which is in direct contrast to the clean and covered tanks in East Malaysia.
Our findings also showed that not washing hands before eating or after handling/playing with animals were significant risk factors for Giardia infection among the study population. Although previous studies have suggested zoonotic transmission for Giardia infection, studies among Malaysian aborigines have previously found no such association44,45. Giardia cysts can remain infective in the environment for a very long period of time, meaning they could easily get picked-up on the fur of animals such as cats and dogs, who were observed as moving about in the contaminated environment while also mixing freely with the members of their households. Hence, not washing hands after handling or playing with these animals could facilitate the spread of the infections. Similarly, not wearing shoes when outside the house may also contribute to the contamination of houses with cysts, and this was also identified as a significant predictor of Giardia infection by the present study.
It is also worth noting that a significantly higher prevalence of Giardia infection was reported among participants with low educational levels (either non educated or with only primary levels of education), as well as in areas in which indiscriminate defecation was common, in areas with indiscriminate garbage disposal systems, in large households (>7 members), and when not washing vegetables/fruits before consumption. However, these associations were not retained by the logistic regression model. Previous studies among aboriginal communities in West Malaysia have identified these variables as significant predictors of Giardia and other intestinal parasitic infections20,31,32,46.
In view of this, when analysing the risk factors according to location, it is remarkable to find out that all of the variables were retained as significant risk factors of Giardia infection in West Malaysia, while only one significant risk factor was retained among the participants from East Malaysia (that is not washing vegetables/fruits before consumption). It is also interesting to note that while eating raw vegetables and fruits has been identified as significant risk factors of Giardia infection among Orang Asli in West Malaysia31,47, our study revealed that the significant association is actually in the practice of not washing vegetables or fruits prior to consumption, which appeared to be a more tenable reason when compared to the simple consumption of this nutritious food. Most of the fruits in these communities are tropical peeled fruits like rambutan (Nephelium lappaceum), longan (Dimocarpus longan) and mangosteen (Garcinia mangostana). We observed people collecting dropped fruits (rambutan, longan) from the ground, opening the soft shell with their mouths and eating the fruit directly without first washing it. Thus, fruits and vegetables could be the medium of transmission in cases where the surface carries the parasites infective stages, especially when the fruit has been in contact with the contaminated ground followed by the direct consumption of the fruit itself, both of which increase the chances of transmission from contaminated hands.
The molecular findings of the current study showed that assemblages A and B were present at equal frequency (69/138). Indeed, our findings contradict previous genotyping data using a single locus (i.e. tpi gene)30,31. A recent community-based study identified two-thirds of 98 Giardia-positive isolates as assemblage A and the rest were assemblage B30. By contrast, a previous study using SSU rRNA locus identified only one specimen as assemblage A in 42 specimens while the rest were assemblage B31. This is, however, different from the proportion of assemblages A and B reported globally where assemblage B (~58%) has a higher prevalence than assemblage A (~37%)48.
We found a significant association between Giardia infection and diarrhoea among the studied population, with significantly higher frequency of diarrhoeal cases among Giardia-infected participants from West Malaysia when compared to their counterparts from East Malaysia. However, about two-thirds of the Giardia cases were mixed infection with at least one parasite species. Therefore, it was not possible to confirm the causal relationship between Giardia and diarrhoea in the present study, due to the limitation of the cross-sectional design used to gather results. With regard to genotypes, gastrointestinal symptoms such as diarrhea, vomiting, abdominal pain and dysentery were reported in 18.8% and 14.5% of assemblage A and B respectively. We found no significant difference in the prevalence of both assemblages among symptomatic infections. Similar findings were also reported by previous studies49,50,51,52. By contrast, a previous study among Orang Asli in Malaysia reported a strong association between the clinical symptoms of gastroenteritis and assemblage B47. To date, there is still a lack of clear association between the assemblage and the clinical outcome, with contradictory results. While previous studies conducted in Bangladesh, Australia and Spain have reported a significant association between assemblage A and the presence of symptoms28,53,54, other studies from various regions also suggest a correlation between the presence of symptoms and infection with assemblage B29,55,56,57,58. Hence, conclusive inference with regard to the genotype-dependent pathogenecity may only be drawn after further well-designed studies12.
Besides that, the present study showed that the majority of Giardia–positive individuals were asymptomatic (83.8%). This high percentage of asymptomatic infections should be taken with apprehension from the public health standpoint as the infected individuals can act as carriers and excrete infective cysts in faeces. Infection is acquired by ingestion of the resilient cysts via contaminated food, water or hands. It could have an adverse impact especially to the family and the community, if the symptom-free cases go unnoticed and contaminate the environment. The cysts have been reported to have a low infectious dose of as few as ten cysts to establish an infection and become immediately infectious upon being released in stools59. More significantly, this health hazard could be long lasting because the transmissive stage of this parasite can persist between 7 to 18 days in faeces, 7 weeks in soil and up to 3 months in water60,61. Yet there has been no study conducted to investigate what genotype of Giardia infection sheds higher number of cysts or has higher level of resistance to withstand the external environment and maintain longer periods of infectivity. If these factors are genotype-related, one will expect that a particular assemblage will exist in greater amounts in the environment and potentially lead to a higher chance of transmission. One relevant study by Haque et al. reported that assemblage B infections produced a higher load of DNA and higher overall prevalence28.
The present study provides a community-based picture on the epidemiology of Giardia infection among the indigenous people in rural Malaysia. Overall, our findings show that Giardia infection in these communities was mostly associated with poor hygienic practices that were often coupled with poor sanitary facilities as well. From the general characteristics of both groups, it is clear that the indigenous people in East Malaysia are more educated, have a higher monthly household income, live in better housing conditions and have a cleaner environment when compared to the indigenous people in West Malaysia. Hence, the lifestyle interventions already implemented in East Malaysia might be the explanation as to why there is a significantly lower prevalence of Giardia reported in that region.
We do acknowledge some limitations of the present study. In many cases only a single faecal sample was collected, instead of the ideal three consecutive samples, because of a limitation of resources and the cultural belief of some Orang Asli against giving their faecal samples. Therefore, the prevalence of Giardia infection might be underestimated due to the variation in cyst shedding per days. Many indigenous villages are located in deeply remote areas, with no road access, and therefore were not involved in our study, though it is worth noting that even higher prevalence rates of intestinal parasitic infections have been previously reported in these remote areas when compared to the villages involved in our study33. We speculate that our findings can be generalised to other rural indigenous children in other states. That said, it is possible that these findings may not be generally applicable to the entire Malaysian rural population, as ethnic groups other than the indigenous people tend to have better socioeconomic and environmental situations. Hence, further studies are required in order to confirm these conjectures.
In conclusion, the findings reveal that the prevalence of Giardia infection is still high and of public health concern among indigenous populations in rural Malaysia. The prevalence was found to be higher among the aboriginal population in West Malaysia when compared to the indigenous people in East Malaysia. It was most commonly found among children and those living in poor sanitation conditions who also had poor standards of personal hygiene. Different control measures are required in order to combat current levels of infection, including health education pertinent to good personal hygiene and good sanitary practices, as well as education aimed at improving general awareness about parasitic infections. Besides which, if re-allocation to new settlements is not possible, providing proper sanitation, as well as making provisions for clean and safe drinking water, are crucial for maintaining the health of indigenous communities in West Malaysia.
Methods
Study areas
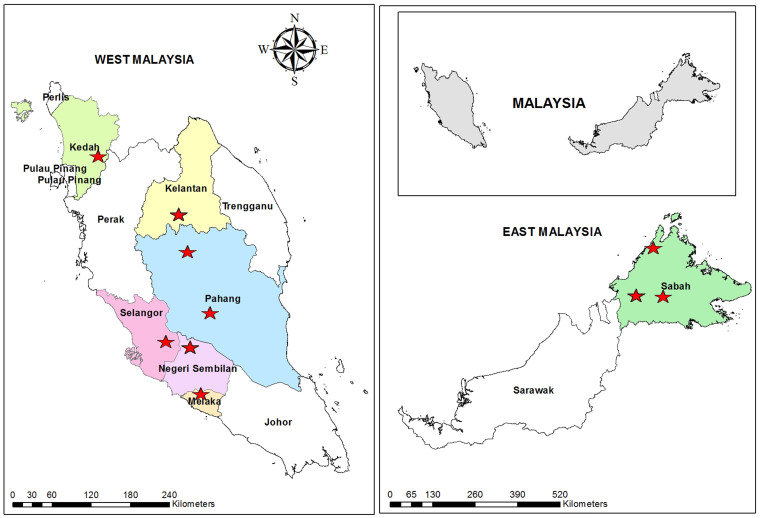
This community-based cross-sectional study was carried out among indigenous communities in rural parts of Malaysia (both in West and East Malaysia) from April 2011 to February 2013. Overall, 28 villages from seven states of Malaysia, namely Pahang, Selangor, Negeri Sembilan, Kelantan, Kedah, Malacca and Sabah were involved (Figure 1). The populations in the areas under study lived with disparate lifestyles and environmental exposures, especially in terms of the indigenous groups in Peninsular Malaysia (West Malaysia) and Sabah (East Malaysia). In particular, the overall living standard was lower among the indigenous groups in West Malaysia compared to their counterparts in East Malaysia, though the housing conditions vary among the villages. The climate is equatorial with hot-humid conditions and rainfall throughout the year. The vegetation is the thick rain forest type and there are few water streams in the area.
Figure 1. A geographic map showing the location of the districts (stars) and states (coloured) involved in the study.
The map was created using the Esri ArcMap 10.2.1 software.
Study population
The aboriginal communities in West Malaysia are recognized specifically as ‘Orang Asli', a collective Malay term translated as ‘original or first people'. This term is used to address the heterogeneous minorities that are classified in to three main groups, namely Negrito (2.8%), Proto-Malay (42.3%) and Senoi (54.9%). Each of these groups consists of six tribes, with an estimated population of 178,197, which makes up 0.7% of the total Malaysian population according to the population census 2010. In contrast with West Malaysia, the indigenous peoples form more than 50.0% of the total population of Sabah and Sarawak (East Malaysia). In Sabah, there are 72 ethnic and sub-ethnic groups, with Kadazandusun (17.7%), Bajau (14.0%) and Murut (3.2%) making up the major composition of the Sabah population62.
The lifestyles and means of pursuing a livelihood are diverse among the indigenous groups, though they tend to have a close connection with the various tribes traditional habitats and natural resources. Some of the aborigines in the Peninsular Malaysia still live in remote areas, however, due to implementation of development programmes that were initiated by the government, an increasing number of these minorities are moving to the periphery of urban areas where they are integrating with urban communities. The common economic activities engaged in by the present Orang Asli are rubber tapping, small-scale cultivation of local crops (e.g. cassava and banana), wage-earning jobs in private sectors (e.g. oil palm/coco plantation, construction site and factory), as well as forest produce collection and selling (e.g. fruits and bamboo) to a lesser extent63. Conversely, the indigenous communities in the rural areas of Sabah employ a more diversified subsistence economy. The coastal and riverine communities engage largely in the fishing industry, with a recent expedition in to seaweed cultivation. Whilst those living in the interior areas depend on farming, gardening and collecting forest resources, both for their own consumption and in order to sell the surplus for cash. Some of them are involved in cash crops plantation projects, such as oil palm, cocoa and rubber.
Sample size and sampling strategy
The minimum sample size required for this study was calculated according to the formula provided by Lwanga and Lemeshow64. At a 5% level of significance and a 95% confidence level, the minimum number of subjects required for this study was estimated as being 983, assuming that the prevalence of Giardia infection was 20%; as recently reported among three different Aboriginal tribes20. Overall, 1,330 individuals agreed to voluntarily participate in this study who met the inclusion criteria (signed written consent, completed questionnaire and delivered stool samples for examination).
The states and villages involved in this study were randomly selected from the available official administrative list in collaboration with the Department of Orang Asli Development (Jabatan Kemajuan Orang Asli, JAKOA) and Sabah Health Department (JKN SABAH), with consideration of the following criteria for villages: located in a rural area, accessible from the main roads and each village has more than 20 houses or ≥100 residents. The probability proportional to size sampling method was used to select the participants from each state, based on the total number of the indigenous population in the states and districts. Before the commencement of the sampling surveys, the villages were visited by researchers accompanied by officers from JAKOA and JKN SABAH, to meet the head of the village so as to explain and prepare the villagers for data and sample collections, as well as to obtain primary information on the existing conditions in the villages.
Questionnaire survey
A pretested questionnaire was used to collect information on the demographic (e.g. age, sex and number of household members), socioeconomic (e.g. household monthly income, occupation and educational status), environmental (e.g. availability and types of toilets in the household, types of water supply, garbage disposal and presence of domestic animals), personal hygiene (e.g. washing hands before eating, after defecation and after playing with animals, washing vegetables and fruits before consumption, boiling water before consumption and bathing place), and general health status of the participants(i.e. symptoms related to intestinal parasitic infections such as diarrhoea, nausea, vomiting, abdominal pain and a history of receiving anthelmintics treatment). The questionnaire was designed in the English language and then translated into the Malay language. Two research assistants from the Department of Parasitology, University of Malaya were trained for the purpose of this study.
Faecal samples collection and examination
Fresh faecal samples were collected in 60 ml clearly labelled containers with wide mouths and screw-caps. The containers were distributed and the participants were informed on the proper method of sample collection e.g. not to mix their stool sample with urine or water, and to provide a sample amount of at least a thumb-size. Participants were invited to bring their early morning stool samples the next day. Samples were transported in suitable cool boxes to the Department of Parasitology, Faculty of Medicine, University of Malaya and stored in a cold room at 4°C. When immediate transfer of samples to the department was not possible, samples were kept in a refrigerator at local clinics or health offices.
The collected faecal samples were processed based on the formalin-ether sedimentation technique previously described by Cheesbrough65. Pellets of the sediment were used for examination by emulsifying them in 1–2 drops of iodine solution, then examining the results using a light microscope with the aim of finding the presence of Giardia cysts and/or trophozoites, as well as other intestinal parasites. For quality control, duplicate slides were prepared from 20% of the samples and the slides were read by another microscopist.
Molecular analysis
DNA extraction and molecular analysis were carried out using PCR relevant protocols published elsewhere66. In brief, DNA was extracted directly from faecal samples either using PowerSoil®DNA Isolation Kit (MoBio Laboratories Inc., Carlsbad, California) or MACHEREY-NAGEL NucleoSpin® Soil (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) to a final volume of 50 μl. Multilocus genotyping was performed using markers that amplify 432-bp fragment of the gdh gene54, 530-bp fragment of the tpi gene67 and 511-bp fragment of the bg gene68,69 to analyse microscopy Giardia- positive samples.
Statistical analysis
Data analysis was done by using SPSS for WINDOWS (version 18.0). Data was entered and reviewed by two different researchers. For descriptive analysis, the prevalence of infections and other categorical variables were expressed in percentages, while mean (standard deviation; SD) was used to present the quantitative data, with results being presented in tables. Pearson's Chi Square test was used to investigate the association between Giardia infection as the dependent variable and demographic, socioeconomic, environmental and personal hygiene factors as the independent variables. All the variables used in the survey were coded in a binary manner as dummy variables. For example, Giardia infection (positive = 1, negative = 0); gender (male = 1, female = 0); availability of piped water supply of toilet in the house (no = 1, yes = 0) and washing hands before eating (no = 1, yes = 0). Family size was categorized into two groups (>7 and ≤7 members), and age of participants was also categorized into two groups (≤12 and >12 years)23,31,32. Furthermore, a monthly household income of <RM500 was considered as being low based on the poverty income threshold in Malaysia32. Odd ratios (OR) and 95% confidence intervals (CI) were calculated using univariate and multivariable logistic regression analyses. All tests were considered significant at P < 0.05.
Ethical consideration
The protocol of this study was approved by the Medical Ethics Committee of the University of Malaya Medical Centre (Ref. no: 788.74 and 878.19). Prior to the commencement of the survey questionnaire and sample collection, permissions were obtained from the heads of the villages. Then, the participants were informed about the objectives and methods of the study. They were informed that their participation was totally voluntary and that they could withdraw from the study at any time without citing any reason whatsoever. Written and signed or thumb-printed informed consent was obtained from those who agreed to participate, or from parents or guardians on behalf of their children, and these procedures were approved by the Medical Ethics Committee of the University of Malaya Medical Centre. The methods used in this research were carried out in accordance with the approved guidelines.
Author Contributions
J.S., H.M.A., M.A.K.M. and S.H.C. conceived and designed the experiments. S.H.C. and N.A.N. collected the samples and performed the experiments. S.H.C. and H.M.A. analyzed the data and wrote the paper. M.S. provided logistic support for data collection and field work. H.M.A., J.S. and Y.A.L.L. revised the article critically for important intellectual content.
Acknowledgments
The authors would like to acknowledge the Department of Orang Asli Development (JAKOA), Ministry of Rural and Regional Development, Kuala Lumpur, and Sabah Health Department, Malaysia for their generous cooperation during this study. Thanks also are indebted to the participants their voluntary involvements in this study. Special thanks go to Dr Romano Ngui, Ms Lee Soo Ching, Ms Yap Nan Jiun, Ms Goh Xiang Ting, Ms Lorraine Angal and Ms Reena Richard from the Department of Parasitology, University of Malaya for their fruitful help during field work. This research was financially supported by the University of Malaya High Impact Research Grant UM-MOHE UM.C/625/1/HIR/MOHE/MED/18 from the Ministry of Higher Education Malaysia, and University of Malaya student grant (PV063- 2012A).
References
- WHO. The World Health Report 1996-Fighting Disease, Fostering Development. World Health Organization Geneva (1996). [PubMed]
- Upcroft P. & Upcroft J. A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14, 150–164 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L., Smith H. & Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative'. Trends. Parasitol. 22, 203–208 (2006). [DOI] [PubMed] [Google Scholar]
- Daly E. R. et al. Outbreak of giardiasis associated with a community drinking-water source. Epidemiol. Infect. 138, 491–500 (2010). [DOI] [PubMed] [Google Scholar]
- Yoder J. S., Gargano J. W., Wallace R. M. & Beach M. J. Giardiasis surveillance-United States, 2009-2010. MMWR 61, 13–23 (2012). [PubMed] [Google Scholar]
- Nygard K. et al. A large community outbreak of waterborne giardiasis-delayed detection in a non-endemic urban area. BMC Public Health 6, 141 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcioglu I. C. et al. Incidence of giardiasis among siblings in Turkey. Pediatr. Int. 45, 311–313 (2003). [DOI] [PubMed] [Google Scholar]
- Duffy T. L., Montenegro-Bethancourt G., Solomons N. W., Belosevic M. & Clandinin M. T. Prevalence of giardiasis in children attending semi-urban daycare centres in Guatemala and comparison of 3 Giardia detection tests. J. Health Popul. Nutr. 31, 290–293 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakianathan M. R. & McMillan A. Intestinal protozoa in homosexual men in Edinburgh. Int. J. STD. AIDS. 10, 780–784 (1999). [DOI] [PubMed] [Google Scholar]
- Escobedo A. A., Almirall P., Alfonso M., Cimerman S. & Chacin-Bonilla L. Sexual transmission of giardiasis: a neglected route of spread? Acta Trop. 132, 106–111 (2014). [DOI] [PubMed] [Google Scholar]
- Gardner T. B. & Hill D. R. Treatment of giardiasis. Clin. Microbiol. Rev. 14, 114–128 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buret A. G. Pathophysiology of enteric infections with Giardia duodenalius. Parasite 15, 261–265 (2008). [DOI] [PubMed] [Google Scholar]
- Adam R. D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14, 447–475 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel D., Treluyer J. M. & Richard-Lenoble D. Parasitic diarrhea in normal and malnourished children. Fundam. Clin. Pharmacol. 17, 189–197 (2003). [DOI] [PubMed] [Google Scholar]
- Al-Mekhlafi H. M., Surin J., Sallam A. A., Abdullah A. W. & Mahdy M. A. K. Giardiasis and poor vitamin A status among aboriginal school children in rural Malaysia. Am. J. Trop. Med. Hyg. 83, 523–527 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mekhlafi H. M. et al. Burden of Giardia duodenalis infection and its adverse effects on growth of schoolchildren in rural Malaysia. PLoS Negl. Trop. Dis. 7, e2516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quihui L. et al. Could giardiasis be a risk factor for low zinc status in schoolchildren from northwestern Mexico? A cross-sectional study with longitudinal follow-up. BMC Public Health 10, 85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman D. S., Lescano A. G., Gilman R. H., Lopez S. L. & Black M. M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359, 564–571 (2002). [DOI] [PubMed] [Google Scholar]
- Faustini A. et al. The impact of the Catholic Jubilee in 2000 on infectious diseases. A case-control study of giardiasis, Rome, Italy 2000–2001. Epidemiol. Infect. 134, 649–658 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuar T. K. et al. Giardiasis among different tribes of Orang Asli in Malaysia: Highlighting the presence of other family members infected with Giardia intestinalis as a main risk factor. Parasitology. Int. J. Parasit. 42, 871–880(2012). [DOI] [PubMed] [Google Scholar]
- Bisseru B. & Abdul Aziz bin A. Intestinal parasites, eosinophilia, haemoglobin and gamma globulin of Malay, Chinese and Indian schoolchildren. Med. J. Malaya 25, 29–33 (1970). [PubMed] [Google Scholar]
- Norhayati M., Penggabean M., Oothuman P. & Fatmah M. S. Prevalence and some risk factors of Giardia duodenalis infection in a rural community in Malaysia. Southeast Asian J. Trop. Med. Public Health 29, 735–738 (1998). [PubMed] [Google Scholar]
- Al-Mekhlafi M. S. H. et al. Giardiasis as a predictor of childhood malnutrition in Orang Asli children in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 99, 686–691 (2005). [DOI] [PubMed] [Google Scholar]
- Mohammed Mahdy A. K., Lim Y. A., Surin J., Wan K. L. & Al-Mekhlafi M. S. Risk factors for endemic giardiasis: highlighting the possible association of contaminated water and food. Trans. R. Soc. Trop. Med. Hyg. 102, 465–470 (2008). [DOI] [PubMed] [Google Scholar]
- Feng Y. & Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 24, 110–140 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis P. T., Caccio S. M. & Thompson R. C. Variation in Giardia: towards a taxonomic revision of the genus. Trends Parasitol. 25, 93–100 (2009). [DOI] [PubMed] [Google Scholar]
- Lasek-Nesselquist E., Welch D. M. & Sogin M. L. The identification of a new Giardia duodenalis assemblage in marine vertebrates and a preliminary analysis of G. duodenalis population biology in marine systems. Int. J. Parasitol. 40, 1063–1074 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R. et al. Giardia assemblage A infection and diarrhea in Bangladesh. J. Infect. Dis. 192, 2171–2173 (2005). [DOI] [PubMed] [Google Scholar]
- Pelayo L. et al. Giardia infections in Cuban children: the genotypes circulating in a rural population. Ann. Trop. Med. Parasitol. 102, 585–595 (2008). [DOI] [PubMed] [Google Scholar]
- Anuar T. S. et al. Molecular epidemiology of giardiasis among Orang Asli in Malaysia: application of the triosephosphate isomerase gene. BMC Infect. Dis. 14, 78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdy A. K. et al. Molecular characterization of Giardia duodenalis isolated from Semai Pahang Orang Asli (Peninsular Malaysia aborigines). Parasitol. 136, 1237–1241 (2009). [DOI] [PubMed] [Google Scholar]
- Ngui R., Ishak S., Chuen C. S., Mahmud R. & Lim Y. A. Prevalence and risk factors of intestinal parasitism in rural and remote West Malaysia. PLoS Negl. Trop. Dis. 5, e974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor Azian M. Y. et al. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop. Biomed. 24, 55–62 (2007). [PubMed] [Google Scholar]
- Sinniah B. et al. Determining the prevalence of intestinal parasites in three Orang Asli (Aborigines) communities in Perak, Malaysia. Trop. Biomed. 29, 200–206 (2012). [PubMed] [Google Scholar]
- Shahrul Anuar T. et al. Prevalence and risk factors associated with Entamoeba histolytica/dispar/moshkovskii infection among three Orang Asli ethnic groups in Malaysia. PLoS One 7, e48165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuar T. S., Salleh F. M. & Moktar N. Soil-transmitted helminth infections and associated risk factors in three Orang Asli tribes in Peninsular Malaysia. Sci. Rep. 4, 4101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn F. L. Intestinal parasitism in Malayan aborigines (Orang Asli). Bull. World Health Organ. 46, 99–113 (1972). [PMC free article] [PubMed] [Google Scholar]
- Sagin D. D. et al. Intestinal parasitic infection among five interior communities at upper Rejang River, Sarawak, Malaysia. Southeast Asian J. Trop. Med. Public Health 33, 18–22 (2002). [PubMed] [Google Scholar]
- Baldursson S. & Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004–2010. Water Res. 45, 6603–6614 (2011). [DOI] [PubMed] [Google Scholar]
- Ahmed A. et al. The burden of moderate-to-heavy soil-transmitted helminth infections among rural malaysian aborigines: an urgent need for an integrated control programme. Parasit. Vectors 4, 242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes E. et al. Risk factors for Giardia intestinalis infection in agricultural villages practicing wastewater irrigation in Mexico. Am. J. Trop. Med. Hyg. 62, 388–392 (2000). [DOI] [PubMed] [Google Scholar]
- Lim Y. A. & Aahmad R. A. Occurrence of Giardia cysts and Cryptosporidium oocysts in the Temuan Orang Asli (aborigine) River System. Southeast Asian J. Trop. Med. Public Health 35, 801–810 (2004). [PubMed] [Google Scholar]
- Lim Y. A. L., Ahmad R. A. & Smith H. V. Current status and future trends in Cryptosporidium and Giardia epidemiology in Malaysia. J. Water Health 6, 239–254 (2008). [DOI] [PubMed] [Google Scholar]
- Traub R. J. et al. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitol. 128, 253–262 (2004). [DOI] [PubMed] [Google Scholar]
- Cedillo-Rivera R. et al. Seroepidemiology of giardiasis in Mexico. Am. J. Trop. Med. Hyg. 80, 6–10 (2009). [PubMed] [Google Scholar]
- Nasr N. A., Al-Mekhlafi H. M., Ahmed A., Roslan M. A. & Bulgiba A. Towards an effective control programme of soil-transmitted helminth infections among Orang Asli in rural Malaysia. Part 1: prevalence and associated key factors. Parasit Vectors 6, 27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Mahdy A. K. et al. Giardia intestinalis genotypes: Risk factors and correlation with clinical symptoms. Acta Trop. 112, 67–70 (2009). [DOI] [PubMed] [Google Scholar]
- Ryan U. & Caccio S. M. Zoonotic potential of Giardia. Int. J. Parasitol. 43, 943–956 (2013). [DOI] [PubMed] [Google Scholar]
- Ajjampur S. S. et al. Giardia duodenalis assemblages associated with diarrhea in children in South India identified by PCR-RFLP. Am. J. Trop. Med. Hyg. 80, 16–19 (2009). [PMC free article] [PubMed] [Google Scholar]
- Kohli A. et al. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans. R. Soc. Trop. Med. Hyg. 102, 718–725 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbad M. et al. Dominance of Giardia assemblage B in Leon, Nicaragua. Acta Trop. 106, 44–53 (2008). [DOI] [PubMed] [Google Scholar]
- Yang R. et al. High prevalence Giardia duodenalis assemblage B and potentially zoonotic subtypes in sporadic human cases in Western Australia. Int. J. Parasitol. 40, 293–297 (2010). [DOI] [PubMed] [Google Scholar]
- Sahagun J. et al. Correlation between the presence of symptoms and the Giardia duodenalis genotype. Eur. J. Clin. Microbiol. Infect. Dis. 27, 81–83 (2008). [DOI] [PubMed] [Google Scholar]
- Read C. et al. Correlation between genotype of Giardia duodenalis and diarrhoea. Int. J. Parasitol. 32, 229–231 (2002). [DOI] [PubMed] [Google Scholar]
- Gelanew T. et al. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 102, 92–99 (2007). [DOI] [PubMed] [Google Scholar]
- Minvielle M. C. et al. First genotyping of Giardia lamblia from human and animal feces in Argentina, South America. Mem. Inst. Oswaldo. Cruz. 103, 98–103 (2008). [DOI] [PubMed] [Google Scholar]
- Molina N. et al. High prevalences of infection with Giardia intestinalis genotype B among children in urban and rural areas of Argentina. Ann. Trop. Med. Parasitol. 105, 299–309 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puebla L. J. et al. Correlation of Giardia duodenalis assemblages with clinical and epidemiological data in Cuban children. Infect. Genet. Evol. 23, 7–12 (2014). [DOI] [PubMed] [Google Scholar]
- Rendtorff R. C. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am. J. Hyg. 59, 209–220 (1954). [DOI] [PubMed] [Google Scholar]
- deRegnier D. P., Cole L., Schupp D. G. & Erlandsen S. L. Viability of Giardia cysts suspended in lake, river, and tap water. Appl. Environ. Microbiol. 55, 1223–1229 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. E. et al. Giardia Cyst and Cryptosporidium Oocyst Survival in Water, Soil, and Cattle Feces. J. Environ. Qual. 28, 1991–1996 (1999). [Google Scholar]
- Department of Statistics Malaysia. Profile of Orang Asli in Peninsular Malaysia, Kuala Lumpur (http://www.statistics.gov.my). Accessed: 23 April 2014.
- Chong Y. F. The Orang Asli of Malaysia: Poverty, sustainability and capability approach. Master thesis. Lund University, Lund, Sweden, 2010.
- Lwanga S. K. & Lemeshow S. Sample size determination in health studies: a practical manual. (World Health Organization, 1991). [Google Scholar]
- Cheesbrough M. District laboratory practice in tropical countries. 2nd edn, (Cambridge University Press, 2005). [Google Scholar]
- Huey C. S. et al. Multilocus genotyping of Giardia duodenalis in Malaysia. Infect. Genet. Evol. 17, 269–276 (2013). [DOI] [PubMed] [Google Scholar]
- Sulaiman I. M. et al. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 9, 1444–1452 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccio S. M., De Giacomo M. & Pozio E. Sequence analysis of the beta-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 32, 1023–1030 (2002). [DOI] [PubMed] [Google Scholar]
- Lalle M. et al. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 35, 207–213 (2005). [DOI] [PubMed] [Google Scholar]