Abstract
Objective
The purpose of this study was to retrospectively review cases of intracerebral hemorrhage (ICH) medically treated at our institution to determine if the CT angiography (CTA) 'spot sign' predicts in-hospital mortality and clinical outcome at 3 months in patients with spontaneous ICH.
Methods
We conducted a retrospective review of all consecutive patients who were admitted to the department of neurosurgery. Clinical data of patients with ICH were collected by 2 neurosurgeons blinded to the radiological data and at the 90-day follow-up.
Results
Multivariate logistic regression analysis identified predictors of poor outcome; we found that hematoma location, spot sign, and intraventricular hemorrhage were independent predictors of poor outcome. In-hospital mortality was 57.4% (35 of 61) in the CTA spot-sign positive group versus 7.9% (10 of 126) in the CTA spot-sign negative group. In multivariate logistic analysis, we found that presence of spot sign and presence of volume expansion were independent predictors for the in-hospital mortality of ICH.
Conclusion
The spot sign is a strong independent predictor of hematoma expansion, mortality, and poor clinical outcome in primary ICH. In this study, we emphasized the importance of hematoma expansion as a therapeutic target in both clinical practice and research.
Keywords: Intracerebral hemorrhage, Spot sign, Outcome, Mortality
INTRODUCTION
Intracerebral hemorrhage (ICH) is the most disabling form of stroke. Approximately 40% of patients with intracerebral hemorrhage die within 30 days, and the majority of survivors are left with severe disability4,11). Hematoma expansion occurs in up to 70% of patients who have ICH documented by computed tomographic (CT) scanning performed within 3 hours after the onset of symptoms4,6,18). Other predictors of poor outcome include age, initial hematoma volume of hematoma, Glasgow Coma Scale (GCS), intraventricular hemorrhage (IVH), warfarin use, and infratentorial hemorrhage10,14). Recently, several studies suggested that contrast extravasation on CT angiography (CTA) is a crucial predictor of hematoma expansion and mortality7,8,12,30). The presence of active contrast extravasation into the hematoma at the time of multi-detector CT angiography (MDCTA), the spot sign, is an indicator of active hemorrhage and has been associated with an increased risk of hematoma expansion and mortality in patients with ICH6,7,8,12,30). The purpose of this study was to retrospectively review cases of ICH treated at our institution to determine if the CTA 'spot sign' predicts in-hospital mortality and clinical outcome at 3 months in patients with spontaneous ICH.
MATERIALS AND METHODS
Patient selection
We used our institutional medical data search system to identify all adult patients who were admitted for medical treatment of ICH between January 1, 2008 and January 31, 2012. Our study was approved by institutional review board. We conducted a retrospective review of all consecutive patients who were admitted to the department of neurosurgery. To be eligible for the study, patients with ICH needed to meet the following inclusion criteria : 1) evidence of nontraumatic ICH on a noncontrast CT (NCCT) examination of the head, 2) evaluation with CTA of the intracranial circulation within 24 hours of presentation, and 3) age between 18 and 80 years. The exclusion criteria were : 1) deep coma (GCS 3-5); 2) brainstem hemorrhage; 3) a history of previous intracerebral hemorrhage; 4) pure intraventricular hemorrhage; 5) secondary intracerebral hemorrhage such as arteriovenous malformation, moyamoya disease, tumor bleeding, and venous sinus thrombosis; 5) incomplete standard CT protocol including NCCT and MDCTA; 6) previous stroke history; and 7) surgically treated patients.
Clinical data
Clinical data of patients with ICH were collected by 2 neurosurgeons blinded to the radiological data and at the 90-day follow-up. The collected demographic and clinical variables included sex, age, alcohol and smoking use, history of hypertension, diabetes, liver disease (liver cirrhosis and hepatocellular carcinoma), coronary heart disease, and medications (antihypertensives, antiplatelet, and anticoagulation agents). The systolic, diastolic, and mean arterial blood pressures of patients were recorded. Stroke severity on admission was evaluated by the GCS and National Institutes of Health Stroke Scale (NIHSS). Laboratory tests on admission included serum glucose, activated partial thromboplastin time (aPTT), and prothrombin time (PT) as expressed by the international normalized ratio (INR). Clinical outcomes were assessed by the modified Rankin Scale (mRS) and Glasgow Outcome Scale (GOS) on discharge and 90-day follow-up. Poor clinical outcome was defined as mRS >2 and GOS <4. In-hospital stay lengths were recorded.
Radiological data
NCCT and MDCTA acquisitions were performed according to standard departmental protocols on 16- or 64-section General Electric helical CT scanners (General Electric Medical Systems, New York, USA). Imaging was performed as follows : 1) initial and 24-hour follow-up NCCT scans were performed using 4.5 mm contiguous axial sections from skull base to vertex parallel to the inferior orbitomeatal line. Parameters for pre- and postcontrast CT were 120 kVp; 340 mA; 4×5 mm collimation; 1 second/rotation; and table speed of 15 mm/rotation. CTA was performed following 0.7 mL/kg of iohexol (300 mg I/mL; Omnipa-que; GE Healthcare, Piscataway, NJ, USA) injected intravenously at 4 mL/s rate via power injector through an intravenous line, using the following parameters : 120 kVp; 240 mAs; section thickness, 1.25 mm; and section-acquisition interval, 1 mm. All images were prospectively and independently reviewed on picture archiving and communication system (PACS) workstations by 2 neurosurgeons blinded to clinical data. The presence of contrast extravasation and spot sign score was determined according to the criteria from a prior study on MDCTA source or reconstructed images8). Hematoma location was classified as supratentorial or infratentorial. Supratentorial location was further subclassified as lobar or deep. The presence of intraventricular hemorrhage (IVH) was recorded. Determination of initial and follow-up ICH volumes was performed independently by investigators blinded to the initial NCCT and follow-up CT. Each reviewer measured the volume of hemorrhage in milliliters using the ABC/2 method, where A is the greatest diameter of hemorrhage on the CT section with the largest area of hemorrhage, B is the diameter perpendicular (90°) to A, and C is the number of sections with hemorrhage multiplied by the section thickness17). C was calculated by a comparison of each CT section with hemorrhage, with the CT section demonstrating the largest area of hemorrhage on that scan. An increase of hematoma volume >33% or >12.5 mL was considered a hematoma expansion5,12,15).
The spot sign was defined according to four criteria : 1) serpiginous or spot-like appearance within the margin of a parenchymal hematoma without connection to an outside vessel; 2) contrast density greater than 1.5 mm in diameter in at least one dimension; 3) contrast density (Hounsfield units, HU) at least double that of the background hematoma; and 4) no hyperdensity at the corresponding location on non-contrast CT29).
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences for Microsoft Windows (Version 12.0; SPSS Inc., Chicago, IL, USA). Patients were classified according to survival versus fatality and good versus poor clinical outcomes (mRS 0-2 versus 3-6, GOS 1-3 versus 4-5) on discharge and at 90-day follow-up. The relationships between spot sign with clinical outcomes and in-hospital mortality were examined by the chi-square test for categorical variables or Mann-Whitney U-test for continuous variables. The multivariate logistic regression analysis was repeated for the prediction of in-hospital mortality and poor outcome among survivors at 3-month follow-up including, as an additional variable, first the presence of any spot sign, and then mortality. The adjusted odds ratios (ORs) and their 95% confidence intervals (95% CIs) were calculated. Significance was set at a p value of less than 0.05.
RESULTS
From January 1, 2008 until January 31, 2012, a total of 227 patients presented to the department of neurosurgery with spontaneous ICH on NCCT and were evaluated with MDCTA of the intracranial circulation within 24 hours of admission (Fig. 1). No adverse events were attributable to the CTA. Forty patients were excluded from the primary analysis for the following reasons : 20 patients were treated with surgical evacuation before follow-up CT; 10 patients died before follow-up CT, and 10 patients did not have a follow-up CT for unknown reasons. A total of 187 patients met our inclusion criteria, with a mean age of 60.45±14.49 years (median 60.45 years, range 19-80 years). The median time from emergency department admission to MDCTA evaluation was 1.33 hours (mean 2.5 hours, range 0.25-8 hours), and median length of hospital stay was 14 days (mean 17.72 days, range 2-95 days). CTA demonstrated 61 CTA spot sign-positive patients (61/187; 32.6%) and 126 patients without the spot sign (126/187; 67.4%) (Fig. 1). Median time to presentation was 120 minutes (33-312 minutes). ICH was deep, lobar, or within the posterior fossa in 46 (34.6%), 120 (64.2%), and 21 (11.2%) patients, respectively. Baseline demographic data are indicated in Table 1. Follow-up results demonstrated 47 patients (25.1%) with clinically important hematoma growth; 35 of these demonstrated spot sign (74.46%) on the initial CTA (Table 1) (Fig. 2). Patients with clopidogrel use were more likely to have spot sign (p=0.006), but the small sample size (n=11) was a limiting factor. Univariate analyses demonstrated the spot sign (p<0.001), and clopidogrel use (p= 0.001) were associated with hematoma expansion, whereas a history of hypertension, diabetes mellitus, antiplatelet use, anticoagulants, PT/aPTT, INR, mean arterial blood pressure (MABP) ≥120 mm Hg, in-hospital stay, and glucose ≥8.3 mmol/L had no association with hematoma expansion. Hematoma expansion occurred significantly more frequently in patients with the spot sign than in those without (p<0.001). In multivariate logistic regression analysis, we found that the spot sign may play an important role indicating the presence of volume expansion (OR 5.010; 95% CI 1.993-12.599; p=0.001), mRS (OR 7.706; 95% CI 1.021-7.169; p=0.045), and in-hospital mortality (OR 8.870; 95% CI 2.554-30.804; p=0.001) (Table 2). The associations between clinical, laboratory, and imaging variables and 90-day outcomes are shown in Table 3. The predictors of poor clinical outcome at 90-day follow-up include GCS, NIHSS, systolic blood pressure (SBP), diastolic blood pressure (DBP), MABP, prothrombin time (PT), INR, IVH, IVH volume, ICH location, ICH volume, hematoma expansion, spot sign, and treatment modality (Table 3). Multivariate logistic regression analysis identified predictors of poor outcome; we found that hematoma location (OR 2.258; 95% CI 1.190-4.284; p=0.013), spot sign (OR 3.883; 95% CI 1.467-10.275; p= 0.006), IVH (OR 2.994; 95% CI 1.295-6.922; p=0.010) were independent predictors of poor outcome (Table 4). In-hospital mortality was 57.4% (35 of 61) in the CTA spot-sign positive group versus 7.9% (10 of 126) in the CTA spot-sign negative group. We found that presence of spot sign (OR 10.197; 95% CI 2.572-41.157; p=0.001) and presence of volume expansion (OR 11.832; 95% CI 2.591-54.034; p=0.001) were independent predictors for the in-hospital mortality of ICH (Table 5). Mortality and unfavorable outcome rates were high in spot sign-positive and volume expansion-positive patients. Positive predictive values from the previous studies varied considerably (24-77%, 77.78% in this study), whereas negative predictive values were lower (96-98%, 81.68% in this study) (Table 6)12,16,30).
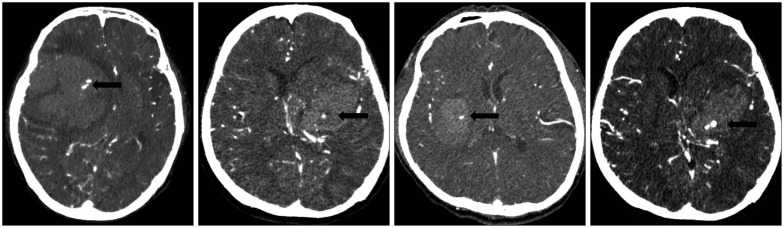
Fig. 1.
The appearance of a spot sign on CT angiography in a patient with intracerebral hemorrhage. The spot sign (black arrow) assesses diameter and Hounsfield units. The spot sign is located within the hematoma, has no connection to any outside vessel, and is absent on baseline non-contrast CT.
Table 1.
Baseline characteristics
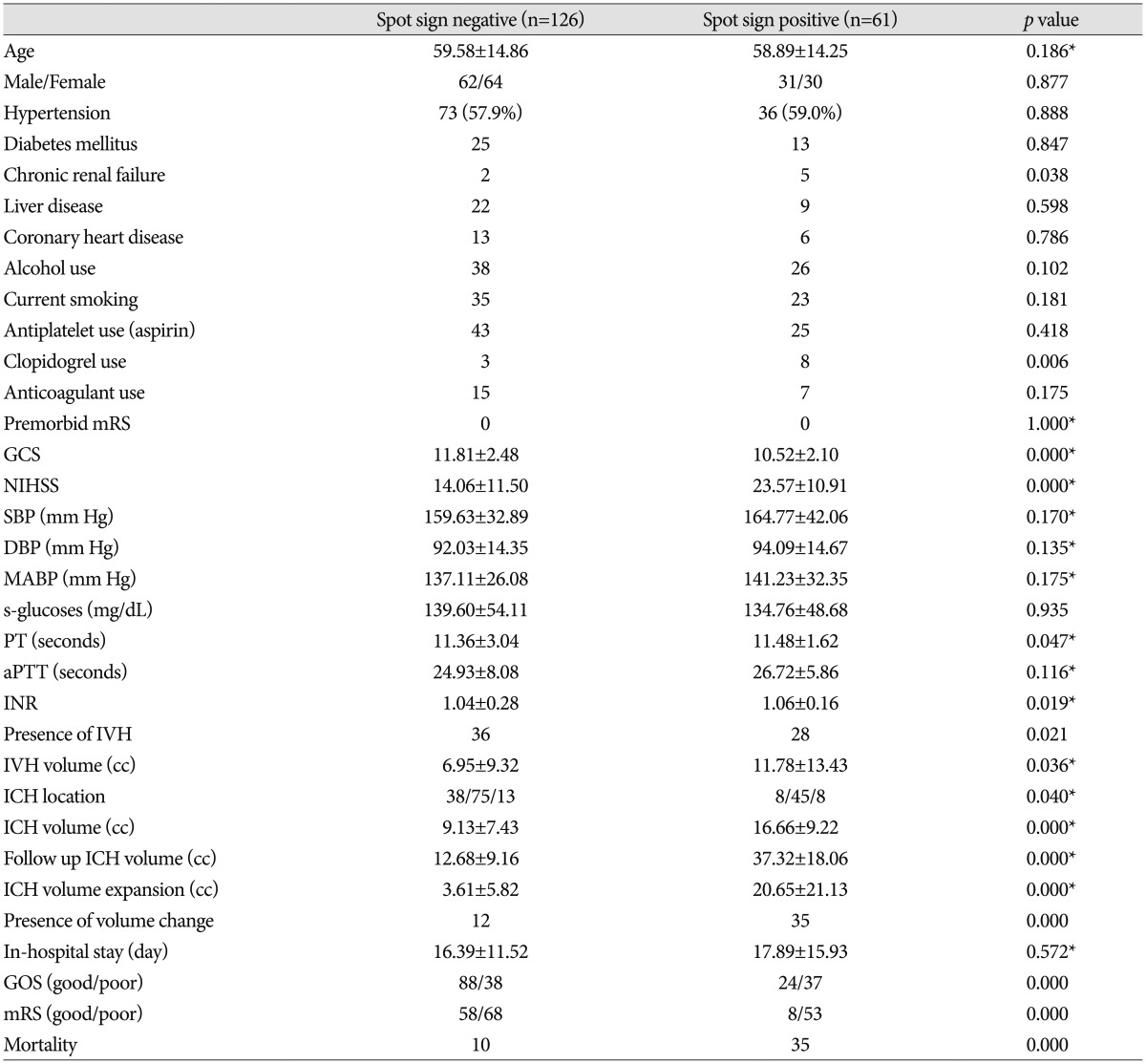
Chi-square test was used for dichotomizing variables, and Mann-Whitney test (*) was used for continuous variables. For comparison of outcome, we divided patients into two groups with good (GOS 4-5, mRS 0-2) and poor (GOS 1-3, mRS 3-6) outcomes. Hematoma location : supratentorial lobar/supratemtorial deep/infratentorial. GCS : Glasgow Coma Scale, NIHSS : National Institutes of Health Stroke Scale, SBP : systolic blood pressure, DBP : diastolic blood pressure, MABP : mean arterial blood pressure, PT : prothrombin time, aPTT : activated partial thromboplasin time, INR : international normalized ratio, IVH : intraventricular hemorrhage, ICH : intracerebral hemorrhage, GOS : Glasgow Outcome Scale, mRS : modified Rankin Scale
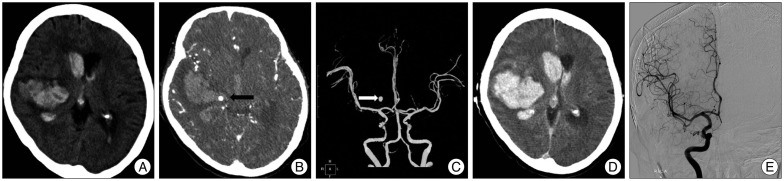
Fig. 2.
A : A 61-year-old man underwent imaging 2 hours following onset of left-sided paralysis. NCCT demonstrates a right basal ganglia ICH (34 mL) with associated IVH (19 mL). B : Axial CTA source image in spot windows demonstrates 1 foci of contrast pooling within the ICH with an attenuation 176 HU (arrowheads), consistent with spot signs (a total of 4 spot signs were identified). The largest spot sign measured 5.7 mm in maximum axial dimension and had an attenuation of 245 HU (spot sign score, 4). C : Axial CTA image shows that the spot sign looks like aneurysmal sac. D : Non-contrast CT 4 hours after the baseline CTA demonstrates marked interval expansion of both the ICH (86 mL) and IVH (42 mL). E : Conventional angiographic image demonstrates absence of aneurysmal sac. NCCT : noncontrast CT, ICH : intracerebral hemorrhage, IVH : intraventricular hemorrhage, CTA : CT angiography, HU : Hounsfield units.
Table 2.
Multivariable analysis of association with volume expansion
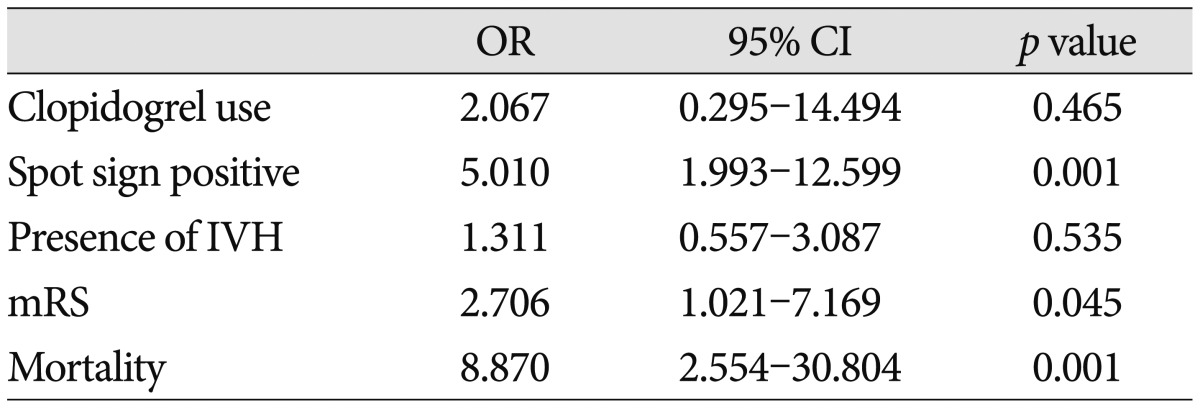
OR : odds ratio, CI : confidence interval, IVH : intraventricular hemorrhage, mRS : modified Rankin Scale
Table 3.
Risk factors of 90-day clinical outcome in primary ICH
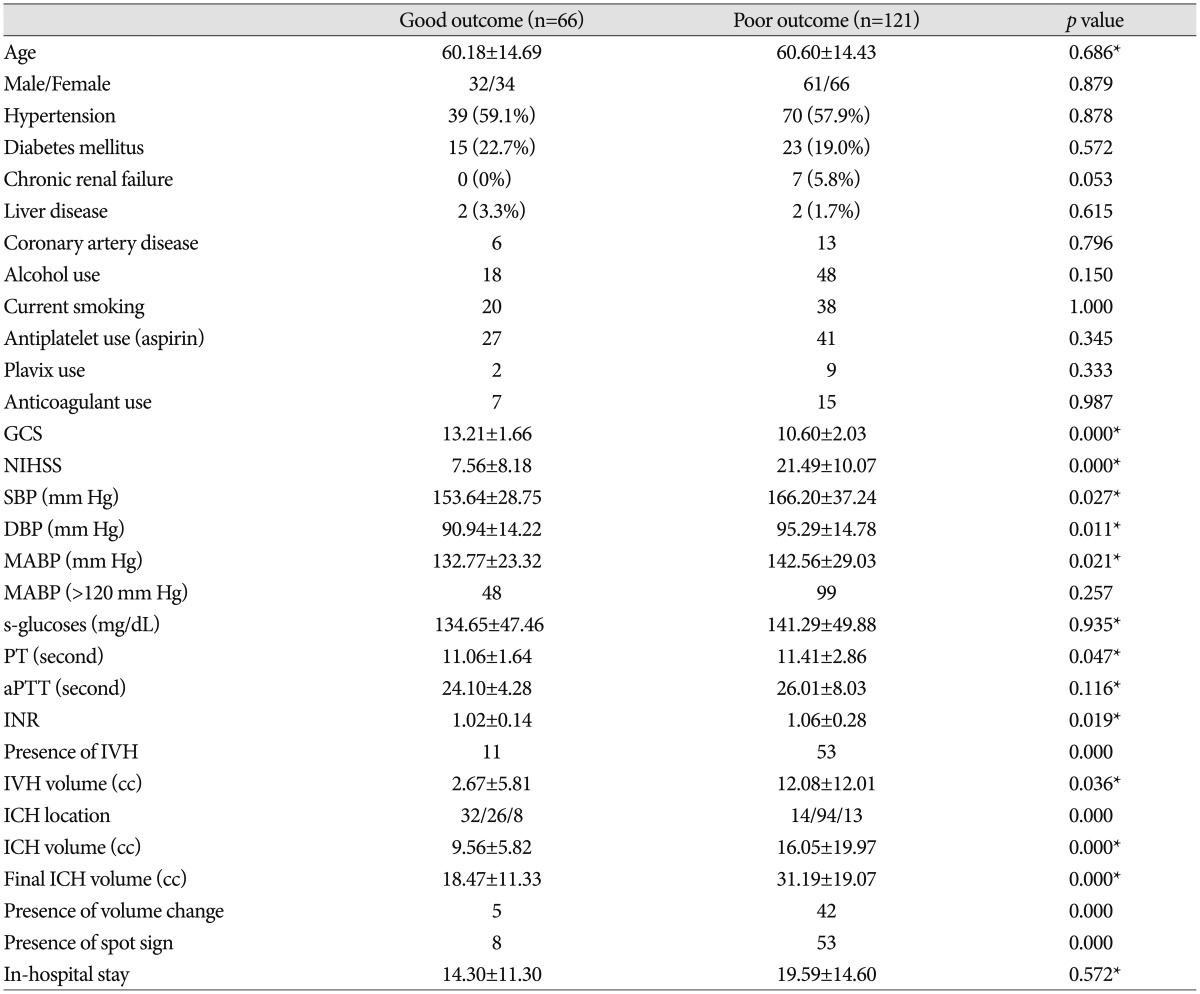
Chi-square test was used for dichotomizing variables, and Mann-Whitney test (*) was used for continuous variables. For comparison of outcome, we divided patients into two groups with good (GOS 4-5, mRS 0-2) and poor (GOS 1-3, mRS 3-6) outcomes. Hematoma location : supratentorial lobar/supratemtorial deep/infratentorial. GCS : Glasgow Coma Scale, NIHSS : National Institutes of Health Stroke Scale, SBP : systolic blood pressure, DBP : diastolic blood pressure, MABP : mean arterial blood pressure, PT : prothrombin time, aPTT : activated partial thromboplasin time, INR : international normalized ratio, IVH : intraventricular hemorrhage, ICH : intracerebral hemorrhage
Table 4.
Multivariate analysis of predictors of poor outcome
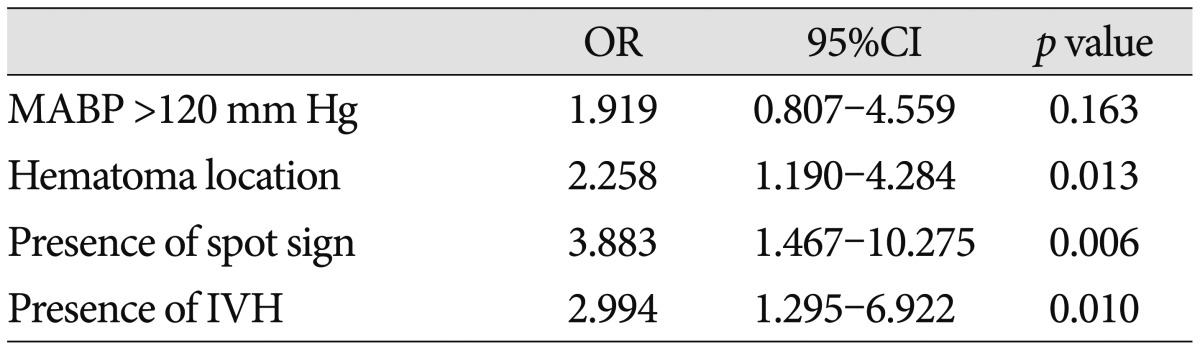
OR : odds ratio, CI : confidence interval, MABP : mean arterial blood pressure, IVH : intraventricular hemorrhage
Table 5.
Multivariate analysis of predictors of mortality
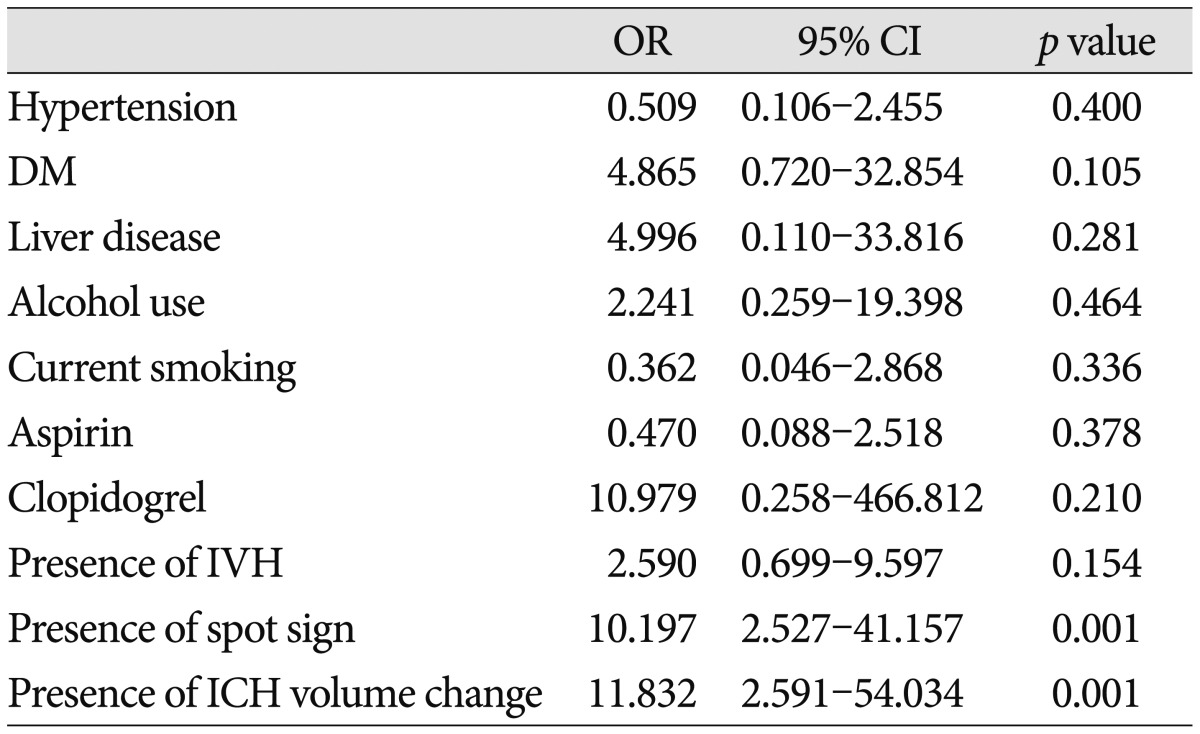
OR : odds ratio, CI : confidence interval, DM : diabetes mellitus, IVH : intraventricular hemorrhage, ICH : intracerebral hemorrhage
Table 6.
Accuracy of the spot sign for the prediction of volume expansion, mortality, and poor outcome in primary ICH
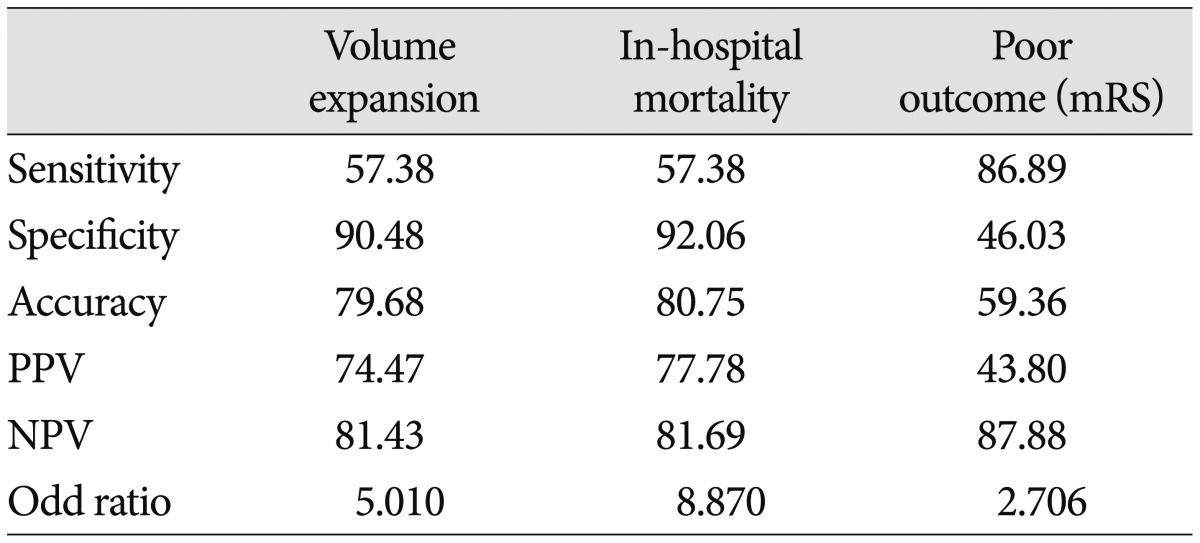
ICH : intracerebral hemorrhage, mRS : modified Rankin Scale
DISCUSSION
We found that the spot sign is the strongest significant predictor of hematoma volume expansion, poor outcome (mRS >2), and increased in-hospital mortality. The spot sign is also associated with larger hemorrhage, and a more severe clinical presentation. Most recently, Demchuk et al.9) concluded that the CTA spot sign is highly predictive of hematoma expansion, irrespective of hematoma expansion definition and for both intraparenchymal and intraventricular hemorrhage growth. The CTA spot sign is associated with poor prognosis, high rates of early clinical deterioration and mortality, often occurring within days after onset9).
Hematoma expansion occurs in up to 70% of patients with ICH documented by CT performed within 3 hours after the onset of symptoms4,5,6). ICH accounts for 10% to 20% of all strokes and is more fatal and disabling than ischemic stroke or subarachnoid hemorrhage (SAH)24). There is no proven treatment for patients with ICH. Because of the rapid and severe devastation associated with ICH, innovative treatments need to be developed and evaluated. Major predictors of increased early mortality and adverse outcome during the acute phase of ICH are hematoma expansion, IVH with obstructive hydrocephalus, and hyperglycemia3,5,20,28). At least 38-70% of patients had >33% growth in the volume of parenchymal hemorrhage during the first 24 hours after symptom onset5,6). A previous study reported that the volume of ICH is the strongest predictor of 30-day outcome for all locations of spontaneous ICH18). Hematoma expansion is associated with early neurologic deterioration and is an independent predictor of poor outcome and increased morbidity4,20,26). The precise mechanism of early hematoma expansion during the acute phase is poorly understood. Mayer et al.20) proposed that hematoma expansion is associated with dysregulation of hemostasis via inflammatory cascade activation and matrix metalloproteinase (MMP) overexpression, breakdown of the blood-brain barrier, a sudden increase in ICP leading to local tissue distortion and disruption, and vascular engorgement due to reduced venous outflow. Hematoma expansion can also result from an increased plasma concentration of cellular fibronectin (c-FN) and the inflammatory mediator interleukin-6 (IL-6)27). Treatments to restrict hematoma expansion include hemostatic therapy, cautious lowering of high BP, quick reversal of prior anticoagulation, and surgical evacuation22). There have been a few clinical trials to restrict hematoma expansion. Treatment options to restrict hematoma expansion can be divided into surgical and nonsurgical approaches.
Clinical trials to restrict hematoma expansion in ICH and a meta-analysis have shown that the use of recombinant factor VII (rFVIIa) limits the extent of hematoma expansion in patients with non-coagulopathic ICH13,19,20,31). However, there was an increase in thromboembolic risk with no clear clinical benefit in unselected patients. Reports from the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT) and Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trials show that SBP reduction might restrict hematoma expansion in the hyperacute phase of ICH1,2,25). Many previous studies reported that spot sign is an independent predictor of hematoma expansion and mortality7,8,12,16,23,28,29,30). Brott et al.5) found the majority of relevant growth of the hematoma volume on admission CT, to occur within 4 hours after symptom onset. This suggests that growth occurs early in the course of spontaneous intracerebral hemorrhage (SICH) and that early CT scan repetition is warranted to detect it. The timing of surgical treatment for ICH is a key benchmark for hematoma expansion prevention because treatment delays are associated with an increased risk of mortality. Early prevention may be an ideal target for medical or surgical hemostatic therapy in patients who exhibit high average rates of hematoma expansion. The timing of surgical treatment varies widely because of patient factors (delayed symptom recognition and presentation to medical attention), physician factors (delayed diagnosis and referrals), resource availability (rapid access to vascular imaging), and institutional/system-level factors related to operating room availability and staffing. In our study, all patients arrived at the hospital within 10 hours of the clinical event. However, hematoma expansion during referrals occurred in over 35% of patients. Theoretically, early surgical treatment putatively reduces hematoma expansion, reduces mortality, and improves clinical outcome. The role of surgical treatment for ICH is controversial. Many clinical trials have failed to show an outcome benefit over conservative treatment. A report from the Surgical Trial in Intracerebral Haemorrhage (STICH) trial showed no overall benefit of early surgical clot evacuation compared with initial conservative treatment in patients with ICH21). However, a subgroup analysis showed a potential benefit for surgery in lobar ICH within 1 cm of the cortical surface21). Guidelines from the American Heart Association and American Stroke Association suggest that indications of surgical intervention are as follows : 1) for most patients with ICH, the usefulness of surgery is uncertain; 2) patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression and/or hydrocephalus from ventricular obstruction should undergo surgical removal of the hemorrhage as soon as possible; 3) for patients presenting with lobar clots >30 mL and within 1 cm of the surface, evacuation of supratentorial ICH by standard craniotomy might be considered; 4) the effectiveness of minimally invasive clot evacuation utilizing either stereotactic or endoscopic aspiration with or without thrombolytic usage is uncertain and is considered investigational22).
Clinical trials that are tailored more individually with regard to patient characteristics and SICH features are still necessary to elucidate the role of surgery. Further clinical trials should include surgical versus medical treatment in spot sign-positive patients. The use of recombinant factor VII (rFVIIa) limits the extent of hematoma expansion in spot sign-positive patients.
This study is limited by its retrospective design. We excluded patients with GCS scores of 3-5. The number of 187 patients was relatively small, and the data set was heterogeneous with regard to time to scan. A prospective study with a larger number of cases is needed to confirm the association of spot sign to poor clinical outcomes.
CONCLUSION
The spot sign is a strong independent predictor of hematoma expansion, mortality, and poor clinical outcome in primary ICH. Several trials have been conducted to restrict hematoma expansion. Unfortunately, all measures to restrict hematoma expansion have so far failed to improve outcome in randomized controlled trials. In this study, we emphasized the importance of he-matoma expansion as a therapeutic target in both clinical practice and research. Clinical trials of surgical interventions and hemostatic treatment, such as rFVIIa, should be done in ICH patients with a positive spot sign on CTA.
References
- 1.Anderson CS, Huang Y, Arima H, Heeley E, Skulina C, Parsons MW, et al. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage : the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT) Stroke. 2010;41:307–312. doi: 10.1161/STROKEAHA.109.561795. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT) : a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 3.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD STICH Investigators. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage : results from the STICH trial. Acta Neurochir Suppl. 2006:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 4.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 5.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 7.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion : the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT) : a prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 10.Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 11.Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage : can modification to original score improve the prediction? Stroke. 2006;37:1038–1044. doi: 10.1161/01.STR.0000206441.79646.49. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 13.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial : efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score : a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 15.Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–1787. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Smith A, Hemphill JC, 3rd, Smith WS, Lu Y, Dillon WP, et al. Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol. 2008;29:520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 18.Leira R, Dávalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage : predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 19.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 20.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 21.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH) : a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage : a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SY, Kong MH, Kim JH, Kang DS, Song KY, Huh SK. Role of 'spot sign' on CT angiography to predict hematoma expansion in spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc. 2010;48:399–405. doi: 10.3340/jkns.2010.48.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham A, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage : results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67:570–576. doi: 10.1001/archneurol.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Luna D, Rubiera M, Ribo M, Coscojuela P, Piñeiro S, Pagola J, et al. Ultraearly hematoma growth predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2011;77:1599–1604. doi: 10.1212/WNL.0b013e3182343387. [DOI] [PubMed] [Google Scholar]
- 27.Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Dávalos A, et al. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 28.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage : risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–773. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773-774. [DOI] [PubMed] [Google Scholar]
- 29.Thompson AL, Kosior JC, Gladstone DJ, Hopyan JJ, Symons SP, Romero F, et al. Defining the CT angiography 'spot sign' in primary intracerebral hemorrhage. Can J Neurol Sci. 2009;36:456–461. doi: 10.1017/s0317167100007782. [DOI] [PubMed] [Google Scholar]
- 30.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography "spot sign" predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 31.Yuan ZH, Jiang JK, Huang WD, Pan J, Zhu JY, Wang JZ. A meta-analysis of the efficacy and safety of recombinant activated factor VII for patients with acute intracerebral hemorrhage without hemophilia. J Clin Neurosci. 2010;17:685–693. doi: 10.1016/j.jocn.2009.11.020. [DOI] [PubMed] [Google Scholar]