Abstract
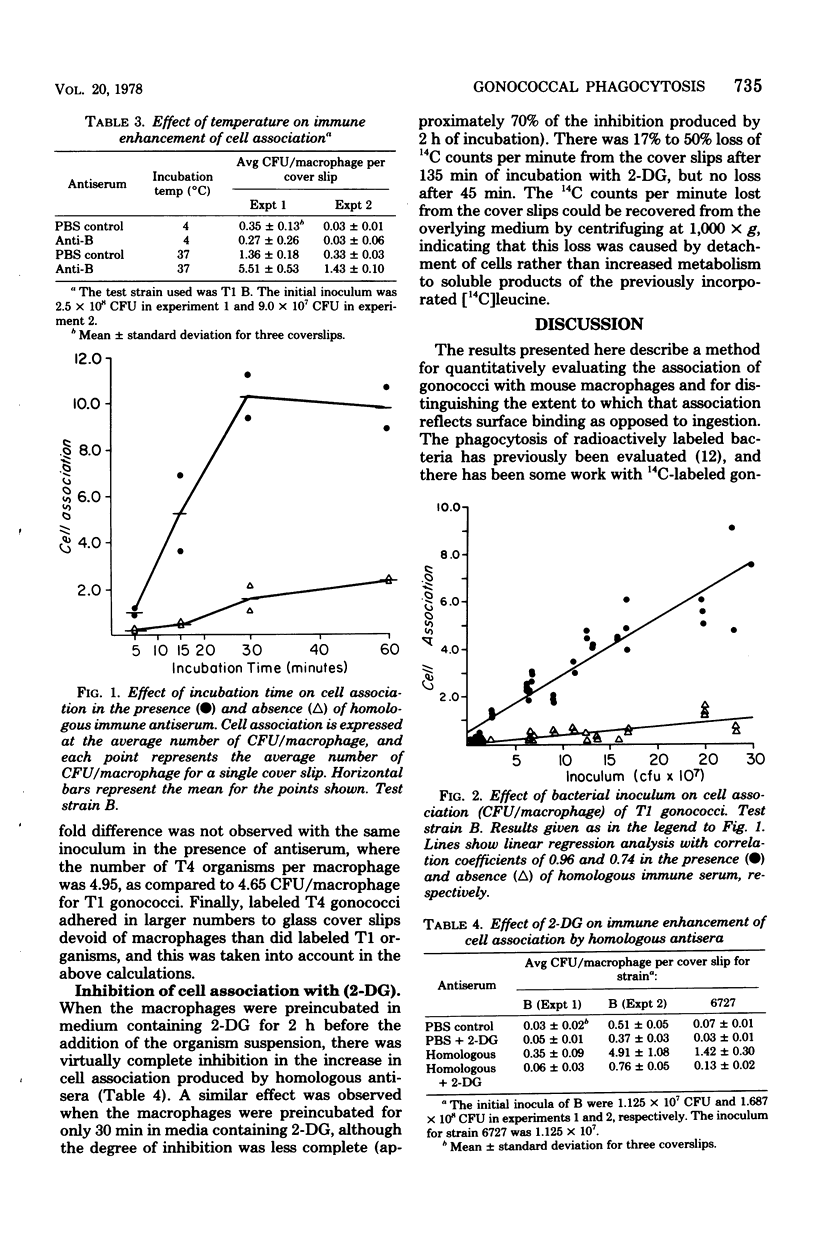
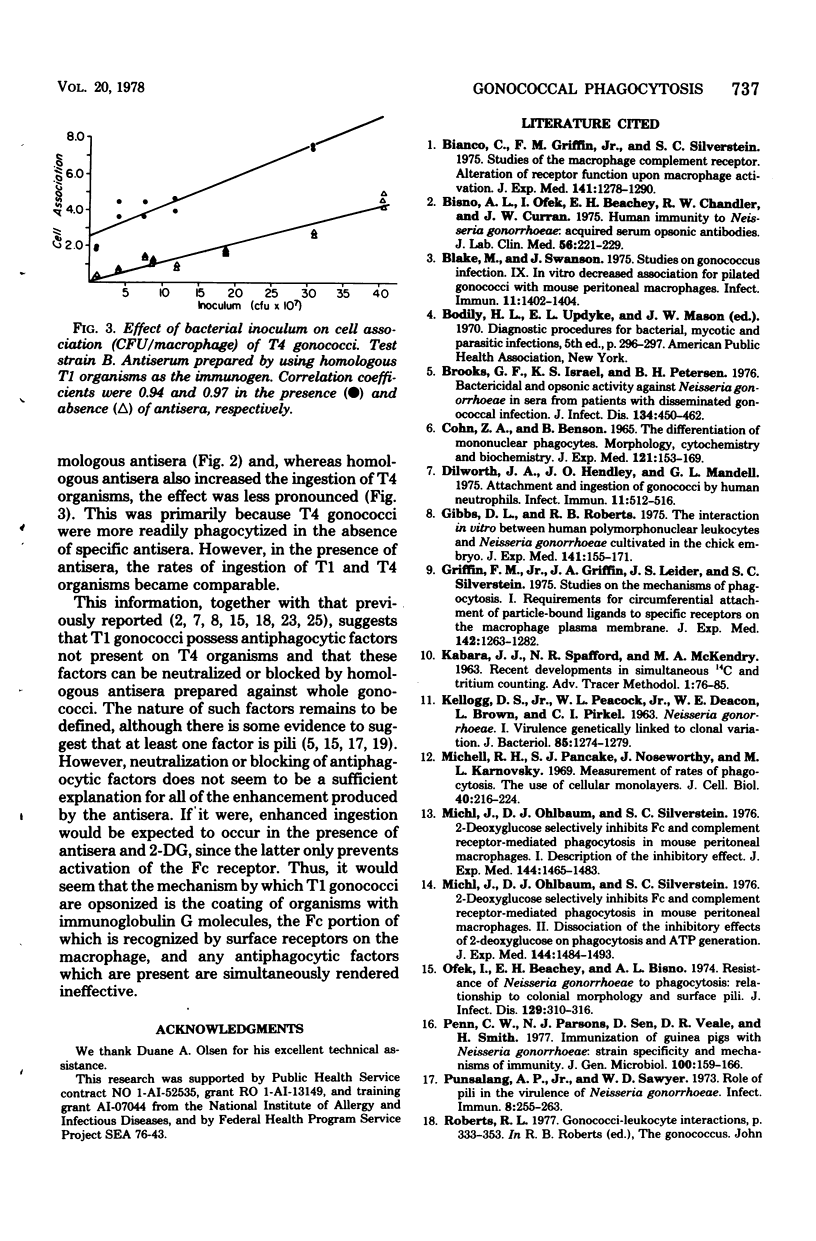
The simultaneous labeling of gonococci with [6-3H]uracil and of mouse peritoneal macrophages with L-[U-14C]leucine permits a quantitative assessment of the association of gonococci with macrophages under various experimental conditions. Colony-type T1 (piliated) gonococci associated more than T4 (nonpiliated) organisms at 4 degrees C, but at 37 degrees C the association of T4 gonococci with macrophages exceeded that for the T1 organisms. The association of T1 gonococci with macrophages could be enhanced as much as 70-fold by homologous rabbit antisera prepared against whole, formaldehyde-treated organisms. This immune enhancement represented primarily increased phagocytosis rather than surface attachment, as shown by its inhibition at 4 degrees C or with 2-deoxyglucose. The data further suggested that this enhanced phagocytosis was mediated via the Fc receptor for immunoglobulin G.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L., Ofek I., Beachey E. H., Chandler R. W., Curran J. W. Human immunity to Neisseria gonorrhoeae: acquired serum opsonic antibodies. J Lab Clin Med. 1975 Aug;86(2):221–229. [PubMed] [Google Scholar]
- Blake M., Swanson J. Studies on Gonococcus infection. IX. In vitro decreased assocation of pilated gonococci with mouse peritoneal macrophages. Infect Immun. 1975 Jun;11(6):1402–1404. doi: 10.1128/iai.11.6.1402-1404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G. F., Israel K. S., Petersen B. H. Bactericidal and opsonic activity against Neisseria gonorrhoeae in sera from patients with disseminated gonococcal infection. J Infect Dis. 1976 Nov;134(5):450–462. doi: 10.1093/infdis/134.5.450. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D. L., Roberts R. B. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975 Jan 1;141(1):155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABARA J. J., SPAFFORD N. R., McKENDRY M. A., FREEMAN N. L. Recent development in simultaneous C-14 and tritium counting. Adv Tracer Methodol. 1963;1:76–85. [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976 Dec 1;144(6):1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Parsons N. J., Sen D., Veale D. R., Smith H. Immunization of guinea pigs with Neisseria gonorrhoeae: strain specificity and mechanisms of immunity. J Gen Microbiol. 1977 May;100(1):159–166. doi: 10.1099/00221287-100-1-159. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K., Veale D. R., Finch H., Penn C. W., Sen D., Smith H. Resistance of Neisseria gonorrhoeae grown in vivo to ingestion and digestion by phagocytes of human blood. J Gen Microbiol. 1976 Oct;96(2):341–350. doi: 10.1099/00221287-96-2-341. [DOI] [PubMed] [Google Scholar]



