Abstract
Background:
Using of CD10 in accordance with clinical and histological features of thyroid lesions could be used as both diagnostic and prognostic tool, which consequently influence the management and their prognosis for survival of patients with thyroid neoplasms especially papillary thyroid carcinoma (PTC). The aim of this study was to determine its expression in PTC and different benign thyroid lesions.
Materials and Methods:
In this descriptive-analytic, cross-sectional study, paraffin-embedded tissues of patients with definitive pathologic diagnosis of different benign thyroid lesions and PTC were retrieved. Immunostained sections of each slides was performed using immunohistochemistry methods and expression of CD10 was compared in two groups of benign thyroid lesions and PTC.
Results:
From selected cases 134 sections studied in two groups of PTC (n = 67) and benign thyroid lesions (n = 67). CD10 were immunohistochemically positive in 29.9% of PTC cases, but in none of the thyroid benign lesions (0%) (P < 0.001). There was not significant relationship between expression of CD10 with age and sex of the studied population (P > 0.05).
Conclusion:
The results of the current study indicate that due to the higher expression of CD10 in PTC than benign thyroid lesions it might be used for differentiating mentioned lesions. But for using it as a diagnostic tool further studies with larger sample size and determination of its sensitivity, specificity and cut-off point is necessary.
Keywords: CD10, immunohistochemistry, papillary thyroid carcinoma
INTRODUCTION
Thyroid cancer considered as the most common endocrine malignancy. It includes papillary thyroid carcinoma (PTC) (80%), follicular carcinoma (15%), poorly differentiated carcinoma (<1%) and anaplastic carcinoma (<2%).[1]
Thyroid carcinoma constitutes for 1% of all cancers. Its incidence has significant geographic variation.[2] It is estimated that the incidence rate of thyroid carcinoma ranging from 0.5-10 cases per 100, 000, world-wide.[3] The most common origin of thyroid carcinoma is epithelial which originates from the hormone-producing follicular cells of the thyroid gland.[4]
PTC is the most common form of malignant thyroid tumor.[5] The incidence rate of PTC has been increased during the past few decades. One of the causes of this increasing rate is an improvement of its diagnostic tools.[6]
Though there are characteristic morphologic features for differentiation different thyroid lesions, but many studies indicated that differentiation of PTC from thyroid gland benign lesions based on their morphology is often problematic. A great verity of observer variation has been reported in this field. It considered as one of the diagnostic challenges even among experienced pathologists.[7,8,9,10]
To overcome the problem different cytological, immunohistochemistry (IHC) and molecular studies have been developed.[11,12,13] Various biomarkers of malignancy in thyroid tumors have been investigated in surgical as well as fine-needle aspiration cytology specimens for improving diagnostic accuracy. Among them different immunohistochemical markers have been evaluated. Though the utility of some of the markers in differentiating benign from malignant thyroid lesions have been reported but considering some advantages and limitations of the markers, practically useful markers have not been established, yet.[14,15,16,17]
CD10, a membrane-bound zinc metalloproteinase, is one of the studied markers which originally was used for diagnosis and classification of malignant lymphomas and leukemia's.[18] Recently, the diagnostic utility of CD10 in different in non-hematopoietic lesions including breast, liver and uterus has been reported.[19,20,21,22] The usefulness of CD10 marker in discrimination of different benign and malignant thyroid lesions demonstrated in some reports.[23,24]
Using of CD10 in accordance with clinical and histological features of thyroid lesions could be used as both diagnostic and prognostic tool which consequently influence the management and their prognosis for survival of patients with thyroid neoplasms specially PTC. The aim of this study was to determine its expression in PTC and different benign thyroid lesions.
MATERIALS AND METHODS
In this descriptive-analytic, cross-sectional study, paraffin-embedded tissues of 134 patients with definitive pathologic diagnosis of different benign and malignant (PTC) thyroid lesions were retrieved from the archives of the Department of Pathology of Al-zahra Hospital affiliated with Isfahan University of Medical Sciences, from 2006 to 2011. All sections selected by consecutive sampling method.
The Medical Ethics Committee of the Isfahan University of Medical Sciences approved the study protocol (Research project number; 390201).
The slides had been stained by H and E staining methods. The diagnosis of studied cases re-evaluated by two pathologists. Cases with typical history and histological pattern were selected. Characteristics of studied cases were recorded. IHC was performed for all the cases and expression of CD10 was compared in two groups of benign thyroid lesions and PTC.
For immunohistochemical staining
3-4 μm thick sections of selected formalin-fixed, paraffin-embedded tissues were prepared. The sections mounted on glass slides and poly-l-lysin coated slides for H and E and IHC staining, respectively. The sections deparaffinized, rehydrated by immersion in xylene and rinsed in tap water. For antigen retrieval deparaffinized sections immersed in citrate buffer (pH = 9.0) for 15 min at 95°C. The sections incubated with hydrogen peroxide 3% for 60 min to inactivate endogenous catalyzes. The sections then incubated with primary antibody for 24 h (clone 56C6, RTU-CD10-270 Novocastra Lot: 6004843) diluted at a 1:150 concentration and then secondary antibody (N-vision, Dako system) for 40or 60 min at room temperature. After each step of antibody treatment, the sections drained with phosphate buffered saline. Finally, they incubated with diaminobenzidine in a chromogen solution for 5 min and counterstained with hematoxylin. Brown cytoplasmic with or without membrane staining defined as CD10 positivity. For each case, 10 high power fields were evaluated. Mean percentage of positive cells <10% and ≥10% considered as negative and positive, respectively. The immunoreactivity interpreted based on the percentage of the stained cells irrespective of the intensity of the staining.
Obtained data analyzed using SPSS version 18 for windows software and t-test, Chi-square, ANOVA and Fisher's exact test. P ≤ 0.05 was considered to be statistically significant.
RESULTS
From selected cases 134 sections studied in two groups of PTC (n = 67) and benign thyroid lesions (n = 67). Benign thyroid lesions including follicular adenoma (n = 40), nodular goiter (n = 26) and hurthle cell adenoma (n = 1).
Demographic characteristics and CD10 immunoreactivity in two studied groups were presented in Table 1.
Table 1.
Demographic characteristics and CD10 immunoreactivity in PTC (n=67) and benign thyroid lesions (n=67)
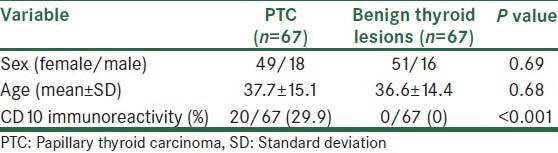
Mean age and sex distribution of cases with positive and negative CD10 immunoreactivity in PTC group is presented in Table 2. There was not significant relationship between age and sex with CD10 immunoreactivity (P > 0.05).
Table 2.
Mean age and sex distribution of cases with positive and negative CD10 immunoreactivity in patients with PTC

DISCUSSION
In the present study, we investigate whether CD10 could be a useful diagnostic tool in differentiating PTC and benign thyroid lesions. We have demonstrated that CD10 were immunohistochemically positive in 29.9% of PTC cases, but in none of the thyroid benign lesions (0%).
As reported by Rezk and Khan considering the fact that morphologic diagnosis of some thyroid benign and malignant lesions is challenging and also subjective, so using ancillary procedures such as IHC for some markers including CD10 with conventional pathologic evaluation could be more reliable for diagnosis and classification of different thyroid lesions.[25]
Moreover, mentioned immunohistochemical markers could be used as prognostic tools, because during progression of thyroid carcinomas to more poorly differentiated and undifferentiated phenotype, the tumors have not enough and appropriate diagnostic morphologic features of thyroid carcinoma.[25] Thus, in this study we evaluate the diagnostic utility of CD10 marker in this regard. The results of studies in this field is controversial, some confirmed its utility whereas others did not report any case of CD10 immunoreactivity in thyroid tumors.
Our results were in accordance with the report of Tomoda et al. in Japan. They examined the expression of CD10 in 70 thyroid neoplasm. Their findings indicated that CD10 was not positive in benign lesions. CD10 was positive in 80% and 77% of follicular carcinomas and follicular variant of PTC, respectively. That was the first report on the expression of CD10 marker in thyroid neoplasm. They concluded that CD10 expression by IHC considered as a useful marker for classification and diagnosis of benign and malignant thyroid lesions.[23]
In research in Turkey, Yegen et al. have investigated the staining pattern of CD10 in different benign (n = 14) and malignant (n = 61) thyroid lesions. According to their results CD10 was negative in adenomatous nodules, minimally invasive follicular carcinoma and well-differentiated carcinoma. It was positive in conventional papillary carcinomas (64.2%), follicular variant of papillary carcinomas (16.6%), papillary microcarcinomas (50%), widely invasive follicular carcinomas (11.1%) and follicular adenomas (30%). They concluded that, though CD10 had strong positivity in conventional papillary carcinoma but it could not be used as a useful marker for differentiating benign and malignant thyroid lesion.[24]
Though our results indicated that expression of CD10 is significantly higher in PTC than benign lesions, but the rate of its positivity, i.e. 29.9% in comparison with mentioned studies with 60-70% positivity is low. It may be due to our methods of study.
Chu and Arber have studied the expression of CD10 in 505 non-hematopoietic neoplasms including 55 thyroid tumors {follicular adenoma (n = 24), papillary carcinoma (n = 10), medullary carcinoma (n = 16) and Follicular carcinoma (n = 5)} by IHC. According to their results CD10 expression was negative in all studied thyroid tumors.[26]
Similarly, Yasuda et al. in Japan have investigated the availability of CD10, as a histopathological marker, in different non-hematopoietic neoplasms. They showed that CD10 was not present in thyroid tumors and has not any diagnostic value for this group of non-hematopoietic neoplasms.[27]
The limitation of current study was that the different benign lesions were studied together because the subgroups have small sample size and in the PTC group different subgroups was not evaluated separately. In addition, we did not evaluate other malignant lesions (follicular or other malignant tumors) of thyroid gland. However, we studied lesions that represent nuclear changes and mentioned malignant lesions have not the changes.
In sum, the results of current study indicate that due to the higher expression of CD10 in PTC than benign thyroid lesions it might be used in making distinction between benign thyroid lesions and PTC. But for using it as diagnostic tool further studies with consideration of mentioned limitations, larger sample size and determination of its sensitivity, specificity and cut-off point is necessary. Moreover, we recommended to study the expression of CD10 in invasive and indolent disease of thyroid gland.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Nikiforov YE. Thyroid tumors: Classification and general considerations. In: Nikiforov YE, Biddinger PW, Thompson LD, editors. Diagnostic Pathology and Molecular Genetics of the Thyroid. Philadelphia, PA: Lippincott Williams; 2009. pp. 94–102. [Google Scholar]
- 2.Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U. S and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000;89:202–17. doi: 10.1002/1097-0142(20000701)89:1<202::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Baloch ZW, LiVolsi VA. Pathology of thyroid gland. In: Livolsi VA, Asa SL, editors. Endocrine Pathology. New York: Churchill Livingstone; 2002. pp. 61–88. [Google Scholar]
- 4.Khan A, Nose V. Pathology of thyroid gland. In: Lloyd RV, editor. Endocrine Pathology: Differential Diagnosis and Molecular Advances. 2nd ed. New York: Springer; 2010. pp. 181–236. [Google Scholar]
- 5.Classification of Tumours. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors; Kleihues P, Sobrin LH, editors. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: World Health Organization, IARC Press; 2004. [Google Scholar]
- 6.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5:51–6. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVita VT, Hellman JS, Rosenberg SA. Philadelphia, Pa: Lippincott Williams and Wilkins; 2008. Cancer: Principles and Practice of Oncology; pp. 1674–99. [Google Scholar]
- 8.Franc B, de la Salmonière P, Lange F, Hoang C, Louvel A, de Roquancourt A, et al. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol. 2003;34:1092–100. doi: 10.1016/s0046-8177(03)00403-9. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–40. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 10.Baloch ZW, Livolsi VA. Follicular-patterned lesions of the thyroid: The bane of the pathologist. Am J Clin Pathol. 2002;117:143–50. doi: 10.1309/8VL9-ECXY-NVMX-2RQF. [DOI] [PubMed] [Google Scholar]
- 11.Maruta J, Hashimoto H, Yamashita H, Yamashita H, Noguchi S. Diagnostic applicability of dipeptidyl aminopeptidase IV activity in cytological samples for differentiating follicular thyroid carcinoma from follicular adenoma. Arch Surg. 2004;139:83–8. doi: 10.1001/archsurg.139.1.83. [DOI] [PubMed] [Google Scholar]
- 12.El Demellawy D, Nasr A, Alowami S. Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol. 2008;3:5. doi: 10.1186/1746-1596-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barden CB, Shister KW, Zhu B, Guiter G, Greenblatt DY, Zeiger MA, et al. Classification of follicular thyroid tumors by molecular signature: Results of gene profiling. Clin Cancer Res. 2003;9:1792–800. [PubMed] [Google Scholar]
- 14.Barut F, Onak Kandemir N, Bektas S, Bahadir B, Keser S, Ozdamar SO. Universal markers of thyroid malignancies: Galectin-3, HBME-1, and cytokeratin-19. Endocr Pathol. 2010;21:80–9. doi: 10.1007/s12022-010-9114-y. [DOI] [PubMed] [Google Scholar]
- 15.Magro G, Cataldo I, Amico P, Torrisi A, Vecchio GM, Parenti R, et al. Aberrant expression of TfR1/CD71 in thyroid carcinomas identifies a novel potential diagnostic marker and therapeutic target. Thyroid. 2011;21:267–77. doi: 10.1089/thy.2010.0173. [DOI] [PubMed] [Google Scholar]
- 16.Sethi K, Sarkar S, Das S, Mohanty B, Mandal M. Biomarkers for the diagnosis of thyroid cancer. J Exp Ther Oncol. 2010;8:341–52. [PubMed] [Google Scholar]
- 17.Fryknäs M, Wickenberg-Bolin U, Göransson H, Gustafsson MG, Foukakis T, Lee JJ, et al. Molecular markers for discrimination of benign and malignant follicular thyroid tumors. Tumour Biol. 2006;27:211–20. doi: 10.1159/000093056. [DOI] [PubMed] [Google Scholar]
- 18.Mechtersheimer G, Möller P. Expression of the common acute lymphoblastic leukemia antigen (CD10) in mesenchymal tumors. Am J Pathol. 1989;134:961–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Moritani S, Kushima R, Sugihara H, Bamba M, Kobayashi TK, Hattori T. Availability of CD10 immunohistochemistry as a marker of breast myoepithelial cells on paraffin sections. Mod Pathol. 2002;15:397–405. doi: 10.1038/modpathol.3880536. [DOI] [PubMed] [Google Scholar]
- 20.Iwaya K, Ogawa H, Izumi M, Kuroda M, Mukai K. Stromal expression of CD10 in invasive breast carcinoma: A new predictor of clinical outcome. Virchows Arch. 2002;440:589–93. doi: 10.1007/s00428-002-0639-4. [DOI] [PubMed] [Google Scholar]
- 21.Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33:1175–81. doi: 10.1053/hupa.2002.130104. [DOI] [PubMed] [Google Scholar]
- 22.McCluggage WG, Oliva E, Herrington CS, McBride H, Young RH. CD10 and calretinin staining of endocervical glandular lesions, endocervical stroma and endometrioid adenocarcinomas of the uterine corpus: CD10 positivity is characteristic of, but not specific for, mesonephric lesions and is not specific for endometrial stroma. Histopathology. 2003;43:144–50. doi: 10.1046/j.1365-2559.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- 23.Tomoda C, Kushima R, Takeuti E, Mukaisho K, Hattori T, Kitano H. CD10 expression is useful in the diagnosis of follicular carcinoma and follicular variant of papillary thyroid carcinoma. Thyroid. 2003;13:291–5. doi: 10.1089/105072503321582105. [DOI] [PubMed] [Google Scholar]
- 24.Yegen G, Demir MA, Ertan Y, Nalbant OA, Tunçyürek M. Can CD10 be used as a diagnostic marker in thyroid pathology? Virchows Arch. 2009;454:101–5. doi: 10.1007/s00428-008-0698-2. [DOI] [PubMed] [Google Scholar]
- 25.Rezk S, Khan A. Role of immunohistochemistry in the diagnosis and progression of follicular epithelium-derived thyroid carcinoma. Appl Immunohistochem Mol Morphol. 2005;13:256–64. doi: 10.1097/01.pai.0000142823.56602.fe. [DOI] [PubMed] [Google Scholar]
- 26.Chu P, Arber DA. Paraffin-section detection of CD10 in 505 nonhematopoietic neoplasms. Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol. 2000;113:374–82. doi: 10.1309/8VAV-J2FU-8CU9-EK18. [DOI] [PubMed] [Google Scholar]
- 27.Yasuda M, Itoh J, Satoh Y, Kumaki N, Tsukinoki K, Oqane N, et al. Availability of CD10 as a histopathological marker. Acta Histochem Cytochem. 2005;38:17–24. [Google Scholar]


