Abstract
Background:
Amoxicillin is a semisynthetic antibiotic, which is used as an antimicrobial drug. This study was designed to formulate amoxicillin effervescent tablets, aimed at improved patient compliance and increased drug stability.
Materials and Methods:
In this study, nine effervescent tablet formulations were prepared from amoxicillin trihydrate. The effervescent base was comprised of various amounts of citric acid and sodium bicarbonate. Powders and granules were evaluated for their particle size, bulk density, tapped density, compressibility index, Hausner's ratio and angle of repose. The effervescent tablets were then prepared from powders and granules of acceptable quality by direct compression and fusion methods. The tablets were evaluated for weight variation, friability, pH of solution, carbon dioxide (CO2) content, hardness, effervescence time, thickness, assay, content uniformity, water content and equilibrium moisture content.
Results:
The results indicated better flowability of granules prepared by fusion method as compared with the direct compression. The percent weight variations of tablets were within the acceptable limit of 0.5%. The friability was less than 1% in all formulations. The solution pH of tablets prepared by direct compression and fusion methods ranged from 4.55 to 5.74 and 4.74-5.84, respectively. The CO2 amounts generated by of fusion method tablets were smaller as compared to the direct compression method. The hardness of tablets was 40.66-56 for direct compression method and 60.6-74.6 for fusion method. The tablets produced by the fusion method had a larger thickness and lower water content than tablets produced by direct compression method.
Conclusion:
Tablets prepared by the fusion method exhibited superior pre- and post-compression characteristics as compared to tablets prepared by direct compression method.
Keywords: Amoxicillin, direct compression method, effervescent tablets, fusion method
INTRODUCTION
As per revised definition proposed to US Food and Drug Administration “effervescent tablets are tablet intended to be dissolved or dispersible in water before administration.”[1] Effervescent tablets are uncoated tablets that contain acid substances (citric, tartaric, malic acid or any other suitable acid or acid anhydrate) and carbonates or bicarbonates (sodium, potassium or any other suitable alkali metal carbonate or bicarbonate) that react rapidly in the presence of water by releasing carbon dioxide (CO2).[2] Occasionally, an active ingredient itself could act as the acid or alkali metal component for effervescent reaction. Effervescent tablets have some advantages over suspension dosage forms. They are already in solution at the time they are consumed; thus, the drug absorption is faster and more complete. Faster absorption means faster onset of action so, they are helpful in treating acute symptoms such as infections and pain.[3,4,5] They are more easily transported; good stability is inherent with the effervescent formulations. This kind of drug delivery has predictable and reproducible pharmacokinetic profiles.[6] Effervescent systems provide a pleasant taste in comparison to liquids, mixtures and suspensions because carbonation helps to mask the bad taste of drugs and effervescent tablets retain their flavor after lengthy storages.[7] Effervescence reaction is favored by many patients, especially children; thus, patients are more likely to complete their course of treatment.[8] Furthermore, some pharmaceuticals such as antibiotics and amino acids are sensitive to changes in gastric pH and low gastric pH can lead to their destruction, resulting in their reduced activity. Effervescent products can increase gastric pH and avoid or minimize the destruction of materials, prone to gastric pH.[9,10,11]
Amoxicillin (α-amino-hydroxi benzyl penicillin) is a semisynthetic antibiotic; belonging to β-lactam family. Amoxicillin is effective against many different bacteria including Heamophilus influenza, Neisseria gonorrhea, Escherichia coli, Salmonella, Proteus mirabilis, Enterococci, Streptococci, Listeria, Helicobacter pylori and certain strain of Staphylococci. It inhibits cross-linkage between peptidoglycan polymer chains that make up a major component of the cell walls of both gram-positive and gram-negative bacteria. Amoxicillin is used for infections of middle ear, tonsils, upper and lower respiratory tract, urinary tract, skin and stomach in adult and pediatric patients.[12,13,14]
Amoxicillin is commercially available in trihydrate form. Amoxicillin is used as powder for suspension at two strengths of 125 mg and 250 mg in children. An important disadvantage of suspensions is inter-dose variability, often seen when patient forgets shaking the bottle before taking the medication. Phase separation and difficulty of reconstituting suspension are other problems of the dosage form. The therapeutic effect of amoxicillin suspensions may change and decline from the 1st day to the last day of treatment. In addition, patients may avoid carrying the medication with them when away from home, resulting in poor compliance and the failure of therapy.
Therefore, this study was designed to develop effervescent tablets as an alternative dosage form of amoxicillin for achieving improved patient compliance and drug stability.
MATERIALS AND METHODS
Chemicals
Amoxicillin trihydrate was provided from Farabi Pharmaceutical Company (Isfahan, Iran). Sodium bicarbonate, citric acid, tartaric acid, polyvinylpyrrolidone (PVP), mannitol, aspartame and polyethylene glycol (PEG-6000) were provided from Merck Company (Germany).
Preparation of the standard curve of amoxicillin trihydrate
A total of 10 mg amoxicillin was added to phosphate buffer pH 5 in a 100 ml volumetric flask to obtain drug concentration of 100 μg/ml. From this solution, concentrations of 10, 15, 20, 25, 30 and 40 μg/ml were made and their absorbances determined by a ultraviolet spectrophotometer at 229.8 nm against blank. This experiment was repeated for 3 days to evaluate intra and inter day variations.[15]
Direct compression method
Fine powder of amoxicillin trihydrate was prepared to slug. Then, the slugs crushed and passed through a sieve No. 20. Other components were weighed and mixed with amoxicillin granules to obtain a homogeneous powder. Finally, lubricant (PEG 6000) was added to the mixed powder.[5,16]
Fusion method
Certain amount of citric acid and sodium bicarbonate were mixed together and heated at 54°C for about 15 min. The granules were dried for ½ h in the oven at 60°C. Finally, amoxicillin dry granules and remaining ingredients of formulation were added and mixed, to which the lubricant was added.[16,17]
Pre-compressional evaluation
Determination of particle size
Sieve method was used for this test. Sieves of different meshes (20, 25, 30, 35, 40, 70 and 100) were selected and placed on top of each other. 100 g of powder was placed on the upper sieve. After 10 min of mild shaking the amount remaining on each sieve was collected. Average diameter of powder was calculated by the following equation:[18,19]
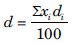
xi = Average size of both the upper and lower sieve
di = Percent of the value i in that range of bulk.
Bulk density
A quantity of accurately weighed powder from each formula was introduced in to the measuring cylinder and then the volume was noted. Bulk density was expressed in g/ml and determined by the following formula:[20]
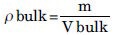
Tapped density
A quantity of accurately weighed powder from each formulation was introduced into a measuring cylinder. The cylinder was hit from the height of 2.5 cm every 2 s, up to volume plateau. Tapped density was calculated from the following formula:[20]
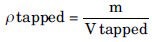
Compressibility index and Hausner's ratio
These terms explain flow properties of the powders. Compressibility index was expressed in percentage. They were given by the following equation:[15]
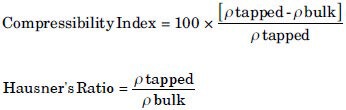
Angle of repose
It was measured by fixed funnel method. A funnel was secured with its tip at a given height H, above graph paper that was placed on a flat horizontal surface. Granules were carefully poured through the funnel until the apex of the conical pile just touches the tip of the funnel. The angle of repose (α) was then calculated by measuring the height (h) and radius (r) of the heap of the formed powder or granules and putting the values in the equation.[20]
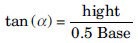
Where, α = Angle of repose.
Post-compressional evaluation
Weight variation
Twenty tablets were weighed individually and the average weight was calculated. The individual weights were then compared with the average weight. The tablets will pass the test if not more than two tablets fall outside the percentage limit and none of tablet differs by more than double percentage limit.[6,21]
Friability
A total of 20 tablets were weighed and placed in the friabilator (Erweka, TAP, Germany) and then operated at 25 rpm for 4 min. The tablets were then weighed again. The difference in the two weights was used to calculate friability as follows:[15]
Friability percent = (Weight of tablets before test − Weight after test)/(Weight of tablets before test) ×100.
pH of solution
One tablet was dissolved in purified water. After complete dissolution, the solution pH was measured by a pH meter (Metrohm, 632, Switzerland). This test was repeated 3 times for each formulation.[19]
Carbon dioxide content
One effervescent tablet was dissolved in 100 ml sulfuric acid 1 N and weight variation was measured before and after dissolution. CO2 content was presented as mg. This experiment was conducted on three tablets for each formulation.[19]
Hardness
The tablet hardness was determined by Erweka (24-TB, Germany).[6,15]
Effervescence time
A tablet was placed in a glass containing purified water and effervescent time was measured by a stopwatch.[19]
Thickness
Thickness was measured by using a calibrated dial caliper. 10 tablets of each formulation were evaluated.[7]
Assay
A tablet was placed in a 100 ml volumetric flask and dissolved in phosphate buffer pH 5. After dilution, the amount of the drug was determined by UV spectrophotometer at 229.8 nm against blank. 10 tablets of each formulation were evaluated.[15,20]
Content uniformity
After selecting 10 tablets randomly, the content of each tablet was separately determined. Then, the above procedure was followed.[15]
Water content
A total of 10 tablets of each formulation were weighed before and after placing in a desiccator containing activated silica gel for 4 h. The percentage of their water content was calculated from the following equation:[19]
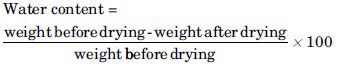
Equilibrium moisture content
Three tablets were placed in three desiccators containing saturated saline solutions, potassium nitrate relative humidity (RH, 90%), sodium chloride (RH, 71%) and sodium nitrite (RH, 60%) at 18°C. After 1 and 7 day, the percent equilibrium moisture content was determined by the Karl-Fisher method using Autotitrator instrument (Mettler, TOLEDO-DL 53, Switzerland).[19,22]
RESULTS
The standard curve of amoxicillin trihydrate was linear (y = 0.026 + 0.3903 [R2 = 0.9986]) at concentrations ranging 10-40 μg/ml. Formulations comprising different stochiometric ratios of citric acid, tartaric acid and sodium bicarbonate were made to determine the pH at which amoxicillin trihydrate had a greater solubility. Formulations are listed in Table 1. In P1-P4 formulations, the amount of citric acid and sodium bicarbonate were fixed and the amount of tartaric acid was changed. In P5-P9, the amount of sodium bicarbonate was fixed and the amount of citric acid was changed according to the previous results. In P10-P18, tartaric acid was omitted from the formulation and the amount of citric acid and sodium bicarbonate were changed.
Table 1.
Preformulation of amoxicillin effervescent tablets
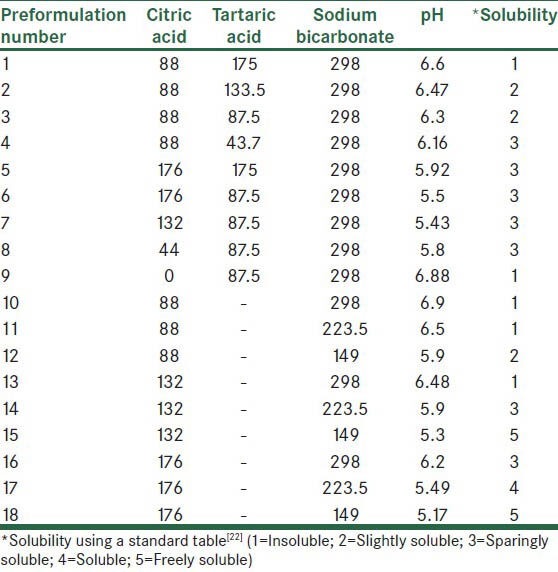
Table 2 shows the compositions of effervescent tablets of amoxicillin, prepared by two methods of direct compression and fusion. Tables 3 and 4 show the results of pre-compression tests on the mixed powders.
Table 2.
Composition of effervescent tablets prepared by direct compression and fusion methods
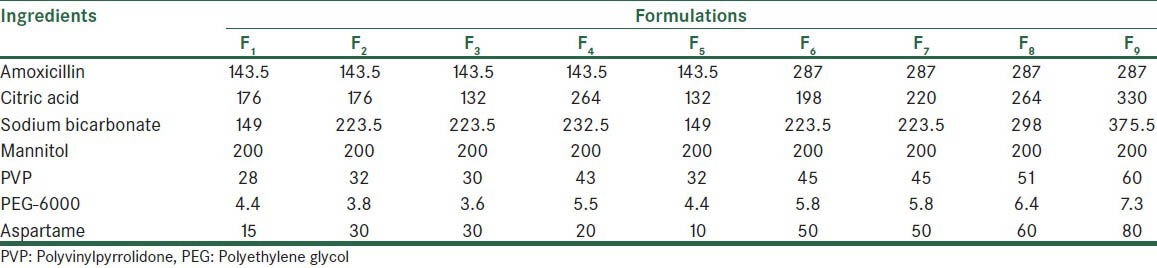
Table 3.
Physical characteristics of the mixed powders in direct compression method
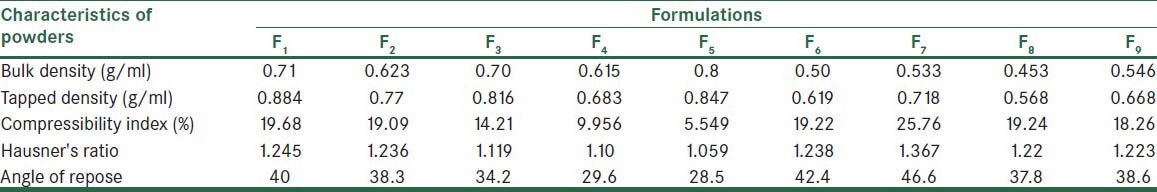
Table 4.
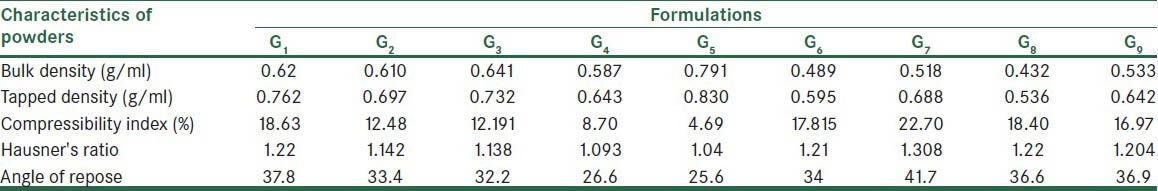
Physical characteristics of the mixed granules in fusion method
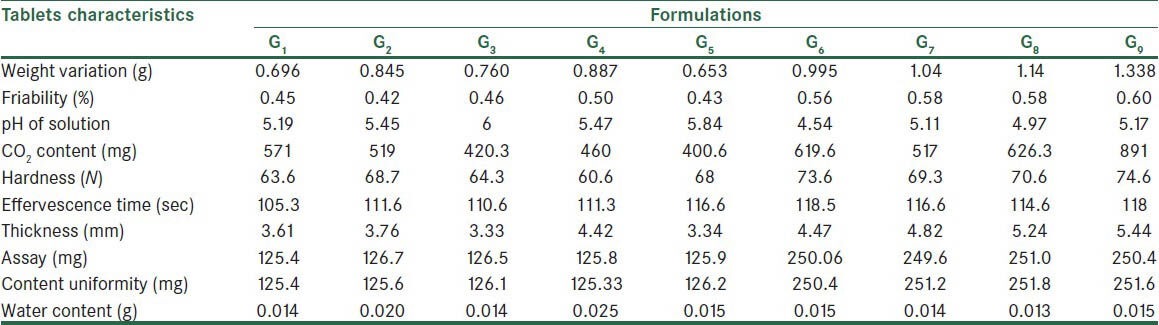
Tables 5 and 6 show the post-compression tests results on tablets. The percent weight variations of all formulations were within the acceptable limits of 0.5% of the weights. The friability was less than 1% in all formulations. In direct compression method, the pH was between 4.55 and 5.74 and for the fusion methods was between 4.74 and 5.84. The CO2 content of the tablets produced by fusion method was lower as compared to of tablets prepared by direct compression method. The hardness of the tablets found to be 40.66-56 for direct compression method and 60.6-74.6 for fusion method. The tablets produced by the fusion method were thicker. Water content in the tablets produced by direct compression method was higher.
Table 5.
Physicochemical characteristics of tablets produced by direct compression method
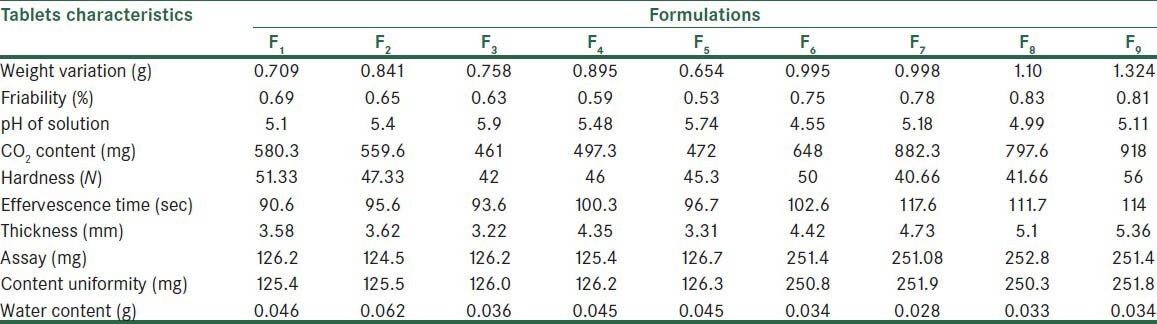
Table 6.
Physicochemical characteristics of the tablets produced by fusion method
DISCUSSION
Active pharmaceutical ingredients can be formulated effervescent tablets if they have specific characteristics including quick aqueous dissolution and absorption in the gastrointestinal. Amoxicillin is available in the market as powder for suspension at 125 mg and 250 mg/5 ml strengths for children. Furthermore, effervescent tablets are attractive and better tolerated by patients and amoxicillin is not available in this form. We decided to formulate and research amoxicillin trihydrate effervescent tablets.
According to European Pharmacopeia, amoxicillin trihydrate has a low solubility in water but good solubility in dilute acid and hydroxide solutions. Different formulations were made from the combination of citric acid, tartaric acid and sodium bicarbonate. When tartaric acid was employed to a greater extent than citric acid, the resulting solution was turbid and a large amount of sediment remained in the bottom of glass. When only citric acid was used as the acid ingredient, the resulting solution was clear and no sediment was remained, but when tartaric acid was used, the resulting solution was turbid and a large amount of sediment was remained. Consequently, tartaric acid was omitted from the effervescent base formulation. Pre-formulation studies indicated that amoxicillin had greater solubility at pHs about 5. The effervescence time was also determined. Thus, formulations that produced lower amounts of sediment were selected.
The effervescence time for effervescent tablet is directed in the British Pharmacopeia to be measured in 200 ml water. However, the amount of water used in our testing was decreased as possible.[19] Since the dosage forms developed were aimed for children, we demonstrated in pre-formulation tests that an effervescent tablet of amoxicillin trihydrate could be dissolved in 50 ml water. Thus, the following tests were carried out in 50 ml water.
To increase amoxicillin solubility PVP was used. The amount of PVP was increased from 1% to 6%, at which only a very small amount of sediment was produced that was dissolved over 1 min.[23] Furthermore, water temperature had a direct effect on the dissolution of effervescent tablets.
Size of the solid particles in pharmaceutical product is the most important factor to achieve a desired pharmaceutical product. The effervescent granules had greater particle size than that of the effervescent powders. The mean diameters in F1-F9 formulations were 299.54-392.79 μ and for G1-G9 formulations were 322.63-414.22 μ. All formulations had normal distribution. Normal distribution graphs of the formulation 1 for the both methods are shown in Figures 1 and 2. Previous studies also reported similar findings.[23,24]
Figure 1.
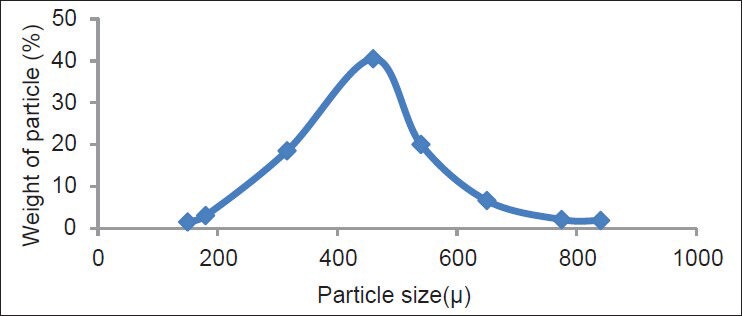
Particle size distribution of F1 granules prepared by direct compression method
Figure 2.
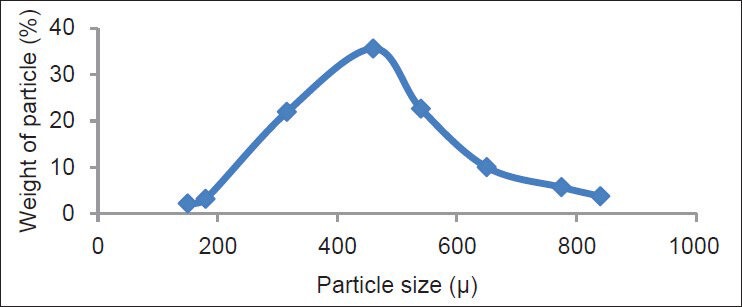
Particle size distribution of G1 granules prepared by fusion method
Bulk and tapped densities in granules due to increased porosity and decreased cohesion force were less than that of the powders. The results are shown in the Tables 4 and 5. Granulation by fusion method produced granules with lower tendency for adhesion, due to their lesser moisture content. On the other hand, the larger particle size of granule than powders resulted in decreased contact area of particles. Therefore, friction between particles decreased and so flowability increased. Angle of repose in the formulations of effervescent granules was in the range of 30-45, indicating acceptable to good flowability. Other studies suggested similar findings.[23,24]
The maximum percentage of weight variation was 5%, which was in the acceptable range of the USP. Weights of tablets prepared by the two methods were not out of this range. One of the main reasons for weight uniformity is appropriate flowability of powders. Tablets prepared from the effervescent granules had small weight variations due to their better flowability.
Actually, granules are secondary particles that made from the primary particles and have porous structure. Therefore, in addition to physical changes in the primary particles, the physical changes in the granules provided better compressibility of granules, resulting in production of the effervescent tablets of suitable hardness.
However, the hardness of tablets cannot demonstrate physical strength of tablets exclusively. To evaluate the physical strength of tablets, in addition to the hardness test, the friability test was also performed. Owing to the light weight of the tablets, all formulations had friability of less than 1%. In the other studies were shown that wet granulation technique could improve compressibility and flowability properties and hardness that the results were in agreement with this study.[23,24]
Since amoxicillin showed a greater solubility at pH 5, the pH of all formulations was adjusted around this value. This pH increases gastric pH temporarily, resulting in shorter gastric residence time of the dosage form. Therefore, drug can be absorbed from the small intestine more effectively. In other studies on effervescent granules containing citric acid and sodium bicarbonate has been done solutions pH, which is obtained from dissolving granules, was measured at about 5.8. It is comparable with the results in this study.[23,24]
The CO2 content of the effervescent granules was less than that of the effervescent powders. This difference may be caused by the manufacturing process of the granules. In the fusion method, water can be released during the process, mediating production of CO2 due to the reaction of partial amounts of citric acid and sodium bicarbonate present in the formulation. The amount of gas depends on the amount of base too.
Effervescence time was between 90.6 s and 114 s for the direct compression method and between 105 s and 118.5 s for the fusion method, these ranges were acceptable for both methods according to the British Pharmacopeia.[19] The shorter effervescence time of tablets produced by the fusion method basically reflects the impact of the process employed.[24]
As compared to the direct compression method, tablets produced by the fusion method were thicker due to greater internal porosity of the granules. Tablets had uniform thickness, which shows that the force used by the tableting machine was uniform.
All formulations had passed assay test successfully. Content uniformity was in an acceptable range, which shows powders and granules were uniformly mixed before tableting.
During drying stage in the fusion method, the unbound water in granules evaporated, which resulted in a lower than tablets produced by the direct compression method. The water content of all effervescent tablet formulations; however, was in the acceptable range of 5% or less.
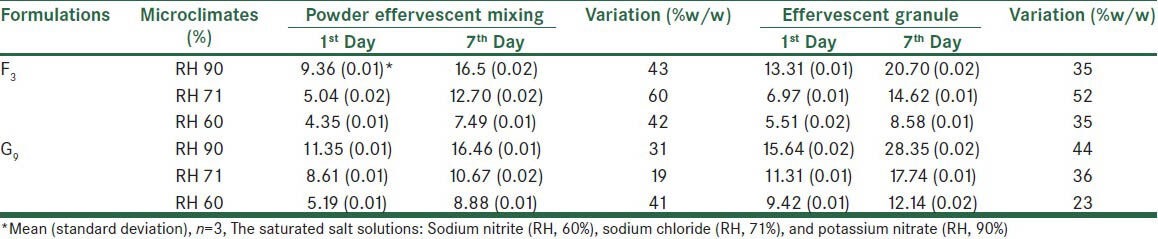
Equilibrium moisture content of a solid is exposed to ambient air changes by changing RH. Formulations of effervescent granules absorbed more moisture because they lost their water in fusion procedure [Table 7].
Table 7.
Equilibrium moisture content (%) in the effervescent powder and granular mixing of the F3 and G9 in temperature 18°C
CONCLUSION
Effervescent amoxicillin trihydrate tablets were prepared by two methods as alternative dosage forms for treatment of bacterial infections in children.
It can be concluded from the results of this study that granules from the fusion method have better flowability and compressibility. Tablets of both dose strengths prepared by the fusion method had better physical characteristics as compared to those prepared by the direct compression method. Amoxicillin showed highest solubility at pH 5. Increasing PVP content of the formulations increased the drug solubility. Our preliminary studies indicate that, in general, the effervescent formulations of amoxicillin exhibited acceptable compendia characteristics and can be introduced as an alternative to amoxicillin suspension, following further investigations.
ACKNOWLEDGMENT
This study was supported by Isfahan University of Medical Sciences as a thesis research project numbered 390180.
Footnotes
Source of Support: This study was supported by Isfahan University of Medical Sciences as a thesis research project numbered 390180
Conflict of Interest: None declared.
REFERENCES
- 1.Palanisamy P, Abhishehn R, Yoganand KD. Formulation and evaluation of effervescent tablets of aceclofenac. Inter Res J Pharmacol. 2011;2:185–90. [Google Scholar]
- 2.5th ed. Strasbourg Cedex, France: Council of Europe; 2007. European Pharmacopeia; p. 749. 1193. [Google Scholar]
- 3.Altomare E, Vendemiale G, Benvenuti C, Andreatta P. Bioavailability of a new effervescent tablet of ibuprofen in healthy volunteers. Eur J Clin Pharmacol. 1997;52:505–6. doi: 10.1007/s002280050327. [DOI] [PubMed] [Google Scholar]
- 4.Hedges A, Kaye CM, Maclay WP, Turner P. A comparison of the absorption of effervescent preparations of paracetamol and penicillin V (phenoxymethylpenicillin) with solid dose forms of these drugs. J Clin Pharmacol. 1974;14:363–8. doi: 10.1002/j.1552-4604.1974.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee RE. Effervescent tablets. [Last accessed on 2011 Dec 20]. Available from: http://www.amerilabtech.com/wp-content/uploads/EffervescentTabletsKeyFacts.pdf .
- 6.Lachman L, Liberman HA, Kaning JL. 3rd ed. Philadelphia: Lea and Febiger; 1987. The theory and practice of industrial pharmacy; pp. 334–410. [Google Scholar]
- 7.Srinath KR, Pooja Chowdary C, Palaniamy P, Krishna V, Aparna S. Formulation and evaluation of effervescent tablets of paracetamol. Inter J Pharmacol Res Dev. 2011;3:76–104. [Google Scholar]
- 8.Strickley RG, Iwata Q, Wu S, Dahl TC. Pediatric drugs – A review of commercially available oral formulations. J Pharm Sci. 2008;97:1731–74. doi: 10.1002/jps.21101. [DOI] [PubMed] [Google Scholar]
- 9.Hespe W, Verschoor JS, Olthoff M. Bioavailability of new formulations of amoxicillin in relation to its absorption kinetics. Arzneimittelforschung. 1987;37:372–5. [PubMed] [Google Scholar]
- 10.Amela J, Salazar R, Cemeli J. Effervescent tablets of ascorbic acid I. Physical study of the possible components to be used. Drug Dev Ind Pharm. 1996;22:407–16. [Google Scholar]
- 11.Eichman JD, Robinson JR. Mechanistic studies on effervescent-induced permeability enhancement. Pharm Res. 1998;15:925–30. doi: 10.1023/a:1011936901638. [DOI] [PubMed] [Google Scholar]
- 12.Kaye CM, Allen A, Perry S, McDonagh M, Davy M, Storm K, et al. The clinical pharmacokinetics of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate. Clin Ther. 2001;23:578–84. doi: 10.1016/s0149-2918(01)80061-8. [DOI] [PubMed] [Google Scholar]
- 13.Cooreman MP, Krausgrill P, Hengels KJ. Local gastric and serum amoxicillin concentrations after different oral application forms. Antimicrob Agents Chemother. 1993;37:1506–9. doi: 10.1128/aac.37.7.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.File TM., Jr The development of pharmacokinetically enhanced amoxicillin/clavulanate for the management of respiratory tract infections in adults. Int J Antimicrob Agents. 2007;30(Suppl 2):S131–4. doi: 10.1016/j.ijantimicag.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Hoizey G, Lamiable D, Frances C, Trenque C, Kaltenbach M. Simultaneous determination of amoxicillin and clavulanic acid in human plasma by HPLC with UV detection. J Pharm Bio Anal. 2002;30:661–6. doi: 10.1016/s0731-7085(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 16.Kraus G, Shimidt PC. Optimization of an effervescent formulation using a central composite design optimization of an effervescent tablet formulation containing spray dried L-lucine and polyethylene glycol 6000 as lubricants using a central composite design. Eur J Pharm Biopharm. 1998;46:85–94. doi: 10.1016/s0939-6411(97)00154-9. [DOI] [PubMed] [Google Scholar]
- 17.Murray RB. New approach to the fusion method for preparing granular effervescent products. J Pharm Sci. 1968;57:1776–9. doi: 10.1002/jps.2600571032. [DOI] [PubMed] [Google Scholar]
- 18.Yanze FM, Duru C, Jacob M. A process to produce effervescent tablets: Fluidized bed dryer melt granulation. Drug Dev Ind Pharm. 2000;26:1167–76. doi: 10.1081/ddc-100100988. [DOI] [PubMed] [Google Scholar]
- 19.Moghimipour E, Akhgari A, Ghassemian Z. Formulation of glucosamine effervescent granules. Sci Med J. 2010;9:21–34. [Google Scholar]
- 20.21st ed. Eastrol, Pennsylvania: Mack Publishing Company; 2006. Remington's Pharmaceutical Sciences, the science and practice of pharmacy; pp. 895–6. 900, 916-7. [Google Scholar]
- 21.29th ed. Washington: The United State Pharmacopeia and Convention; 2006. The United State Pharmacopeia and National Formulary; p. 9. 209, 640-1, 675, 3157-8. [Google Scholar]
- 22.Kschmit M, Heinz-Dieter I. Fischer titration a method for determination the true water content of cereals. Fresenius J Anal Chem. 1998;360:465–9. [Google Scholar]
- 23.Aslani A, Fattahi F. Formulation, characterization and physicochemical evaluation of potassium citrate effervescent tablets. APB. 2013;3:217–25. doi: 10.5681/apb.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslani A, Jahangiri H. Formulation, characterization and physicochemical evaluation of effervescent ranitidine hydrochloride tablets. APB. 2013;3:315–22. doi: 10.5681/apb.2013.051. [DOI] [PMC free article] [PubMed] [Google Scholar]


