Abstract
Background:
Regarding the modulatory effects of tamoxifen (TAM) on the actions of estrogen in the present study, the effects of TAM on learning, memory and brain tissues oxidative damage in ovariectomized (OVX) and naοve female rats was investigated.
Materials and Methods:
The animals were divided into: (1) Sham, (2) OVX, (3) Sham-tamoxifen (Sham-TAM) and (4) ovariectomized-tamoxifen (OVX-TAM). The animals of the Sham-TAM and OVX-TAM groups were treated by TAM (1 mg/kg; 4 weeks).
Results:
In Morris water maze, the escape latency in the OVX group was higher than in the Sham group (P < 0.01). The time latency in the animals of OVX-TAM group was lower than that of OVX group (P < 0.01); however, there were no significant differences between the Sham-TAM and Sham groups. In the probe trial, the time spent in target quadrant (Q1) by the animals of OVX group was lower than that of Sham group (P < 0.01). Interestingly, the animals of OVX-TAM group spent more times in target quadrant (Q1) compared with OVX group (P < 0.01). In passive avoidance test, the animals of OVX group had lower latencies to enter the dark compartment compared with the Sham group (P < 0.05). The time latency to enter the dark compartment by animals of OVX-TAM group was higher than in OVX group (P < 0.01). In OVX-TAM group, the total thiol concentration was significantly higher (P < 0.05) and malondialdehyde concentration was lower (P < 0.01) than OVX group.
Conclusions:
These results allow us to propose that TAM enhances learning and memory of OVX rats. The possible mechanism may be due to the protective effects against brain tissues oxidative damage.
Keywords: Learning, memory, morris water maze, ovariectomy, oxidative damage, passive avoidance, tamoxifen
INTROUDUCTION
Alzheimer's disease (AD) has been recognized as the most common cause of sporadic dementia, involving about 13 million people world-wide.[1] Studies have revealed a significantly higher incidence of AD in postmenopausal women. The neuroprotective effects of estrogen, leading to an increase of interest to evaluate its role in cognitive functioning or dementia.[2] Estrogen can inhibit the accumulation of neurotoxic glutamate or amyloid-beta peptide and it also possesses an antioxidant property; therefore, it protects against cognitive dysfunction of AD.[3,4]
The possibility of using selective estrogen receptor modulators (SERMs) to exert estrogen-like neuroprotective actions in the brain has emerged their use as an alternative to estradiol.[5,6] SERMs bind to estrogen receptors and induce specific changes in their three-dimensional conformation allowing a tissue-selective recruitment of transcriptional cofactors.[7] SERMs may also exert neuroprotective actions by the control of local brain inflammation, which is mainly regulated by microglia and astroglia.[5] Triphenylethylene SERMs, such as tamoxifen (TAM) and its derivatives are also known as the first-generation of SERMs.[7] TAM, a synthetic, non-steroidal estrogen receptor modulator is used extensively in the treatment of breast cancer.[8] In central nervous system, TAM antagonizes estrogen receptors and induces cell death in a variety of cells.[9] While, estrogens enhance the development of hippocampal neurons in CA1 region, it inhibits the development and decreases cell number in the hippocampus.[9,10] However, some human studies also suggest that TAM may decrease the risk of AD.[11] The neuroprotective actions of TAM in different forms of neural injury have also been reported.[4,12,13] In the previous studies, it has been shown that TAM significantly impaired learning and memory abilities in passive avoidance tests in rat.[14] SERMs have also been suggested to have pro-oxidative effects; however, it seems that they sometimes protect tissues against oxidative damages.[15,16,17] Regarding to these controversial findings, the present study was carried out to evaluate the effect of TAM on learning and memory of ovariectomized (OVX) and naïve female rats using Morris water maze (MWM) and passive avoidance test. Brain tissues oxidative damage was also evaluated as a possible mechanism.
MATERIALS AND METHODS
Animals and drugs
In the present study, 60 female Wistar rats, 12-week-old (240 ± 10 g) were used. The animals were housed in 4-5 per standard cages, at room temperature (22 ± 2°C) on a 12 h light/dark cycle. Food and water were available ad libitum properly. Animal handling and all related procedures were approved by the Mashhad Medical University Committee on Animal Research. The animals were divided into four groups: (1) Sham (Sham) (n = 20), (2) OVX (n = 10), (3) Sham-TAM (n = 20) and (4) ovariectomized-tamoxifen (OVX-TAM) (n = 10). In Sham and Sham-TAM groups, 9-10 animals, which had proestrus phase were selected and used for the behavioral studies. The animals in the Sham-TAM and OVX-TAM groups were treated by daily injections of TAM (1 mg/kg; i.p.) for 4 weeks before training in the water maze or passive avoidance test. The animals of Sham and OVX groups received 1 ml/kg of saline instead of TAM.
Ketamine was purchased from Alfasan Company (Holand). TAM was kindly provided by Iran Hormone Company (Tehran, Iran). Other chemicals, which were used for malondialdehyde (MDA) and total thiol concentrations, were purchased from Merck Company.
Surgery
Before surgery, the rats were permitted 15 days for acclimatization to the animal house. The animals were OVX under ketamine anesthesia (150 mg/kg, i.p.).[18] Anesthesia was confirmed by reduced respiratory rate and no response to gentle pinching of foot pad. Abdominal incision was made through the skin of the flank of rats and ovaries and ovarian fats were removed. Ovaries were isolated by ligation of the most proximal portion of the oviduct before removal. The same procedure was performed on the sham rats except that the wound was closed without removing the ovaries.[19]
Vaginal cytology
It was carried out in Sham and Sham-TAM groups to select the animals with proestrus stage for behavioral studies. The female rat estrus cycle is 4-5 days and includes 4 phases: (1) Proestrus stage where estrogen levels are very high and its typical cell pattern is smears include primarily epithelial cells with large nuclei. (2) Estrus stage, typical cell pattern for this stage is that the smears contain primarily cornified epithelial cells. (3) Metaestrus stage comprises primarily cornified cells and sometime a few epithelial cells. (4) Diestrus with typical cell pattern consists of large numbers of leukocytes with scattered nucleated epithelial and cornified cells in smears. To ensure that the female rats were cycling, vaginal cytologies were started 1 week before each experience and continued every day. Rats were held in a non-stress position and quickly lavaged with approximately 1 ml of saline. Slides were read using light microscopy and estrus categories were classified based on cytological characteristics.[20]
MWM apparatus
The MWM was a black circular pool with a diameter of 136 cm and a height of 60 cm, filled with 24 ± 1°C water to a depth of 30 cm. The maze was divided geographically into four equal quadrants and release points were designed at each quadrant as North (N), East (E), South (S), and West (W). A hidden circular platform (10 cm in diameter), made of plexiglass, was located in the center of the southeast quadrant, submerged 1.5 cm beneath the surface of the water. Fixed, outside of the maze visual cues were present at various locations around the maze (i.e., computer, hard wares and posters). A camera was mounted above the center of the maze. A tracking system was used to measure the escape latency and traveled path.[21,22]
MWM test
The MWM task for testing spatial memory was assessed in a water tank as described previously with a minor modification.[23,24] Each rat participated in 16 trials, which were organized into four blocks of four trials (1 trial/start position within a block). Each block was considered as a separate test session and the blocks were separated by 30 min. The platform, situated in the center of the southeast quadrant, was submerged 1.5 cm below the surface of water and therefore invisible. A trial was started by placing a rat into the pool, facing the wall of the tank. Each of four starting positions (N, E, S, W) was used once in a series of four trials and their order was randomized. Each trial was terminated as soon as the rat had climbed onto the platform or when 60 s had elapsed. The animal was allowed to stay on the platform for 15 s. Then, it was taken from the platform and the next trial was started after 20 s. Rats that did not find the platform within 60 s, were put on the platform by the experimenter and were allowed to stay there for 15 s.[21,23,24] The escape latency and traveled path calculated by a computer. In the retention phase, the platform was removed and a 60 s probe trial was conducted to examine how well the rats had learned the exact location of the platform. The animals were allowed to swim for 60 s. The time spent in the target quadrant (Q1) and non-target quadrants (Q2-Q4) was compared between groups.
Passive avoidance test
Passive avoidance learning test is based on negative reinforcement. The apparatus consisted of a light and a dark compartment with a grid floor adjoining each other through a small gate. The rats were accustomed to the behavioral apparatus for 5 min during 2 consecutive days before the training session. On the 3rd day, the animals were placed in light compartment and the time latency to enter the dark compartment was recorded. On a training trial, the rats were placed in the light compartment facing away from the dark compartment. When the rats were entered completely into the dark compartment, they received an electric shock (1 mA, 2 s duration). Then, the rats were returned to their home cage. 1 and 24 h later, the rats were placed in the light compartment and the latency time to enter the dark compartment as well as, the times spent by the animals in dark and light compartments was recorded and defined as retention trial.[25]
Biochemical assessment
Finally, the animals were sacrificed and the cortical and hippocampal tissues were removed, weighed and submitted to determination of total thiol (Sulfhydryl; SH) groups and MDA concentrations. Total SH groups were measured using 2, 2’-dinitro-5,5’-dithiodibenzoic acid (DTNB) as the reagent. This reagent reacts with the SH groups to produce a yellow colored complex, which has a peak absorbance at 412 nm.[26] Briefly, 1 ml Tris-ethylenediaminetetraacetic acid (Tris-EDTA) buffer (pH = 8.6) was added to 50 μl brain homogenate in 1 ml cuvettes and sample absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Then, 20 μl DTNB reagents (10 mM in methanol) were added to the mixture and after 15 min (stored in laboratory temperature) the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mM) was calculated from the following equation.[27]
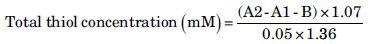
MDA levels, as an index of lipid peroxidation, were measured. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance to produce a red colored complex, which has peak absorbance at 535 nm. A total of 2 ml from reagent of TBA/trichloroacetic acid (TCA)/HCl was added to 1 ml of homogenate and the solution was heated in a water bath for 40 min. After cooling, the whole solutions were centrifuged within 1000 g for 10 min. The absorbance was measured at 535 nm.[28,29,30] MDA concentration was calculated as follows:
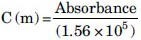
Statistical analysis
All data were expressed as means ± standard error of the mean. The data of different groups during 4 blocks were compared using repeated measures analysis of variance (ANOVA) test with Tukeys’ post-hoc between groups. The data obtained from probe trial was compared using one-way ANOVA and post-hoc test. Data for MDA and total SH groups were evaluated by one-way ANOVA and post-hoc test. Differences were considered statistically significant when P < 0.05.
RESULTS
MWM
The escape latency and traveled path in the OVX group were significantly higher than in the Sham group (P < 0.01 and P < 0.001, respectively) [Figure 1a and b]. The time latency to reach the hidden platform by the animals of OVX-TAM group was significantly lower than that of OVX group (P < 0.01); however, there were no significant differences in time latency and traveled the distance to reach the platform between the animals of the Sham-TAM compared with Sham group [Figure 1a]. The animals of OVX-TAM group also travelled significantly shorter distance to reach the platform compared with OVX group (P < 0.001). There was no significant difference between Sham-TAM and Sham groups in traveled distance [Figure 1b].
Figure 1.
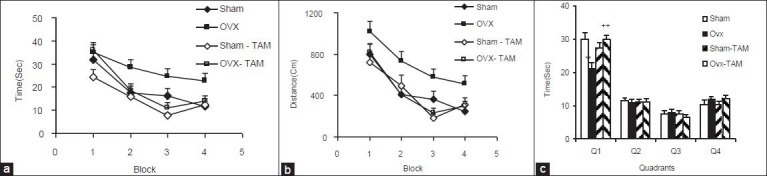
Comparison of time latency (s) (a) the length of swimming path (cm) (b) to reach the platform between Sham, ovariectomized (OVX), Sham-tamoxifen (Sham-TAM) and ovariectomized-tamoxifen (OVX-TAM) groups and the results of the time (s) spent in each quadrant during the probe trial (c). Data are presented as mean ± standard error of the mean (n = 9-10 in each group). The animals of Sham-TAM and OVX-TAM groups were treated by 1 mg/kg tamoxifenfor 4 weeks before Morris water maze test. The latency time and the length of swimming path were significantly higher in OVX group compared with Sham group (P < 0.01 and P < 0.001). The time latency and the travelled distance to reach the hidden platform by the animals of OVX-TAM group were significantly lower than that of OVX group (P < 0.01 and P < 0.001). At the prob trial, the platform was removed, and the time spent in the target quadrant (Q1) and non-target quadrants (Q2-Q4) was compared between the groups. **P < 0.01 compared to Sham group, ++P < 0.01 compared to OVX group
In the probe trial, the time spent in target quadrant (Q1) by the animals of OVX group was significantly lower than that of Sham group [P < 0.01, Figure 1c]. There was no significant difference between the Sham-TAM and Sham groups in the time spent in Q1 [Figure 1c]. Interestingly, the animals of OVX-TAM group spent more times in target quadrant (Q1) compared to OVX group [P < 0.01, Figure 1c]. As the Figure 1c showed there was no significant difference in the time spent in non-target quadrants (Q2- Q4) between four groups.
Passive avoidance test
As shown in Figure 2, before receiving shock, there was no significant difference between groups in time latency to enter the dark compartment. In OVX group, the time latency to enter the dark compartment at 1 h after receiving shock was non-significant and at 24 h after receiving shock was significantly lower than of Sham group [Figure 2, P < 0.05]. The time latency to enter the dark compartment by the animals of OVX-TAM group was higher than in OVX group at 1 h after receiving shock (P < 0.01); however, there was no significant difference at 24 h after shock between these two groups [Figure 2]. There were no significant differences in delay time to enter the dark compartment between Sham-TAM and Sham groups at 1 or 24 h after shock [Figure 2].
Figure 2.
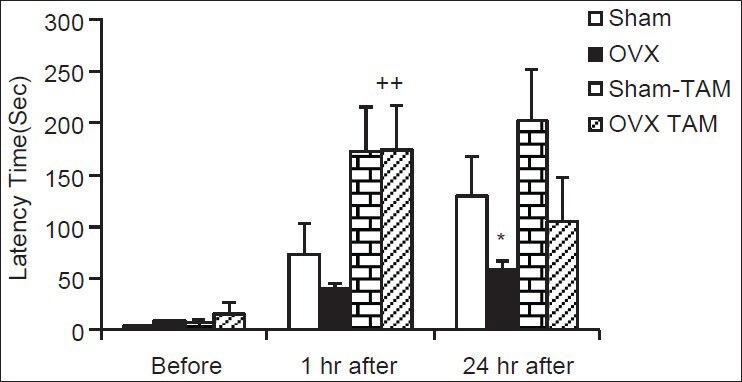
Comparison of time latency for entering the dark compartment at 1 and 24 h after receiving the shock in the experimental groups. Data are presented as mean ± standard error of the mean (n = 9-10 in each group). The animals of Sham-tamoxifen and ovariectomized-tamoxifen groups were treated by 1 mg/kg tamoxifen for 4 weeks. *P < 0.05 compared to Sham group, ++P < 0.01 compared with the ovariectomized group
There was also no significant difference before receiving shock when the total times spent in dark compartment were compared between four groups [Figure 3]. The total time spent in dark compartment by the animals of OVX group was significantly higher than sham group at 24 h after receiving shock (P < 0.001); however, there was no significant difference at 1 h after shock [Figure 3]. There was no significant difference between Sham-TAM and OVX-TAM compared to Sham and OVX groups in the total times spent in dark neither at 1 h nor at 24 h after shock [Figure 3].
Figure 3.
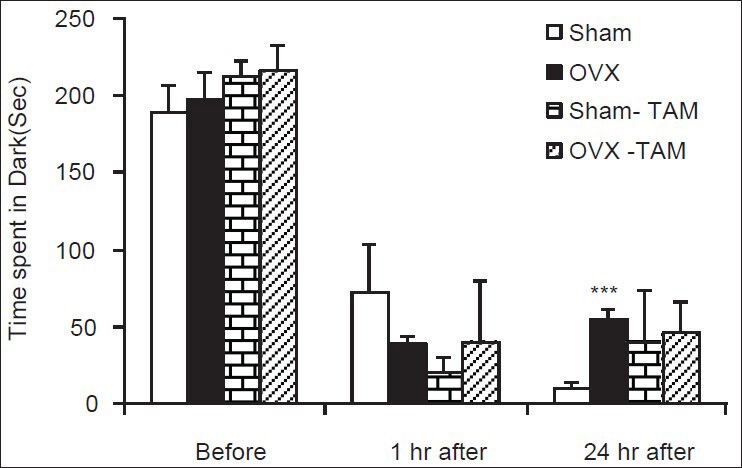
Comparison of the total time spent in the dark compartment at 1 and 24 h after receiving the shock in the experimental groups. Data are presented as mean ± standard error of the mean (n = 9-10 in each group). The animals of Sham-tamoxifen and ovariectomized-tamoxifen groups were treated 1 mg/kg tamoxifen for 4 weeks. ***P < 0.001 compared to Sham group
The Figure 4 shows that there was no significant difference in the total times spent in light compartment before shock. The total time spent in light compartment by the animals of OVX animals was lower than sham ones at both 1 and 24 h after shock [Figure 4, P < 0.01 and P < 0.001]. There was no significant difference in the total times spent in light compartment between Sham and OVX rats pre-treated by TAM compared to non-treated groups [Figure 4].
Figure 4.
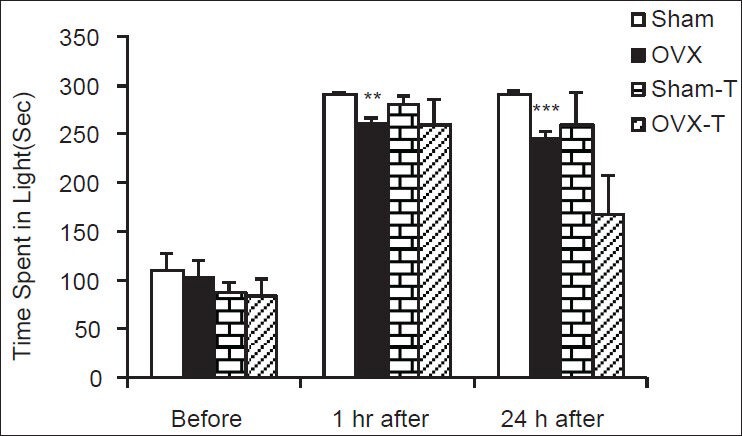
Comparison of the total time spent in the light compartment at 1 and 24 h after receiving the shock in the experimental groups. Data are presented as mean ± standard error of the mean (n = 9-10 in each group). The animals of Sham-tamoxifen and ovariectomized-tamoxifen groups were treated by 1 mg/kg tamoxifen for 4 weeks. **P < 0.01, ***P < 0.001 compared to Sham group
Biochemical results
The total thiol concentration in cortical tissues of OVX rats was significantly lower than sham animals (P < 0.01). In OVX-TAM group, the total thiol concentration was significantly higher than OVX group (P < 0.05); however, there was no significant difference between Sham-TAM and Sham groups thiol group [Figure 5a]. MDA concentration in cortical tissues of OVX animals was higher than sham operated ones (P < 0.001). Treatment of the OVX rats by 1 mg/kg TAM decreased MDA concentration in cortical tissues in comparison with vehicle treated OVX rats (P < 0.01) while there was no significant difference between Sham-TAM and Sham groups [Figure 5b].
Figure 5.
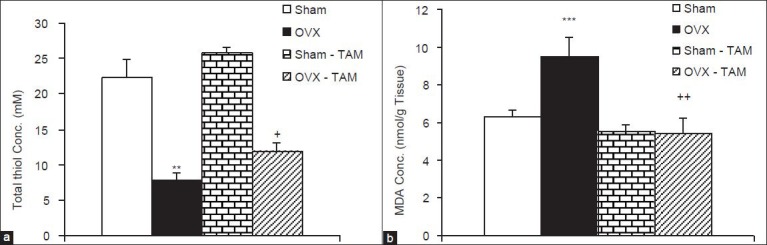
The total thiol concentrations (a) and malondialdehyde concentrations (b) in cortical tissues of 4 groups. Data are shown as mean ± standard error of the mean of 9-10 animals per group. **P < 0.01, ***P < 0.001 compared to Sham group, +P < 0.05and ++P < 0.01 compared with the ovariectomized group
The total thiol concentration in hippocampal tissue of OVX rats was non-significantly lower than that sham-operated ones [Figure 6a]. There were no significant differences between Sham-TAM and OVX-TAM groups compared with Sham and OVX groups respectively in hippocampal thiol concentrations [Figure 6a]. The MDA concentrations in hippocampal tissues of OVX group were significantly higher than that Sham group (P < 0.05); however, there was no significant differences between Sham-TAM and OVX-TAM groups compared with Sham and OVX groups respectively [Figure 6b].
Figure 6.
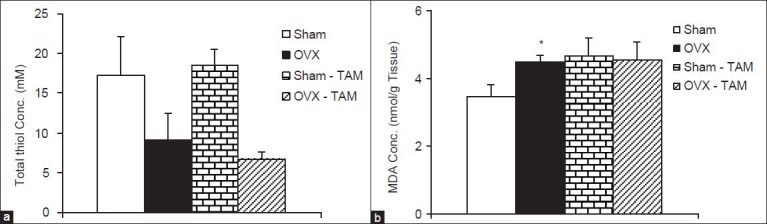
The total thiol concentrations (a) and malondialdehyde concentrations (b) in hippocampal tissues of 4 groups. Data are shown as mean ± standard error of the mean of 9-10 animals per group *P < 0.05 compared to Sham group
DISCUSSION
Modulatory effects of female and male sex hormones on the nervous system functions as well as their effects on neurological disorders such as Parkinson, epilepsy and Alzheimer have been reported.[19,31,32,33,34] Learning and memory impairments, which have been reported in OVX rats confirm the beneficial effects of endogenous estradiol on learning and memory.[35,36,37] The results of previous studies also indicated that estrogen therapy in OVX rats improves spatial learning and memory.[22,38] Other researchers also showed that estrogen administration can reverse the effects of ovariectomy on avoidance and spatial memory.[39,40] Ovarian hormone replacement therapy has been shown to have a protective effect on human memory loss due to aging.[41] In contrast to these findings, it was shown that chronic treatment of female rats with high doses of esradiol has deleterious effects on learning and memory.[36] The results of the present study showed that the female rats with preposterous phase had a better performance in MWM compared with OVX rats. The results of passive avoidance test also showed that learning and memory procedures in animals with proestrus phase were better than OVX ones. The rat estrus cycle is including proestrus, estrus, metaestrus and diestrus.[20] In proestrus phase, the estrogen levels are in the highest level. Therefore, it seems that a high level of endogenous estrogen improve learning and memory. The results of the present study confirm the memory improvement effects of endogenous ovarian hormones.[20,22,38] It has also been previously shown that females in proestrus have better learning and memory performances than those in estrus. However, the other researchers believe that ovarian hormones contribute to functional spatial memory of females in stressed conditions, but they are not necessary for intact spatial memory.[42]
As it was mentioned above, the effects of estradiol on prefrontal cortex function have been widely investigated; however, the possible effects of SERMs on prefrontal cortex-related tasks have not been full assessed. The results of the present study showed that TAM improved learning and memory impairments in OVX rats; however, it was not effective in sham animals, which have a high level of endogenous estradiol. In contrast, it is believed that postmenopausal women receiving TAM for the treatment of breast cancer have an increased risk of cognitive impairment.[43,44] Chen et al. (2002) also confirmed the memory retrieval, but not the acquisition impairments by TAM using MWM.[14]
There is also evidence that the effects of estradiol on learning and memory may change by TAM.[45,46] Other data by Rhodes and Frye (2006), imply that SERMs can enhance spatial memory in the water maze in a manner similar to estradiol.[47] It has been suggested that the effects of SERMs on memory is due to consolidation of memory.[47] It had also been reported that neither latency to enter the bright compartment nor the number of exits nor the total time in the bright compartment were changed in TAM treated mice in comparison to the control groups when the animals were examined in passive avoidance test.[48] Raloxifene and TAM at a dose of 1 mg/kg, but not at doses of 0.5 or 2 mg/kg, also improved acquisition of orchidectomized animals.[49] Any significant effect in learning and memory was also described in the MWM under acute TAM treatment of the mice.[48]
It has also been reported that like estrogen, TAM protects clonal mouse hippocampal (HT-22) cells against both glutamate and amyloid beta protein induced cell death.[50] Furthermore, Sharma and Mehra (2008) showed that estrogen or TAM therapy to the OVX rats for a period of 4 weeks was resulted in a significant decline in the percentage of apoptotic cells in the hippocampus.[51] Murphy and Segal (1996), indicated that TAM blocked the increased in hippocampal cell cultures that would be otherwise induced by estradiol.[52] However, Silva et al. (2000) showed that TAM by its own didn’t affect the hippocampal spine density.[53] Animals treated with raloxifene or TAM showed an increased numerical density of dendritic spines in CA1 pyramidal neurons compared with animals treated with vehicle.[54] Finally, it has been suggested that estradiol, TAM and raloxifene, by their modulation of N-Methyl-D-aspartate (NMDA) receptors in the hippocampal CA1 region and the cingulate cortex, may have a beneficial effect in neurological disorders such as AD and schizophrenia.[55]
It is suggested that modulatory effects of ovarian hormones on learning and memory may be due to neurotransmitter systems such as serotonergic, cholinergic, glutamatergic and nitric oxide.[22,35,36,37,56] It has also been reported that the effects of estrogen may at least in part be due to its effects as an antioxidant.[57] The results of the present study confirmed this hypothesis. In the present study, total thiol concentrations decreased and MDA concentrations were increased in brain tissues of OVX rats. Regarding these facts, we assumed that the beneficial effects of TAM on learning and memory of OVX rats which were seen in the present study may be related to its protective effects against brain tissues oxidative damage. The results showed that treatment by TAM increased thiol concentrations and decreased MDA in brain tissues. In agreement with this, TAM inhibited the increased TBA reactive substances concentrations in Hypoxic-Ischemic rat model.[58] In contrast to these findings, it has been reported that TAM increased lipid peroxidation in B6, but not in B6CBAF1.[59] It has also been reported that raloxifene protected striatal dopaminergic neurons against 6-hydroxydopamine-induced neurotoxicity in the rat. The effect was accompanied by a significant recovery in behavior tests, significant attenuation of neuronal apoptosis and oxidative stress.[57] Oral administration of raloxifene (60 mg/day) for 3 months also lowered the serum MDA levels in postmenopausal women.[16] It was also shown that raloxifene increased the level of GSH in the brain cortex of OVX rats treated by kainic acid.[15] A protective role for raloxifene against oxidative stress associated endothelial dysfunction has also been suggested.[17]
CONCLUSION
The results of the present study showed that treatment with TAM enhances the spatial learning and memory of OVX rats; however, it was not effective in naïve rats. The possible mechanism for the beneficial effects of TAM may be due to its protective effects against brain tissues oxidative damage.
ACKNOWLEDGMENT
The authors would like to thank the Vice Chancellor of Research Affairs of Mashhad University of Medical Science for financial assistance.
Footnotes
Source of Support: Vice Chancellor of Research Affairs of Mashhad University of Medical Science
Conflict of Interest: None declared.
REFERENCES
- 1.Webber KM, Bowen R, Casadesus G, Perry G, Atwood CS, Smith MA. Gonadotropins and Alzheimer's disease: The link between estrogen replacement therapy and neuroprotection. Acta Neurobiol Exp (Wars) 2004;64:113–8. doi: 10.55782/ane-2004-1497. [DOI] [PubMed] [Google Scholar]
- 2.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–58. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J, Wang Q, Johansson JU, Liang X, Woodling NS, Priyam P, et al. Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann Neurol. 2012;72:788–98. doi: 10.1002/ana.23677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–91. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- 6.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994;140:256–61. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 7.Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–82. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 8.Holm A, Andersson KE, Nordström I, Hellstrand P, Nilsson BO. Down-regulation of endothelial cell estrogen receptor expression by the inflammation promoter LPS. Mol Cell Endocrinol. 2010;319:8–13. doi: 10.1016/j.mce.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–15. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: Mechanisms and implications for immunity. Horm Behav. 2012;62:254–62. doi: 10.1016/j.yhbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breuer B, Anderson R. The relationship of tamoxifen with dementia, depression, and dependence in activities of daily living in elderly nursing home residents. Women Health. 2000;31:71–85. doi: 10.1300/J013v31n01_05. [DOI] [PubMed] [Google Scholar]
- 12.Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, et al. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J Neurochem. 2009;109:1658–67. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Connor E, Cox DA, Song J, Mitlak B, Mosca L, Grady D. Raloxifene and risk for stroke based on the framingham stroke risk score. Am J Med. 2009;122:754–61. doi: 10.1016/j.amjmed.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Wu CF, Shi B, Xu YM. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol Biochem Behav. 2002;71:269–76. doi: 10.1016/s0091-3057(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 15.Armagan G, Kanit L, Terek CM, Sozmen EY, Yalcin A. The levels of glutathione and nitrite-nitrate and the expression of Bcl-2 mRNA in ovariectomized rats treated by raloxifene against kainic acid. Int J Neurosci. 2009;119:227–39. doi: 10.1080/00207450802330959. [DOI] [PubMed] [Google Scholar]
- 16.Ozbasar D, Toros U, Ozkaya O, Sezik M, Uzun H, Genc H, et al. Raloxifene decreases serum malondialdehyde and nitric oxide levels in postmenopausal women with end-stage renal disease under chronic hemodialysis therapy. J Obstet Gynaecol Res. 2010;36:133–7. doi: 10.1111/j.1447-0756.2009.01086.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong CM, Yung LM, Leung FP, Tsang SY, Au CL, Chen ZY, et al. Raloxifene protects endothelial cell function against oxidative stress. Br J Pharmacol. 2008;155:326–34. doi: 10.1038/bjp.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alaei H, Hosseini M. Angiotensin converting enzyme inhibitor captopril modifies conditioned place preference induced by morphine and morphine withdrawal signs in rats. Pathophysiology. 2007;14:55–60. doi: 10.1016/j.pathophys.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini M, Sadeghnia HR, Salehabadi S, Alavi H, Gorji A. The effect of l-arginine and l-NAME on pentylenetetrazole induced seizures in ovariectomized rats, an in vivo study. Seizure. 2009;18:695–8. doi: 10.1016/j.seizure.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, et al. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–21. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini M, Hadjzadeh MA, Derakhshan M, Havakhah S, Rassouli FB, Rakhshandeh H, et al. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010;33:463–8. doi: 10.1007/s12272-010-0317-z. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini M, Headari R, Oryan S, Hadjzadeh MA, Saffarzadeh F, Khazaei M. The effect of chronic administration of L-arginine on the learning and memory of estradiol-treated ovariectomized rats tested in the morris water maze. Clinics (Sao Paulo) 2010;65:803–7. doi: 10.1590/S1807-59322020000800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisi P, Alaei H, Babri S, Sharifi MR, Mohaddes G. Effects of treadmill running on spatial learning and memory in streptozotocin-induced diabetic rats. Neurosci Lett. 2009;455:79–83. doi: 10.1016/j.neulet.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Choopani S, Moosavi M, Naghdi N. Involvement of nitric oxide in insulin induced memory improvement. Peptides. 2008;29:898–903. doi: 10.1016/j.peptides.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Naghibi SM, Hosseini M, Khani F, Rahimi M, Vafaee F, Rakhshandeh H, et al. Effect of Aqueous Extract of Crocus sativus L. on Morphine-Induced Memory Impairment. Adv Pharmacol Sci 2012. 2012 doi: 10.1155/2012/494367. 494367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 27.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–9. [PubMed] [Google Scholar]
- 28.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 29.Sharma JB, Sharma A, Bahadur A, Vimala N, Satyam A, Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet. 2006;94:23–7. doi: 10.1016/j.ijgo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Hall ED, Bosken JM. Measurement of oxygen radicals and lipid peroxidation in neural tissues. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0717s48. Chapter: Unit 7.17.1-51. [DOI] [PubMed] [Google Scholar]
- 31.Karami R, Hosseini M, Khodabandehloo F, Khatami L, Taiarani Z. Different effects of L-arginine on morphine tolerance in sham and ovariectomized female mice. J Zhejiang Univ Sci B. 2011;12:1016–23. doi: 10.1631/jzus.B1100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini M, Taiarani Z, Karami R, Abad AA. The effect of chronic administration of L-arginine and L-NAME on morphine-induced antinociception in ovariectomized rats. Indian J Pharmacol. 2011;43:541–5. doi: 10.4103/0253-7613.84969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimzadeh Bideskan AR, Hosseini M, Mohammadpour T, Karami R, Khodamoradi M, Nemati Karimooy H, et al. Effects of soy extract on pentylenetetrazol-induced seizures in ovariectomized rats. Zhong Xi Yi Jie He Xue Bao. 2011;9:611–8. doi: 10.3736/jcim20110606. [DOI] [PubMed] [Google Scholar]
- 34.Hosseini M, Taiarani Z, Hadjzadeh MA, Salehabadi S, Tehranipour M, Alaei HA. Different responses of nitric oxide synthase inhibition on morphine-induced antinociception in male and female rats. Pathophysiology. 2011;18:143–9. doi: 10.1016/j.pathophys.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Saffarzadeh F, Eslamizade MJ, Nemati Karimooy HA, Hadjzadeh MA, Khazaei M, Hosseini M. The effect of L-arginine on Morris water maze tasks of ovariectomized rats. Acta Physiol Hung. 2010;97:216–23. doi: 10.1556/APhysiol.97.2010.2.8. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghian R, Fereidoni M, Soukhtanloo M, Azizi-Malekabadi H, Hosseini M. Decreased nitric oxide levels in the hippocampus may play a role in learning and memory deficits in ovariectomized rats treated by a high dose of estradiol. Arq Neuropsiquiatr. 2012;70:874–9. doi: 10.1590/s0004-282x2012001100010. [DOI] [PubMed] [Google Scholar]
- 37.Azizi-Malekabadi H, Hosseini M, Soukhtanloo M, Sadeghian R, Fereidoni M, Khodabandehloo F. Different effects of scopolamine on learning, memory, and nitric oxide metabolite levels in hippocampal tissues of ovariectomized and Sham-operated rats. Arq Neuropsiquiatr. 2012;70:447–52. doi: 10.1590/s0004-282x2012000600012. [DOI] [PubMed] [Google Scholar]
- 38.Azizi-Malekabadi H, Hosseini M, Saffarzadeh F, Karami R, Khodabandehloo F. Chronic treatment with the nitric oxide synthase inhibitor, L-NAME, attenuates estradiol-mediated improvement of learning and memory in ovariectomized rats. Clinics (Sao Paulo) 2011;66:673–9. doi: 10.1590/S1807-59322011000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velázquez-Zamora DA, Garcia-Segura LM, González-Burgos I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm Behav. 2012;61:512–7. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82:142–9. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–93. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–54. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of Tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J Cancer Surviv. 2008;2:275–82. doi: 10.1007/s11764-008-0070-1. [DOI] [PubMed] [Google Scholar]
- 44.Phillips KA, Ribi K, Sun Z, Stephens A, Thompson A, Harvey V, et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1-98 randomized trial. Breast. 2010;19:388–95. doi: 10.1016/j.breast.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–93. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- 46.Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–64. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 47.Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Vogt MA, Chourbaji S, Brandwein C, Dormann C, Sprengel R, Gass P. Suitability of tamoxifen-induced mutagenesis for behavioral phenotyping. Exp Neurol. 2008;211:25–33. doi: 10.1016/j.expneurol.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Lagunas N, Calmarza-Font I, Grassi D, Garcia-Segura LM. Estrogen receptor ligands counteract cognitive deficits caused by androgen deprivation in male rats. Horm Behav. 2011;59:581–4. doi: 10.1016/j.yhbeh.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Gursoy E, Cardounel A, Al-khlaiwi T, Al-drees A, Kalimi M. Tamoxifen protects clonal mouse hippocampal (HT-22) cells against neurotoxins-induced cell death. Neurochem Int. 2002;40:405–12. doi: 10.1016/s0197-0186(01)00105-x. [DOI] [PubMed] [Google Scholar]
- 51.Sharma K, Mehra RD. Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res. 2008;1204:1–15. doi: 10.1016/j.brainres.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 52.Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–68. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva I, Mello LE, Freymüller E, Haidar MA, Baracat EC. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci Lett. 2000;291:183–6. doi: 10.1016/s0304-3940(00)01410-5. [DOI] [PubMed] [Google Scholar]
- 54.González-Burgos I, Rivera-Cervantes MC, Velázquez-Zamora DA, Feria-Velasco A, Garcia-Segura LM. Selective estrogen receptor modulators regulate dendritic spine plasticity in the hippocampus of male rats. Neural Plast 2012. 2012 doi: 10.1155/2012/309494. 309494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T. Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology. 2001;25:242–57. doi: 10.1016/S0893-133X(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 56.Landry M, Di Paolo T. Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor. Brain Res Mol Brain Res. 2003;112:82–9. doi: 10.1016/s0169-328x(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 57.Baraka AM, Korish AA, Soliman GA, Kamal H. The possible role of estrogen and selective estrogen receptor modulators in a rat model of Parkinson's disease. Life Sci. 2011;88:879–85. doi: 10.1016/j.lfs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Feng Y, Fratkins JD, LeBlanc MH. Treatment with tamoxifen reduces hypoxic-ischemic brain injury in neonatal rats. Eur J Pharmacol. 2004;484:65–74. doi: 10.1016/j.ejphar.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 59.Lee S, Lee MS, Park J, Zhang JY, Jin DI. Oxidative stress in the testis induced by tamoxifen and its effects on early embryo development in isogenic mice. J Toxicol Sci. 2012;37:675–9. doi: 10.2131/jts.37.675. [DOI] [PubMed] [Google Scholar]


