Abstract
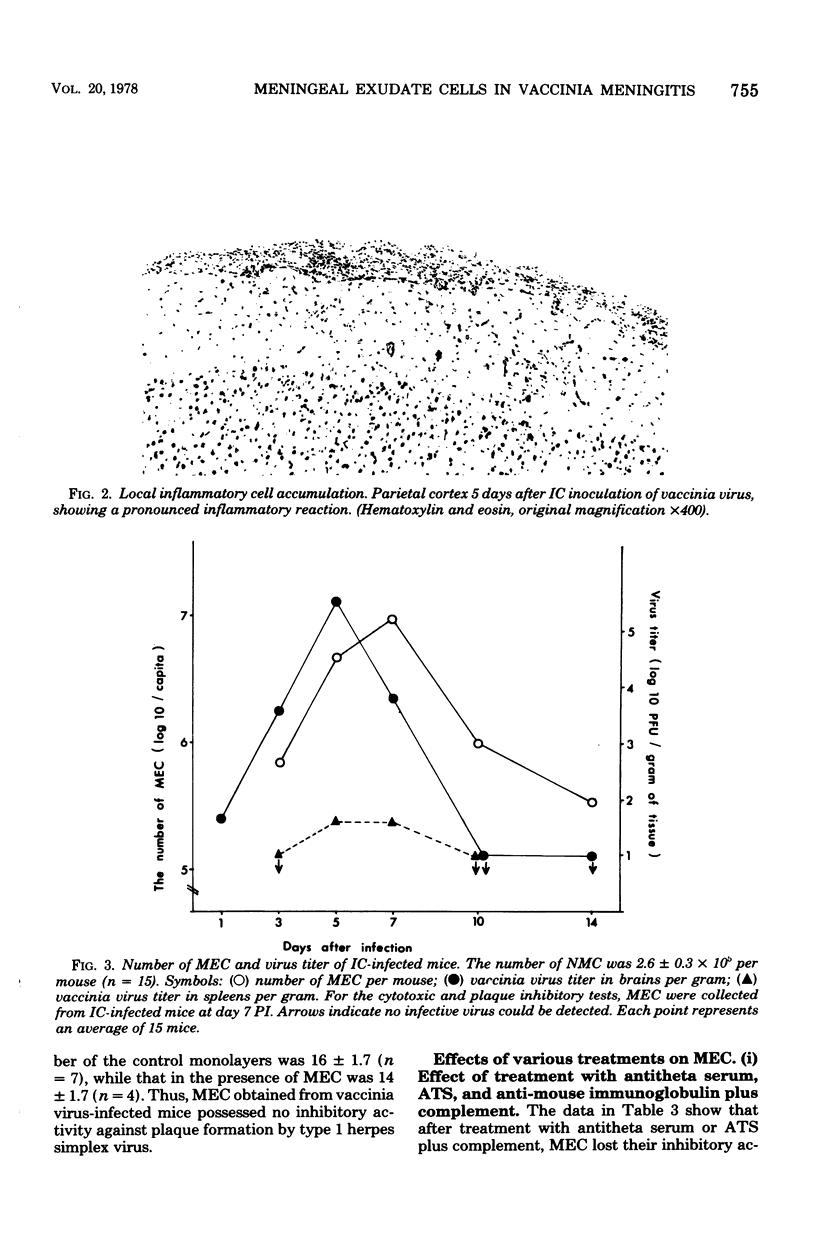
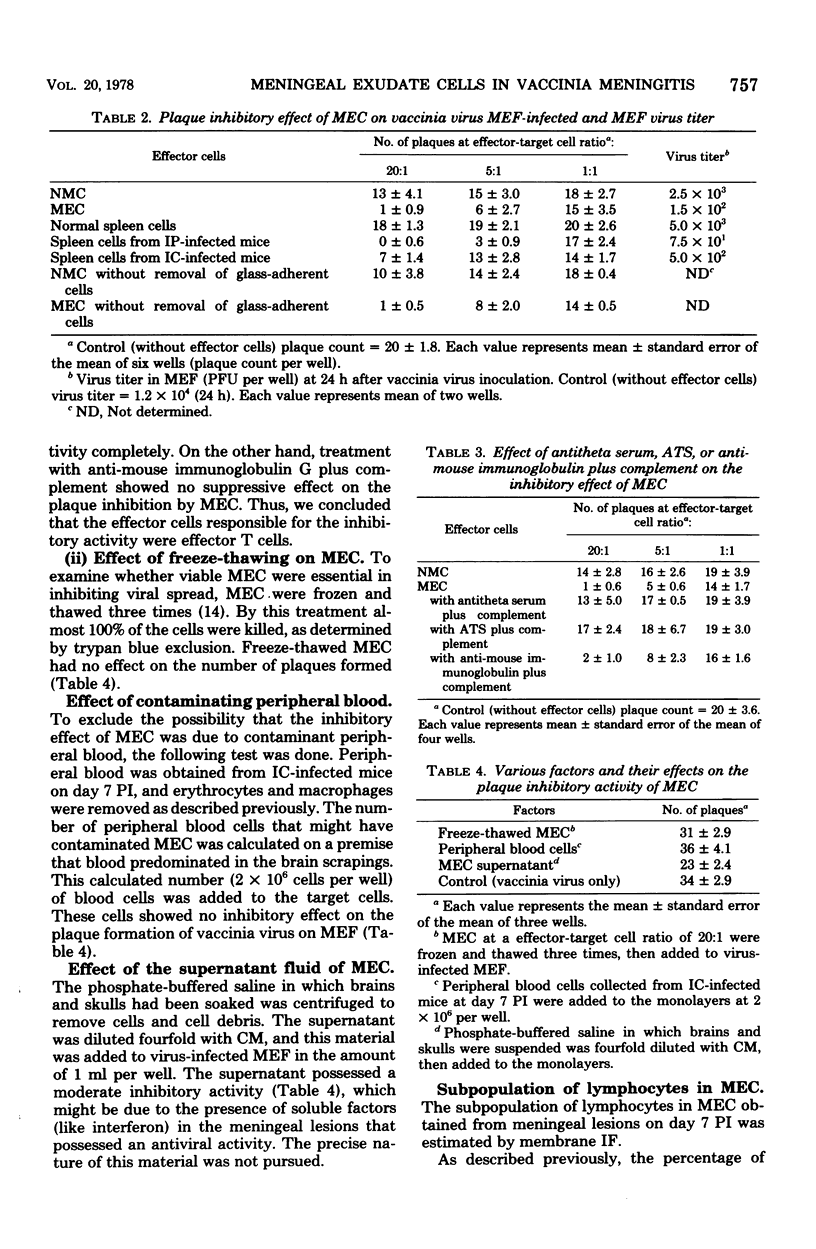
Intracerebral inoculation of vaccinia virus into adult DDD mice produced intensive meningitis several days after infection. The inflammatory reaction could be quantitated by counting cells obtained from this inflammatory lesion. The local virus titer increased until day 5 and subsequently decreased rapidly with time. Concomitant with this titer decrease, numerous meningeal exudate cells appeared in the lesion. The cytotoxic activity of these cells against vaccinia virus infected cells was studied after removal of glass-adherent cells. The results showed that these meningeal exudate cells possessed cytotoxic activity against the virus-infected cells and moreover inhibited plaque formation by vaccinia virus. The magnitude of this activity was much larger than that of spleen cells obtained from the same animals. After treatment with antithymocyte serum, or with antitheta serum plus complement, the meningeal cells lost their inhibitory activity, suggesting that the cells which exerted the effect were mainly T lymphocytes. The meningeal exudate cells obtained on day 7 postinfection were further characterized. A greater part, approximately 80%, of the cell population was composed of theta-positive cells. Less than 1% carried immunoglobulin, 7% possessed neither theta antigen nor immunoglobulin on the surface, and 12% represented glass-adherent cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. F., Raff M. C. Direct demonstration of theta-positive antigen-binding cells, with antigen-induced movement of thymus-dependent cell receptors. J Exp Med. 1973 Jan 1;137(1):69–84. doi: 10.1084/jem.137.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against Herpes simplex virus. I. Control of infection in vitro by senstized spleen cells and antibody. Infect Immun. 1973 Jun;7(6):898–904. doi: 10.1128/iai.7.6.898-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick G. T., Bohl E. H. Local and systemic cell-mediated immunity against transmissible gastroenteritis, an intestinal viral infection of swine. J Immunol. 1976 Apr;116(4):1000–1004. [PubMed] [Google Scholar]
- Ginsberg A. H., Johnson K. P. Vaccinia virus meningitis in mice after intracerebral inoculation. Infect Immun. 1976 Apr;13(4):1221–1227. doi: 10.1128/iai.13.4.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Hapel A., Gardner I. Appearance of cytotoxic T cells in cerebrospinal fluid of mice with ectromelia virus-induced meningitis. Scand J Immunol. 1974;3(3):311–319. doi: 10.1111/j.1365-3083.1974.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Jacobs R. P., Aurelian L., Cole G. A. Cell-mediated immune response to herpes simplex virus: type-specific lymphoproliferative responses in lymph nodes draining the site of primary infection. J Immunol. 1976 Jun;116(6):1520–1525. [PubMed] [Google Scholar]
- Johnson R. T. Inflammatory response to viral infection. Res Publ Assoc Res Nerv Ment Dis. 1971;49:305–312. [PubMed] [Google Scholar]
- Johnson R. T., Mims C. A. Pathogenesis of viral infections of the nervous system. N Engl J Med. 1968 Jan 4;278(1):23–contd. doi: 10.1056/NEJM196801042780106. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F. In vitro studies of cell-mediated immunity in an acute viral infection. J Immunol. 1974 Jul;113(1):173–180. [PubMed] [Google Scholar]
- Rabinowitz S. G., Lipton H. L. Cellular immunity in chronic Theiler's virus central nervous system infection. J Immunol. 1976 Aug;117(2):357–363. [PubMed] [Google Scholar]
- Raff M. C., Owen J. J. Thymus-derived lymphocytes: their distribution and role in the development of peripheral lymphoid tissues of the mouse. Eur J Immunol. 1971 Jan;1(1):27–30. doi: 10.1002/eji.1830010105. [DOI] [PubMed] [Google Scholar]
- Rustigian R., Winston S. H., Bellanti J. A., Clark L. A. Neutralizing antibody and lymphocyte-mediated, colony-forming inhibition responses to measles infection in Cercopithecus aethiops monkeys. J Infect Dis. 1975 Nov;132(5):511–519. doi: 10.1093/infdis/132.5.511. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Woan M. C., Tompkins W. A. Macrophage immunity to vaccina virus: factors affecting macrophage immunity in vitro. J Reticuloendothel Soc. 1974 Jul;16(1):37–47. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Cytotoxic thymus-derived lymphocytes in cerebrospinal fluid of mice with lymphocytic choriomeningitis. J Exp Med. 1973 Nov 1;138(5):1266–1269. doi: 10.1084/jem.138.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]