Abstract
Lung transplantation is a therapeutic modality frequently utilized in end-stage lung disease. Unfortunately, lung transplant recipients have poor clinical outcomes, often due to the development of chronic rejection in the transplanted allograft. This process is characterized by neutrophil influx and extracellular matrix remodeling leading to luminal obstruction and airway inflammation. The molecular mechanisms underlying chronic rejection are poorly understood and disease-specific biomarkers are lacking. We report that in addition to increased levels of interleukin-8 (IL-8), the level of neutrophil chemoattractant proline-glycine-proline (PGP) is elevated in chronic rejection patient bronchoalveolar lavage (BAL) fluid and correlates with loss of lung function. The enzymes responsible for generating PGP, matrix metalloproteases-8 and -9 (MMP-8, -9) and prolyl endopeptidase (PE), are also elevated in chronic rejection samples. Together, IL-8 and PGP account for most of the neutrophil chemoattractant capacity seen in chronic rejection BAL fluid. Using specific neutralizing antibodies to both IL-8 and PGP, we demonstrate that PGP is the major neutrophil chemoattractant found in BAL fluid from individuals at the time of chronic rejection. These findings highlight the influence of a matrix-derived neutrophil chemoattractant in post-transplantation organ rejection and provide opportunities for the development of unique diagnostics and therapeutics to potentially improve disease outcomes.
Introduction
Lung transplantation is a therapeutic modality utilized in approximately 1500 patients per year with end-stage lung disease and severe functional impairment with limited life expectancy (1). The most common conditions requiring lung transplantation are; chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), idiopathic pulmonary fibrosis (IPF), and primary pulmonary hypertension (1). Unfortunately, complications are frequent and result in reduced long-term preservation of graft function and patient survival. National survival statistics from the United Network of Organ Sharing (UNOS) for single lung transplantation are 78% (1-year survival), 61% (3-year survival), and 49% (5-year survival) (2). Major complications contributing to these numbers are ischemia-reperfusion injury in the initial post-transplant period and, thereafter, infection (bacterial, viral, fungal) and chronic rejection (1). Acute allograft rejection characterized by lymphocytic-predominant inflammation, occurs in over 80% of post-lung transplant recipients; it is easily treated with high dose corticosteroids and is not considered a major source of morbidity/mortality in this population (1).
Chronic allograft rejection accounts for poor rates of long-term graft and patient survival. Over 50% of all lung transplant recipients will eventually develop this condition (3). It is a clinical diagnosis of exclusion made with decline in FEV1 to less than 80% of baseline over a 3-week period without other identified etiologies. There is ongoing decline in lung function, most notably during the first 6 months after chronic rejection development (4). The degree of FEV1 decline also classifies patients regarding disease severity (5). It is manifested histologically as obliterative bronchiolitis (OB), a fibroproliferative process targeting small airways that leads to submucosal fibrosis and luminal obliteration. Survival at 5 years after development of chronic rejection is approximately 30% (6).
The specific pathogenic mechanism of chronic rejection is poorly understood but there is damage to both epithelial cells and subepithelial structures. The initial process is a lymphocytic infiltrate of the submucosa, eventually leading to neutrophil attraction and airway damage (7). This neutrophilic influx is felt to contribute to most of the airway damage seen in chronic rejection (8). Chemokines are thought to be important effectors in cellular recruitment in the development of chronic rejection. Specifically, glutamate-leucine-arginine (ELR)+ CXC chemokines, important in neutrophil recruitment (9), seem to play an important role in the pathogenesis of this condition. Patients with chronic rejection demonstrate increased IL-8 levels in bronchoalveolar lavage (BAL) fluid (10, 11). Recently, Belperio et al have described that CXC receptor (CXCR)-2 ligands, such as IL-8, are important in early neutrophil recruitment in chronic rejection and also in later vascular remodeling (12).
Due to the degree of remodeling seen in chronic rejection, interest has turned to the role of proteases in the development of this condition. Several chronic inflammatory conditions are characterized by an imbalance of proteases with their naturally occurring antiproteases (13). This protease/antiprotease hypothesis is believed to define an important component of pathology seen in a variety of conditions such as COPD, cancer, arthritis, and vascular disease (14–17).
Recently, our laboratory has elucidated a unique mechanism of neutrophilic inflammation stimulated by the breakdown of collagen. The release of a tripeptide, acetylated proline-glycine-proline (N-α-PGP), from collagen leads to neutrophil influx (18). Another chemotactic form of this peptide, nonacetylated proline-glycine-proline (PGP) was found in a murine model of pneumonic tularemia (19). Through a series of experiments both in vitro (utilizing transfected cell lines) and in vivo (utilizing knockout mice), it was determined that these peptides act on CXCRs found on the surface of neutrophils (18, 20). Interestingly, these are the same receptors which traditional (ELR)+ neutrophil specific chemokines, such as IL-8, act upon to cause chemotaxis; this similarity in receptor use is due to structural homology seen between PGP/N-α-PGP and ELR+ CXC chemokines (18). We have also recently reported the proteolytic pathway of PGP generation, which involves the coordinated efforts of matrix metalloproteases (MMP-8, MMP-9) and a serine protease, prolyl endopeptidase (PE) (20).
Despite these intriguing findings, the impact of this matrix-derived system of inflammation in clinical disease is not currently known. The aim of the present study was to probe for the presence of these peptides and the proteases required for their generation in lung transplantation recipients to determine their potential role in the neutrophilic inflammation observed in chronic rejection, and their potential to serve as disease biomarkers. In this report, we demonstrate that the appropriate enzymes required for PGP generation are present at both increased levels and activity in chronic rejection samples compared to other transplant populations. We note a significant correlation between both MMP-9 activity with PGP levels and PE activity with PGP levels in chronic lung transplantation rejection, supporting a direct relationship between these enzymes and PGP generation in this condition. These samples demonstrate increased capacity for neutrophil chemotaxis and have elevated levels of both IL-8 and PGP. Utilizing an antibody against PGP, we are able for the first time to determine the role PGP plays in neutrophil chemotaxis in clinical disease. Finally, we demonstrate a unique shift in the chemoattactant profile in the BAL fluid of chronic rejection patients, from an IL-8 predominant phenotype prior to the loss of lung function to a PGP-prominent profile at the time of chronic rejection diagnosis. These findings identify a novel mechanism of neutrophilic inflammation in chronic rejection and may identify disease biomarkers (PE, PGP) to characterize this condition.
Results
Gelatinolytic activity of proteases is increased in chronic rejection lung transplant patients
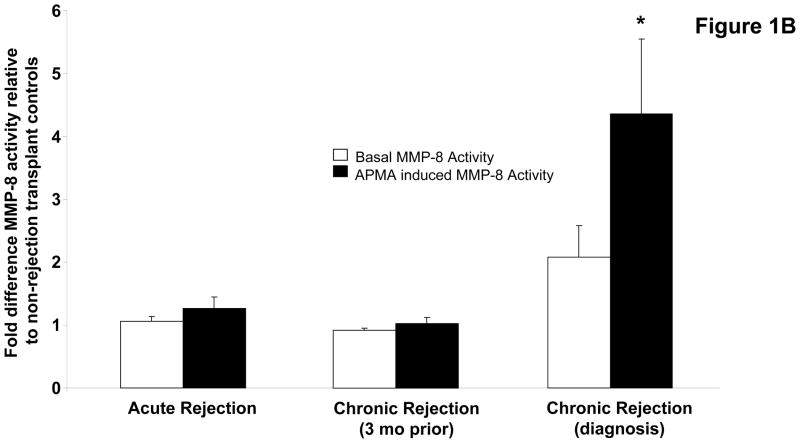
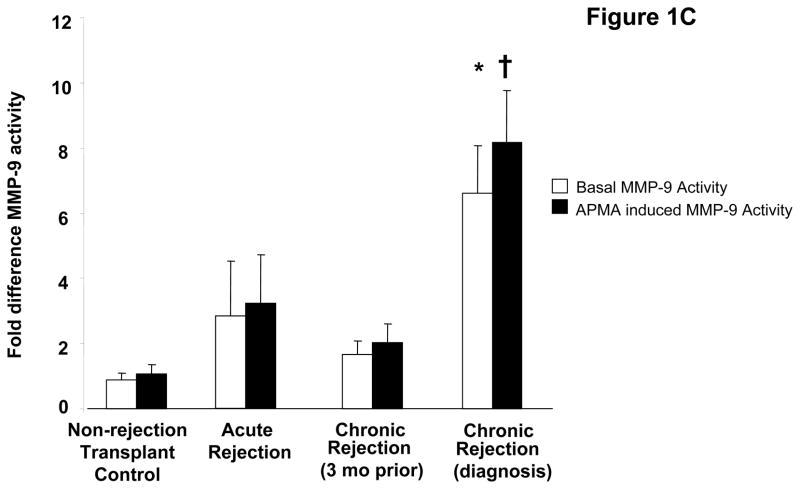
To investigate the role PGP plays in the development of chronic rejection in lung transplant recipients, we began by investigating whether or not enzymes necessary for PGP production are present in bronchoalveolar lavage (BAL) fluid samples from transplant recipients. Using gelatin zymography we demonstrated BAL fluid from chronic rejection patients at the time of diagnosis had a marked increase in gelatinolytic activity as compared to other transplant populations, with a major molecular weight band consistent with the active MMP-9 isoform. This activity was also noticeably increased when compared to matched samples three months prior to the diagnosis of chronic rejection (Figure 1a). We have previously demonstrated that MMP-8 or MMP-9 activity seem to be required for PGP generation, and that these enzymes can act in concert for more efficient generation of PGP (20). We next examined both the activity and concentrations of these enzymes in our transplant populations. MMP-8 concentrations were elevated in chronic rejection BAL fluid samples compared to other populations. The majority of increased enzyme was detected as a zymogen form that was inducible by 4-aminophenylmercuric acetate (APMA) (Figure 1b, p<0.05). In contrast to MMP-8, MMP-9 is constitutively active in chronic rejection BAL fluid. Interestingly, MMP-9 concentrations in all populations were approximately 10-fold higher than the concentration of MMP-8 (data not shown). However, like MMP-8, MMP-9 activity was several fold lower in all other patient samples including chronic rejection BAL fluid from three months prior to diagnosis (Figure 1c, p<0.01). These data suggest that MMP-9 probably plays a prominent role in the generation of PGP after lung transplantation.
Figure 1.
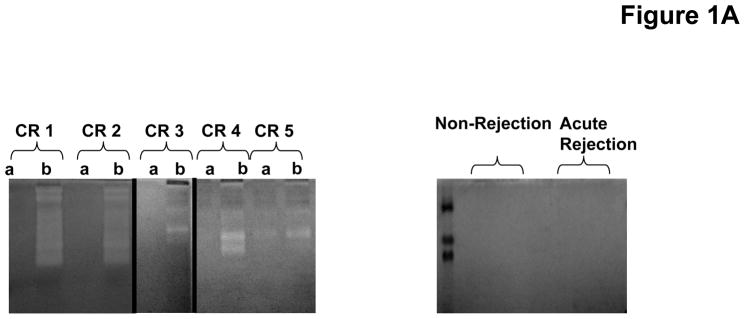
Figure 1A: Zymogram of representative patient BAL. Chronic rejection samples are paired at three months prior to (a) and at time of diagnosis (b) per individual. The majority of the chronic rejection samples collected at the time of diagnosis displayed increased gelatinolytic activity compared to their prior matched samples. None of the non-rejection or acute rejection samples displayed any detectable gelatinolytic activity.
Figure 1B: Fold difference of MMP-8 activity relative to non-rejection transplant controls. Chronic rejection samples collected at the time of diagnosis had elevated levels of MMP-8 activity compared to controls and significantly higher quantities of the zymogen form of the enzyme compared to all patient populations (* p<0.05).
Figure 1C: Relative activity of MMP-9 in BAL fluid from patient populations. The activity of both the active (* p< 0.01) and inducible († p<0.01) forms of MMP-9 are elevated in chronic rejection BAL at the time of diagnosis compared to non-rejection transplant controls.
Prolyl endopeptidase detection and activity are increased in chronic rejection BAL when compared to other patient groups
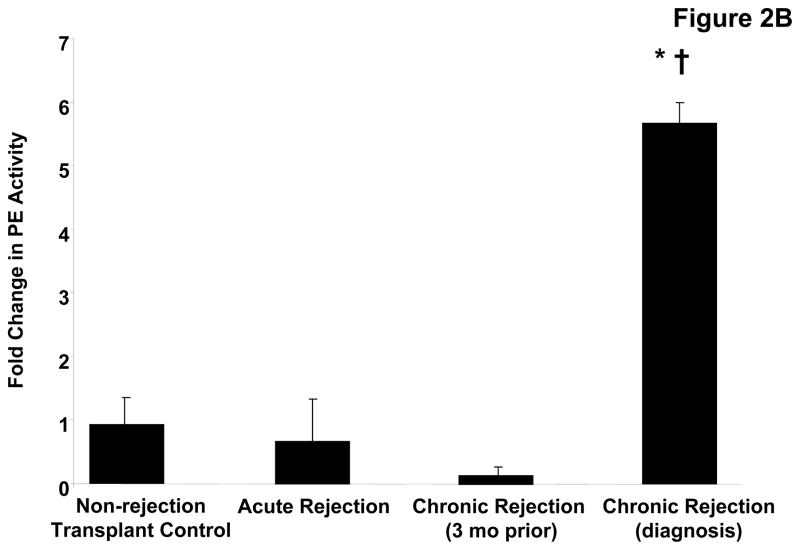
PE, a serine protease, has been previously described to have a central role in PGP generation (20). We examined whether this enzyme, not previously described in transplantation pathology, was present in the transplant patient samples. Western blot analysis identified an 80 kDa band (consistent with the molecular weight of PE) is only present in the chronic rejection samples taken at the time of diagnosis (Figure 2a). The increased detection of this enzyme was complemented by five fold increased activity in chronic rejection BAL fluid at the time of diagnosis compared to normal transplant patients (p< 0.05) or acute rejection and when compared to matched samples from 3 months prior to chronic rejection diagnosis (p<0.01) (Figure 2b). These findings confirm that all of the necessary enzymes for PGP generation are found in chronic rejection BAL fluid.
Figure 2.
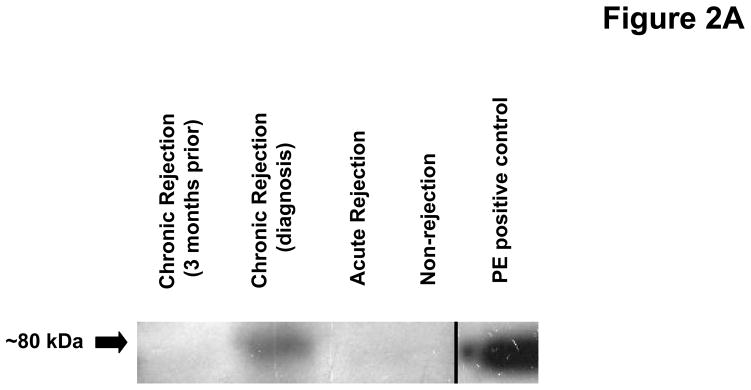
Figure 2A: PE detection in pooled BAL fluid from study populations. BAL fluid was probed using a specific polyclonal antibody for prolyl endopeptidase (PE). Only the chronic rejection samples collected at the time of diagnosis detected PE (~80 kDa).
Figure 2B: PE activity in pooled patient BAL fluid samples relative to transplant controls. Chronic rejection patients at the time of diagnosis demonstrate significantly elevated PE activity compared to non-rejection controls (* p< 0.05), and matched samples collected three months prior to diagnosis († p< 0.01).
Classic and novel chemokine levels are elevated in chronic rejection BAL
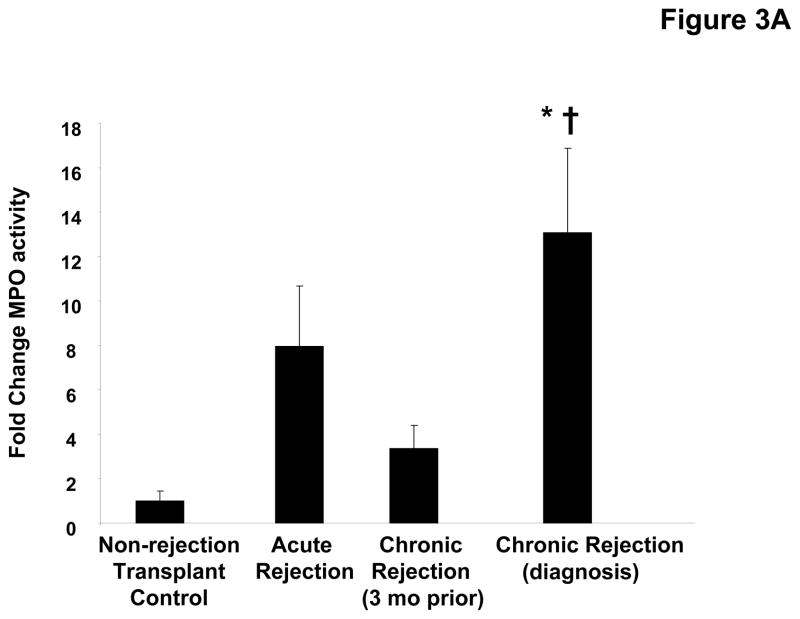
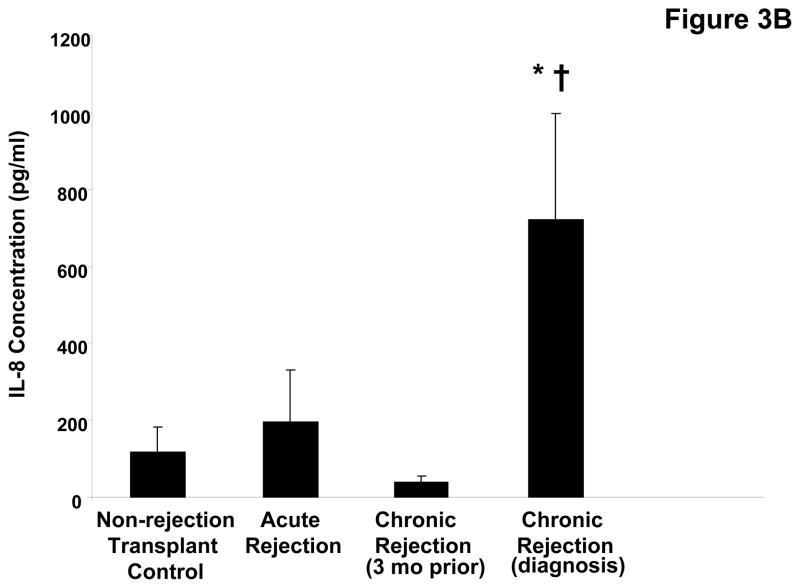
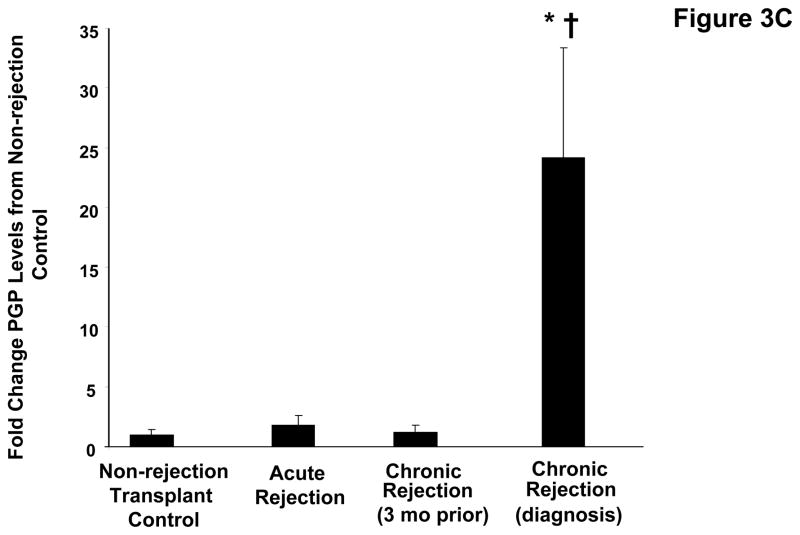
The airway damage and matrix remodeling seen in chronic rejection are thought to be related to an increased neutrophil burden in this condition. Indeed, when myeloperoxidase (MPO), a surrogate of neutrophil influx (21) is measured in the transplant patient populations, there is increased activity seen in chronic rejection versus other groups (p<0.05, Figure 3a). To determine the neutrophil chemoattractants responsible for the increased neutrophil levels observed in these samples, we first examined the presence of IL-8. Levels of IL-8 were approximately 7-fold higher in chronic rejection samples compared to the matched samples obtained three months prior to diagnosis (p<0.05, Figure 3b) and significantly higher than in samples from control populations. We also examined the presence of the neutrophil chemoattractant, PGP, in clinical disease samples utilizing a mass spectrometry technique: electronspray ionization liquid chromatography-tandem mass spectrometry (ESI LC-MS/MS) (18, 20). PGP levels were 25-fold higher in chronic rejection samples compared to their matched samples three months prior to diagnosis and significantly higher compared to other control transplant groups (p<0.05, Figure 3c). Only the non-acetylated form of PGP was found in BAL from lung transplant patients (in contrast with our previous reports of acetylated PGP detection in samples from CF patients).
Figure 3.
Figure 3A: Myeloperoxidase activity in pooled BAL fluid samples relative to transplant controls. Chronic rejection samples from the time of diagnosis are significantly elevated compared to both non-rejection transplant controls (* p< 0.05), and matched samples collected three months prior to diagnosis († p< 0.05).
Figure 3B: IL-8 levels in BAL fluid from lung transplant populations. IL-8 levels were measured in all groups using a commercially available kit as previously described. Chronic rejection BAL collected at the time of diagnosis demonstrated a six-fold increase in IL-8 levels compared to the individual’s prior matched samples († p<0.05) and non-rejection transplant controls (* p<0.05).
Figure 3C: PGP levels in BAL fluid from lung transplant populations. Chronic rejection samples demonstrated a >25 fold increase in PE activity compared to the individual’s prior matched samples († p<0.05) and non-rejection transplant controls (* p< 0.05).
Change in PGP correlates with change in lung function in chronic rejection BAL
We next examined if change in PGP concentrations correlated with change in lung function in chronic rejection individuals. We observed a correlation coefficient (r) of −0.83 (p<0.05) for change in FVC, and an r of −0.67 for change in FEV1 (p=0.14). These coefficients were higher than to those observed to relate to either IL-8 change versus change in FEV1 (r=−0.56, p=0.25) or change in FVC (r=−0.73, p=0.10) in these samples. These findings support the hypothesis that PGP levels are a more robust predictor for loss of lung function compared with IL-8.
PGP levels correlate with both MMP and PE activity in chronic rejection BAL
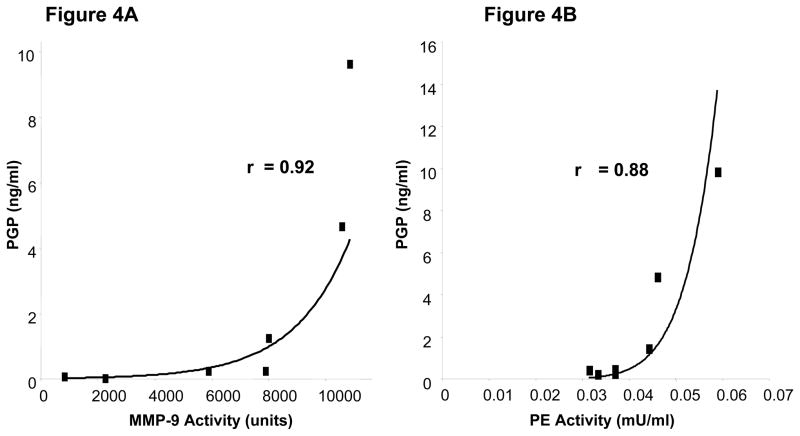
As we have previously described in a murine model and in CF sputum samples ex vivo (19, 20), PGP generation involves the coordinated activity of MMPs and PE. When we examined the correlation between MMP-9 activity and PGP levels in chronic rejection BAL fluid the samples demonstrated a strong correlation (r= 0.92; p<0.05, Figure 4a). Similarly, PE activity demonstrated a strong correlation with PGP concentrations in these samples (r =0.88 (p<0.05, Figure 4b)). This exponential correlation is in keeping with the relationship of an enzyme and its product. Of note, similar r values were seen between MMP-9/PGP and PE activity/PGP in the matched samples three months prior to rejection (r= 0.89 and r=0.67, respectively), although these correlation coefficients were not as robust as that seen at the time of rejection. These findings suggest that these enzymes operate in vivo to generate PGP over the course of the development of chronic rejection following lung transplantation.
Figure 4.
Figure 4A: Correlation of MMP-9 activity and PGP levels. The PGP levels and MMP-9 activity in chronic rejection samples taken at the time of diagnosis displayed a strong correlation r value of 0.92 (p<0.05).
Figure 4B: Correlation of PE activity and PGP levels. The PGP levels and PE activity in chronic rejection samples taken at the time of diagnosis displayed a strong correlation r value of 0.88 (p< 0.05).
Chronic rejection BAL samples are highly chemotactic for peripheral blood neutrophils ex vivo
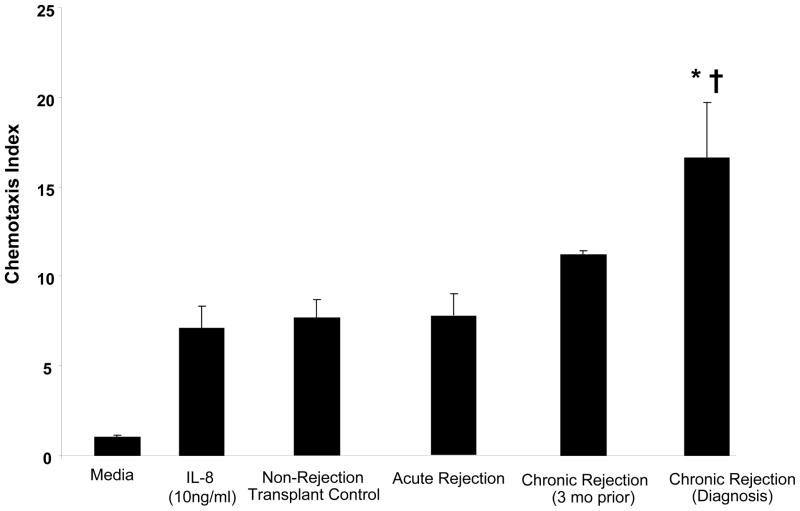
As we have demonstrated the presence of two neutrophil chemoattractants (IL-8 and PGP) in clinical samples, we examined the neutrophil chemotactic potential of BAL fluid from our study populations. BAL fluid from each of the transplant patient groups was used to perform a chemotaxis assay ex vivo on peripheral blood neutrophils (isolated from normal, non-transplant controls). The chronic rejection samples taken at the time of diagnosis demonstrated a 20-fold higher chemotactic index relative to media control (p < 0.01) and was also higher than the other patient populations, including the matched samples from three months prior to diagnosis (p<0.05) (Figure 5). These results are consistent with chemotactic activity of both IL-8 and PGP in these populations’ BAL fluid.
Figure 5. Neutrophil chemotaxis of BAL fluid from transplant populations.
Chronic rejection BAL collected three months prior to and at the time of diagnosis demonstrated elevated chemotaxis indices compared to non-rejection transplant controls. Samples collected at the time of diagnosis demonstrated significantly elevated neutrophil chemotaxis compared to control transplant populations (p< 0.05) and matched samples prior to chronic rejection diagnosis (p< 0.05).
Blockade of IL-8 and PGP ablates the chemoattractive ability of chronic rejection BAL fluid samples
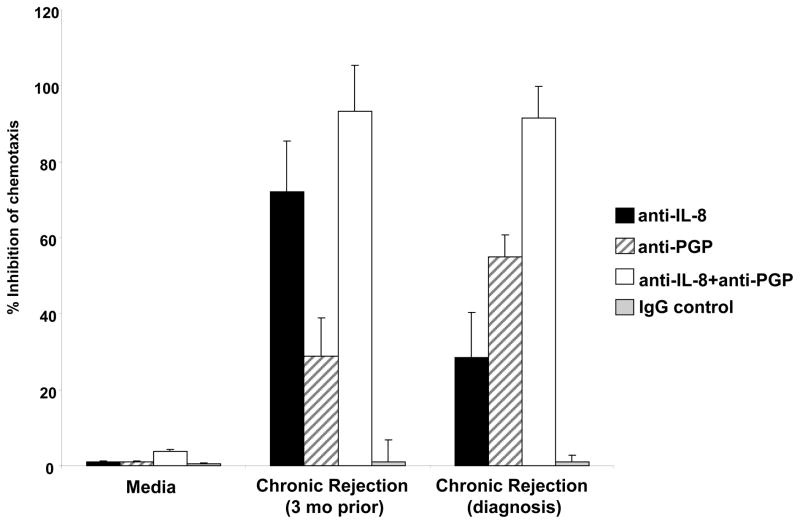
In order to determine the degree by which each of these chemokines alone or in concert influenced the chemotactic properties of chronic rejection BAL fluid, we examined pooled samples at the time of chronic rejection with the matched control samples from three months prior to diagnosis. We investigated the samples’ ability to induce neutrophil chemotaxis in the presence of a specific neutralizing IL-8 antibody and a specific PGP neutralizing antibody developed in our laboratory. Of note, these antibodies demonstrated no cross-reactivity with the alternate chemokine (Supplemental Figure 1). Together, PGP and IL-8 accounted for 90% or more of the chemotactic activity seen in samples at baseline (three months prior), or at the time of chronic rejection diagnosis. By utilizing the specific neutralizing antibodies to both PGP and IL-8, we were able to determine the chemoattractant profile for both time points of the disease. In the samples collected three months prior to diagnosis, the neutralizing antibodies for IL-8 and PGP caused 76.5% and 28.1% reduction in chemotaxis, respectively (Figure 6). When matched samples taken at the time of diagnosis were examined there was a distinct change in the observed chemokine profile. IL-8 neutralizing antibody reduced the chemotactic ability of the samples by 29.2%, while the PGP neutralizing antibody caused a 58.4% inhibition of neutrophil migration. When both antibodies were used in combination in either the early or later chronic rejection samples, we observed almost complete inhibition of chemotaxis activity. These data provide strong evidence that IL-8 is the more dominant chemokine prior to the loss of lung function in chronic rejection and PGP may emerge to play a more prominent role as a neutrophil chemoattractant at the time of diagnosis of chronic rejection.
Figure 6. Relative IL-8 and PGP Chemotaxis in Chronic Rejection BAL fluid.
The samples collected at the time of diagnosis demonstrated a shift in the chemokine profile from samples collected three months prior to chronic rejection. IL-8 neutralizing antibody (1:1000) created a 76.5% and 28.1% inhibition of chronic rejection (three months prior) and chronic rejection (at diagnosis) induced PMN chemotaxis, respectively. In contrast, the inhibition by the PGP neutralizing antibody (1:1000) was 29.2% (in the three months prior samples) and 58.4% (in the samples at time of diagnosis). Together, the antibodies were able to almost completely ablate the chemotactic capacity of the BAL fluid collected three months prior to (93.1%) and at the time of diagnosis (91.4%) of chronic rejection.
Discussion
Chronic rejection in lung transplantation remains the major source of morbidity and mortality in lung allograft recipients (2). It is currently a diagnosis of exclusion made via decline of FEV1 without other clinical explanations. This condition remains poorly understood but it has become increasingly evident that activated neutrophils play important roles in its pathogenesis, not just an epiphenomenon (8). The determination of unique pathways involved in this inflammatory and remodeling response may serve to identify unique biomarkers and to elucidate specific therapeutic targets.
Our work confirms that lavage samples from individuals with chronic rejection have increased proteolytic activity. Although different families of proteases have been implicated in the end-organ damage described in post-lung transplantation pathology, recent attention has turned to matrix metalloproteases (MMPs) as mediators of lung damage (22, 23). This family of zinc containing proteases plays important roles in paracrine and distal hormone signaling, cell matrix turnover, and restoration of normal tissue architecture and function after injury. MMPs are released as tightly regulated zymogens, with secondary regulation provided by direct interactions with small regulatory proteins such as Tissue Inhibitors of MMPs (TIMPs), α-macroglobulin, and other molecules (22).
While MMP dysregulation appears to contribute to extracellular matrix reorganization in many lung diseases (24, 25), few studies have investigated MMPs in post lung transplant organ pathology. Studies have noted increased MMP-9 expression in ischemia reperfusion injury and in lung transplant patients with clinical airway obstruction (26, 27). Another study noted increased MMP-9 expression in patients with chronic rejection (28). Additionally, there is evidence that both MMP-8 and MMP-9 are increasingly elevated prior to the development of chronic rejection in post-transplant individuals (29).
We demonstrate increased MMP-8, and MMP-9 levels and activity in lavage samples from patients at the time of chronic rejection compared to matched samples from three months prior to diagnosis. Utilizing a novel polyclonal anti-PE antibody and an enzymatic assay to determine PE activity, we describe, for the first time, that PE is both detected and upregulated in chronic rejection samples. Our current and previous work suggests that increased activity of these enzymes in clinical samples are important for PGP liberation from collagen in vitro and in vivo (20). Indeed, the statistically significant correlation coefficients seen with either MMP-9 or PE with PGP levels strongly suggest that these enzymes are operative in PGP production in chronic rejection.
There is also increasing evidence that components of the extracellular matrix may serve as modulators of airway inflammation in lung transplantation rejection. Wilkes et al have described the role of cryptic epitopes of type V collagen as a mediator of immune responses seen during chronic lung transplantation rejection (30, 31). Attention has also turned to alternate pathways of inflammation involving fragmentation of matrix scaffolding proteins. Nonspecific collagen-derived fragments have been reported to induce neutrophil chemotaxis in murine models (32, 33). In addition, elastin fragments ending with proline-glycine demonstrate the capacity to cause fibroblast and monocyte chemotaxis and, to a lesser degree, neutrophil chemotaxis in murine models of emphysema (34).
BAL fluid samples from patients at the time of chronic rejection also demonstrated an increased capability to attract neutrophils compared to all other transplant populations. These samples also exhibited high levels of IL-8, a neutrophil chemoattractant well-characterized in chronic rejection. Traditional CXCR ligands are felt to play an important role in both neutrophil influx and propogation of injury via activation of other cellular inflammatory mechanisms (10–12). Here, we show that a extracellular matrix-derived neutrophil chemoattractant, PGP, was elevated in clinical samples from individuals with chronic rejection. To our knowledge, this is the first characterized ECM fragment described in transplantation rejection with the capability to drive neutrophilic inflammation.
We have also developed a novel detection and inhibitory antibody to PGP which distinguishes this peptide from IL-8. Using this reagent and an IL-8 specific antibody, we demonstrate that during the course of the development of chronic rejection there is a change in the chemoattractant profile. The impact of IL-8 as a neutrophil chemoattractant is increased prior to the loss of lung function seen at the diagnosis of chronic rejection. However, at the time of airflow reduction and increased airway remodeling, PGP levels are elevated and account for increased neutrophil recruitment compared with the traditional chemoattractant, IL-8. Together IL-8 and PGP account for virtually all of the chemotactic activity seen in chronic rejection BAL fluid (Figure 6) and may represent logical therapeutic targets.
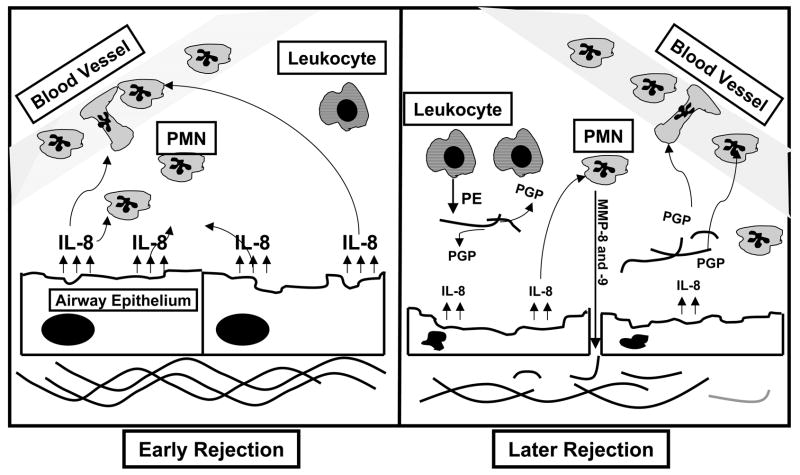
These findings expand our understanding of the role of the ECM as modulators of immune response in lung transplantation rejection, demonstrating that peptide fragments from ECM structural proteins may serve as important mediators of neutrophil recruitment. Our findings support the concept that collagen-derived chemoattractants might have an increased role in neutrophil influx at the time of airflow reduction in chronic rejection due to the significant airway remodeling seen in the disease. As modeled in Figure 7, it is possible that, as chronic rejection persists, the continued remodeling of collagen and subsequent liberation of PGP could lead to a PGP-driven inflammation with traditional chemokines playing a more limited role in ongoing inflammation.
Figure 7. Model of ongoing neutrophilic inflammation in chronic rejection.
In early chronic rejection there is mild damage to the airway and the epithelial cells produce IL-8 which causes PMN chemotaxis. The increased neutrophil burden increases levels of MMPs which further damage the tissue exposing the surrounding collagen. With the collagen breakdown by MMPs and PE, PGP is released causing further PMN influx and a worsening of the fibrosis and matrix turnover already occurring. The dysregulation of this system is most likely a major factor in the morbidity and mortality due to chronic rejection in lung transplant patients.
As such, therapeutics which only target traditional ELR+, CXC chemokine pathways may be inadequate to quell the neutrophilic response during chronic rejection. By targeting proteases involved in generation of PGP, we propose that PGP generation could be reduced, altering the neutrophilic inflammation seen during the development of chronic rejection.
To date, few biomarkers have emerged that are predictive of chronic rejection The correlation of loss of lung function with increasing PGP levels in chronic rejection highlights the possible use of PGP as a measurable marker of clinical features of this condition. Examination of larger cohorts of these populations should allow confirmation of the specificity and sensitivity of the levels/activity of PGP, PE, and MMPs in the development of chronic rejection. Longitudinal studies of PGP production in chronic rejection may also be useful to increase our understanding of disease onset and progression and possibly prognosis.
In summary, we have demonstrated that PGP is increased in the progressive development of chronic rejection in lung transplant recipients. The elevated levels in BAL fluid in chronic rejection patients, along with the high correlation with generating enzymes and diagnosis of disease, make PGP a potential biomarker and therapeutic target for this condition. We have also shown that, while traditional chemokines regulate neutrophils prior to chronic rejection, matrix degradation products, such as PGP, may have a larger role in driving inflammation and neutrophil influx as the condition is established. The identification of this novel neutrophil chemoattractant pathway in chronic rejection following lung transplantation sets the stage for future studies to examine the role of this peptide in disease pathogenesis.
Materials and Methods
Patient populations
University of Alabama at Birmingham (UAB) Institutional Review Board approval (IRB X051014005) was obtained prior to all studies involving human participants and samples. All patients had bronchoalveolar lavage (BAL) samples and basic clinical information collected with a unique patient identifier to maintain patient confidentiality. Clinical data are summarized in Supplemental Table 1. Diagnosis of acute rejection was ≥A2 grade rejection based off of transbronchial biopsy.
Bronchoscopy and bronchoalveolar lavage processing
Bronchoscopy and BAL (4 × 25 ml of 0.9% NaCl) were performed and remnant lavage samples were stored in Core Facility for Collection, Processing, and Storage of Alveolar Fluid (IRB X041026004). These samples were centrifuged at 200 × g for 10 minutes and the cell-free BAL was stored in −80 deg C until analysis.
Materials
Coomassie Brilliant Blue R-250 was obtained from Bio-Rad Laboratories (Hercules, CA). Hyclone PBS (1x) .0067M (PO4) w/o Ca++ and w/o Mg++ was from Hyclone Laboratories (South Logan, UT). Goat Anti-Rabbit Ig-HRP Human Adsorbed antibody was from Southern Biotech (Birmingham, AL). Albumin from Bovine Serum, Cohn V Fraction was obtained from Sigma (St. Louis, MO). Z-Gly-Pro-pNA was from Chem Impex Internation (Wood Dale, IL). Dulbecco’s Modified Eagle’s Medium was from Mediatech (Herndon, VA).
Gelatin Zymography
Porcine skin gelatin (Sigma, St. Louis, MO) at 1mg/ml was added to a 7.5% SDS polyacrylamide solution before casting. Biologic samples were aliquoted and diluted in non-reducing sample buffer, and 25 μl of sample were added to each lane. All samples were electrophoresed at 12 mA for 1 hr. Following electrophoresis, gels were washed in 2.5% Triton X-100 for 1 hr at 4°C, then incubated in 50 mM Tris·Cl for 16 hr at 37°C. Gels were stained in Coomassie blue for 30 min and subsequently destained for 2 hr.
MMP-8 and MMP-9 activity assay
Briefly, MMP-8 and -9 specific ELISA-based activity assays were used to quantify specific MMP activity (R&D Systems, Minneapolis, MN). Samples were diluted to fit manufacturer’s sensitivity for individual kits. Both samples and recombinant enzyme standards were prepared and incubated for 2 hr at room temperature in 96-well plates coated with monoclonal antibody for MMP of interest. After incubation, samples and standards were activated with 1 mM 4-aminophenylmercuric acetate (APMA), a chemical activator of MMPs, and further incubated for 2 hr at 37°C. After incubation, a fluorogenic substrate (Fluor-Pro-Leu-Gly-Leu-Ala-Arg-NH2) was placed in each well and the plate was incubated at 37°C for 18 hr. The plate was then read on a spectrophotometer (SpectraMax Gemini, Molecular Devices, Sunnyvale, CA; excitation and emission wavelength of 320 and 405, respectively) and data was quantified using standard curves provided with the kits.
MPO Assay
Briefly, commercially available assays kits were used to quantify MPO activity in clinical samples (Calbiochem, San Diego, CA). Samples were diluted to fit manufacturer’s sensitivity for kit. Both samples and MPO standards were prepared and placed on 96 well plate coated with polyclonal antibody directed to human MPO. After 2 hr incubation, detection reagent is placed in wells for 1 hr. The samples’ absorbance is then measured at 450 nm wavelength.
Western Blot
All samples were electrophoresed through SDS-polyacrylamide gels (both reducing and non-reducing conditions) and electroblotted onto nitrocellulose membranes. Membranes were blocked in phosphate buffered saline (pH=7.4) containing 5% BSA for one hour. Once washed, they were incubated with primary antibody (rabbit-anti-PE) for one hour at room temperature. The polyclonal rabbit anti-PE antibody was made by EZ Biolab (Westfield, IN) against a synthetic peptide representing residues 190–219 of the C-terminus of mouse PE. This antibody detects recombinant human (whose sequence differs from mouse by one residue between amino acids 190 to 219), but not bacterial PE (whose sequence completely differs from human or mouse PE at residues 190 to 219). After incubation, samples were washed and incubated with goat-anti-rabbit-HRP secondary antibody for one hour. Immunoblots were then developed using ECL chemiluminescent kits (Pierce, Rockford, IL).
PE activity assay
20 μl of BAL was incubated with a specific substrate (2mM Benzylcarboxy-Glycine-Proline-para-Nitroaniline, ZGP-pNa) at 37°C and 5% CO2 and cleavage of para-nitroaniline (pNa) from the substrate by PE was detected using a spectrophotometer at 410 nm and compared to a generated standard curve for PE activity.
IL-8 Levels
Briefly, IL-8 ELISA kits were used to quantify the IL-8 levels in clinical samples (R and D Systems, Minneapolis, MN). Samples were diluted to fit manufacturer’s sensitivity for kit. 50 ul of samples or standards were added to 96 well plate coated with monoclonal antibody against IL-8 for 2 hr at room temperature. Thereafter, 100 ul of IL-8 polyclonal antibody conjugated with horseradish peroxidase are added to wells and incubated for 1 hr. Finally, hydrogen peroxide/chromagen is added to the well and, after 30 min, the absorbance is read at a wavelength of 450 nm.
ESI-LC/MS/MS for PGP detection
PGP and N-α-PGP were measured in sputum samples using a MDS Sciex (Applied Biosystems) API-4000 spectrometer equipped with a Shimadzu HPLC. HPLC was done using a 2.1 × 150 mm Develosi C30 column (with A: 0.1% formic acid and B: acetonitrile +0.1% formic acid). 0 min-0.6 min 20% buffer B/80% buffer A, then increased over 0.6–5 min to 100% buffer B/0% buffer A. Background removed by flushing with 100% isopropanol +0.1% formic acid. Positive electrospray mass transitions are at 270-70 and 270-116 for PGP and 312-140 and 312-112 of N-α-PGP.
Neutrophil chemotaxis assay
Lavage sample is placed in the bottom wells of a 3 μm, 96-well polycarbonate filter plate (Millipore) in 150 μl of DMEM. 2 × 105 neutrophils are added in 100 μl of DMEM to the top portion. These are incubated for 1 hr at 37°C in 5% CO2. The upper portion of the plate is removed and micrographs of the migrated cells are made with an Olympus IX70 microscope. Migration was standardized from cell counts such that chemotactic index=cells per high powered field (experimental)/cells per high powered field (media control), as previously described (18).
Statistical analysis
Descriptive statistics including mean and SEM were made for all quantitative measures Two-tailed Student’s t-test were used for comparisons between 2 groups and ANOVA was utilized for comparing means of 3 or more groups. Pearson’s correlation was utilized to compare relationship between (1) PE activity and PGP (2) MMP-9 activity and PGP (3) change in FEV1 and change in PGP levels, and (4) change in FVC and change in PGP. Means presented ±SEM; statistical significance is considered for p<0.05. Calculations were made utilizing Instat software (Graphpad, San Diego CA) and SPSS version 14 (Chicago, IL). P-values <0.05 were determined to be statistically significant.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Drs. Philip O’Reilly, Robert Snelgrove, Xin Xu, JP Clancy, and James Hagood for their thoughtful comments regarding this manuscript. We would also like to thank Dr. Steven M. Rowe for statistical assistance. Finally, we would like to acknowledge the UAB Lung Transplantation Program for their ongoing support and care of this patient population (Drs. Kevin Leon, Octavio Pajaro, James Kirklin, Joseph Barney, David McGiffin and Ms. Katrina Smith, Theresa Schiller, Melonyssa Hubbard, and Lanier O’Hare).
AG is funded through the UAB CIFA Award (MO1RR00032) and the Cystic Fibrosis Foundation (GAGGAR07A0). JEB is funded through the Cystic Fibrosis Foundation (R464-CR02) and NIH (HL07783 and HL090999). UVD is funded through NIH (T32A107493). Funds for the purchase of mass spectrometers and the operation of the Mass Spectrometry Shared Facility came from the following National Institutes of Health grants to the University of Alabama at Birmingham: S10 RR19231, P30 CA13148, P50 AT00477, U54 CA100949, P30AR050948, and P30 DK74038.
Abbreviations Used
- APMA
4-aminophenylmercuric acetate
- BAL
bronchoalveolar lavage
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- CXC
cysteine-x-cysteine
- CXCR
cysteine-x-cysteine receptors
- ECM
extracellular matrix
- ELR
glutamate-leucine-arginine
- ESI LC- MS/MS
electronspray ionization liquid chromatography-mass spectrometry/mass spectrometry
- FEV1
forced expiratory volume 1 second
- FVC
forced vital capacity
- IL-8
interleukin-8
- IPF
interstitial pulmonary fibrosis
- MMP
matrix metalloprotease
- MPO
myeloperoxidase
- N-α-PGP
acetylated PGP
- OB
obliterative bronchiolitis
- PE
prolyl endopeptidase
- PGP
proline-glycine-proline
- pNa
paranitroaniline
- TIMP
tissue inhibitors of metalloproteases
- UNOS
United Network of Organ Sharing
Footnotes
The content of is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
References
- 1.Arcasoy SM, Kotloff RM. Lung transplantation. NEJM. 1999;340:1081–1089. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the international society for heart and lung transplantation: Twenty-third official adult lung and heart-lung transplantation report 2006. The Journal of Heart and Lung Transplantation. 2006;25:880–892. doi: 10.1016/j.healun.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz MI, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: An update of the diagnostic criteria. The Journal of Heart and Lung Transplantation. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 4.Lama VN, Murray S, Lonigro RJ, Toews GB, Chang A, Lau C, Flint A, Chan KM, Martinez FJ. Course of FEV1 after onset of BOS in lung transplant recipients. Am J Respir Crit Care Med. 2007;175:1192–1198. doi: 10.1164/rccm.200609-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 6.Lau CL, Patterson GA. Current status of lung transplantation. Eur Respir J. 2003;22(S 47):57s–64s. doi: 10.1183/09031936.03.00022103. [DOI] [PubMed] [Google Scholar]
- 7.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- 8.Elssner A, Vogelmeier C. The role of neutrophils in the pathogenesis of obliterative bronchiolitis after lung transplantation. Transpl Infect Dis. 2001;3:168–176. doi: 10.1034/j.1399-3062.2001.003003168.x. [DOI] [PubMed] [Google Scholar]
- 9.Weathington N, Blalock JE. The biology of CXC chemokines and their receptors. Current Topics in Membranes. 2005;55:49–71. [Google Scholar]
- 10.DiGiovine B, Lynch JP, 3rd, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, Morris SB, Kunkel SL, Streiter RM. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157:4194–4202. [PubMed] [Google Scholar]
- 11.Elssner A, Jaumann F, Dobmann S, Behr J, Schwaiblmair M, Reichenspurner H, Fürst H, Briegel J, Vogelmeier C. Elevated levels of interleukin-8 and transforming growth factor-beta in bronchoalveolar lavage fluid from patients with bronchiolitis obliterans syndrome: proinflammatory role of bronchial epithelial cells. Munich Lung Transplant Group. Transplantation. 2000;70:362–367. doi: 10.1097/00007890-200007270-00022. [DOI] [PubMed] [Google Scholar]
- 12.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, Mestas J, Ardehali A, Mehrad B, Saggar R, Lynch JP, III, Ross DJ, Streiter RM. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadek JD, Pacht ER. The protease-antiprotease balance within the human lung. Lung. 1990;168(Suppl):552–64. doi: 10.1007/BF02718178. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogata Y, Miura K, Ohkita A, Nagase H, Shirouzu K. Imbalance between matrix metalloproteinase 9 and tissue inhibitor of metalloproteinases 1 expression by tumor cells implicated in liver metastasis from colorectal carcinoma. Kurume Med J. 2001;48:211–218. doi: 10.2739/kurumemedj.48.211. [DOI] [PubMed] [Google Scholar]
- 16.Ye S, Humphries S, Henney A. Matrix metalloproteinases: implication in vascular matrix remodelling during atherogenesis. Clin Sci (Lond) 1998;94:103–110. doi: 10.1042/cs0940103. [DOI] [PubMed] [Google Scholar]
- 17.Owen CA. Proteinases and oxidants as targets in the treatment of chronic obstructive pulmonary disease. Proc Am Thor Soc. 2005;2(4):373–385. doi: 10.1513/pats.200504-029SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 19.Malik M, Bakshi CS, McCabe K, Catlett SV, Shah A, Singh R, Jackson PL, Gaggar A, Metzger DW, Melendez JA, Blalock JE, Sellati TJ. Matrix metalloproteinase-9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularemia. J Immunol. 2007;178:1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- 20.Gaggar A, Jackson PL, Noerager BN, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662– 5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tauber E, Herouy Y, Goetz M, Urbanek R, Hagel E, Koller DY. Assessment of serum myeloperoxidase in children with bronchial asthma. Allergy. 1999;54(2):177–182. doi: 10.1034/j.1398-9995.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 22.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. Journal of Pathology. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 24.Gaggar A, Li Y, Weathington N, Winkler M, Jackson PL, Blalock JE, Clancy JP. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. AJP – LCMP. 2007;293(1):L96–L104. doi: 10.1152/ajplung.00492.2006. [DOI] [PubMed] [Google Scholar]
- 25.Finlay GA, Russell KJ, McMahon KJ, D’Arcy EM, Masterson JB, Fitzgerald MX, O’Conner CM. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid from emphysematous patients. Thorax. 1997;52:502–506. doi: 10.1136/thx.52.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano M, Omoto Y, Yamakawa Y, Nakashima Y, Kiriyama M, Saito Y, Fujii Y. Increased MMP-9 activity and mRNA expression in lung ischemia-reperfusion injury. Journal of Heart and Lung Transplantation. 2001;20:679–686. doi: 10.1016/s1053-2498(01)00250-9. [DOI] [PubMed] [Google Scholar]
- 27.Beeh KM, Beier J, Kornmann O, Micke P, Buhl R. Sputum levels of MMP-9 and TIMP-1 and their ratio correlate with airway obstruction in lung transplant recipients: relation to TNF-α and IL-10. The Journal of Heart and Lung Transplantation. 2001;20(11):1144–1151. doi: 10.1016/s1053-2498(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 28.Hübner RH, Meffert S, Mundt U, Böttcher H, Freitag S, El Mokhtari NE, Pufe T, Hirt S, Fölsch UR, Bewig B. MMP-9 in bronchiolitis obliterans syndrome after lung transplantation. Eur Respir J. 2005;25:494–501. doi: 10.1183/09031936.05.00091804. [DOI] [PubMed] [Google Scholar]
- 29.Smith GN, Jr, Mickler EA, Payne KK, Lee J, Duncan M, Reynolds J, Foresman B, Wilkes DS. Lung transplant metalloproteinase levels are elevated prior to bronchiolitis obliterans syndrome. Amer J of Transplant. 2007;7(7):1856–1861. doi: 10.1111/j.1600-6143.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 30.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Jr, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: Role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 31.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17–dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagen and collagen-derived peptides. PNAS. 1978;75(2):871–875. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley DJ, Berg RA, Soltys RA, Kerr JS, Gueds HN, Curren SF, Laskin DL. Neutrophil response following intratracheal instillation of collagen peptides into rat lungs. Exp Lung Res. 1988;14:549–563. doi: 10.3109/01902148809087827. [DOI] [PubMed] [Google Scholar]
- 34.Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99:870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.