Abstract
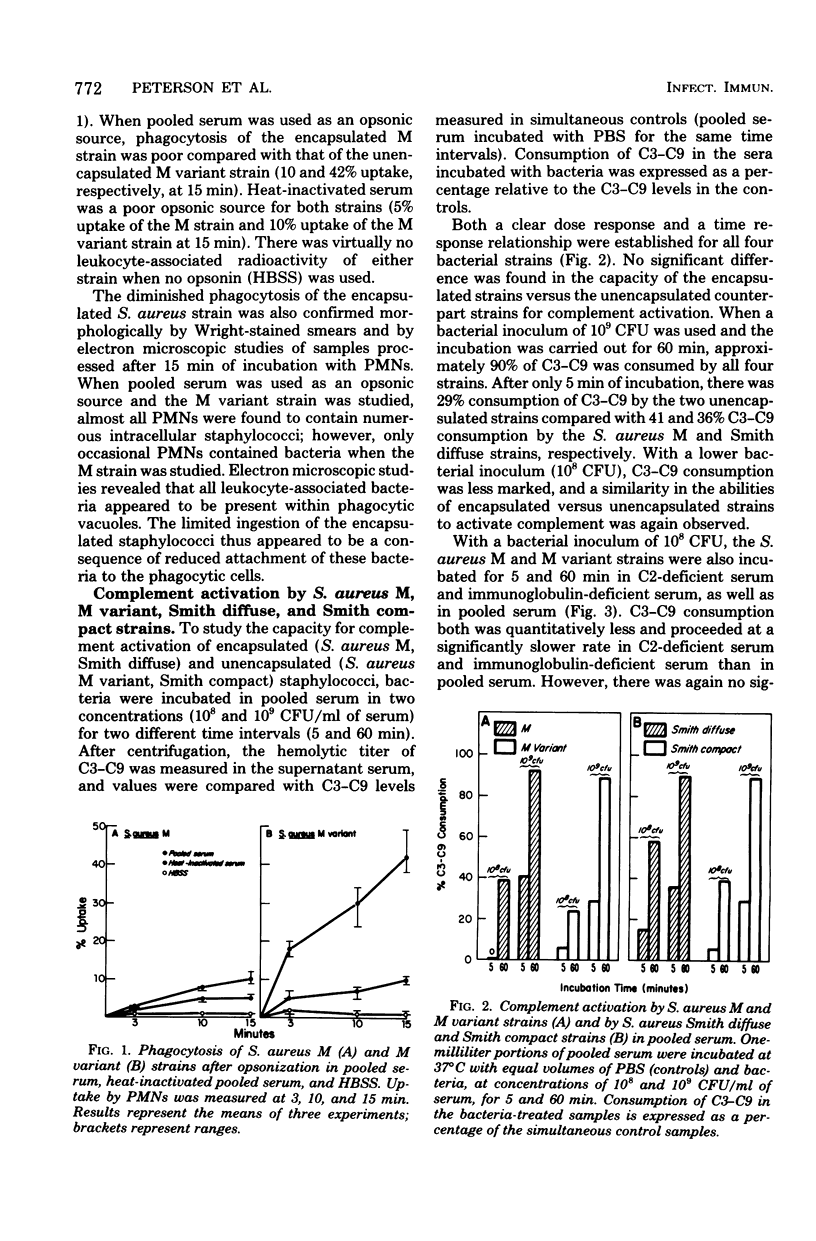
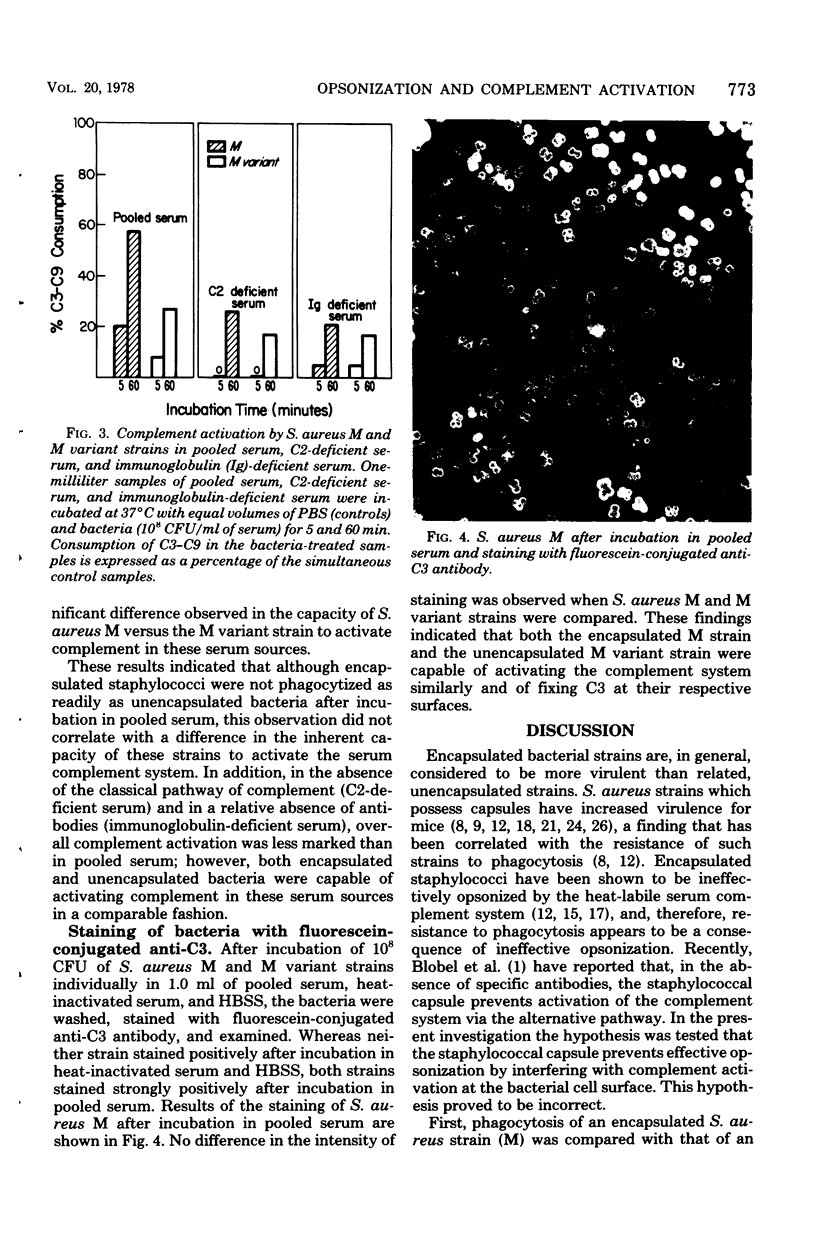
Previous studies have demonstrated that encapsulated Staphylococcus aureus strains are not effectively opsonized by the serum complement system. Encapsulated staphylococci thereby "resist phagocytosis." To test whether this phenomenon might be explained by an inability of encapsulated strains to activate complement, the relationship between staphylococcal opsonization and serum complement activation was studied. Although encapsulation was found to interfere with opsonization by pooled human serum (human polymorphonuclear leukocytes phagocytized significantly fewer encapsulated bacteria than unencapsulated bacteria after incubation in this opsonic source), encapsulated (S. aureus M and Smith diffuse) and unencapsulated (S. aureus M variant and Smith compact) strains had similar capacities for complement activation as measured by C3-C9 consumption. When C2-deficient and immunoglobulin-deficient sera were studied, again C3-C9 consumption was not influenced by the presence or absence of a capsule. In addition, C3 was detected on the surface of both S. aureus M and M variant strains after incubation in pooled serum and staining with fluorescein-conjugated anti-C3 antibody. Thus, encapsulated staphylococci are not effectively opsonized even though complement is activated and C3 is present on the bacterial surface. The exact mechanism by which the capsule interferes with opsonization is still not known; however, inhibition of complement activation appears not to be the explanation of this phenomenon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel H., Brückler J., Kitzrow D., Hasche K. D., Schaeg W. Einige mutmassliche Pathogenitätsfaktoren von Staphylococcus aureus. Zentralbl Bakteriol Orig A. 1976 Aug;235(1-3):91–97. [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Brade V., Lee G. D., Nicholson A., Shin H. S., Mayer M. M. The reaction of zymosan with the properdin system in normal and C4-deficienct guinea pig serum. Demonstration of C3- and C5-cleaving multi-unit enzymes, both containing factor B, and acceleration of their formation by the classical complement pathway. J Immunol. 1973 Nov;111(5):1389–1400. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Gewurz H., Page A. R., Pickering R. J., Good R. A. Complement activity and inflammatory neutrophil exudation in man. Studies in patients with glomerulonephritis, essential hypocomplementemia and agammaglobulinemia. Int Arch Allergy Appl Immunol. 1967;32(1):64–90. doi: 10.1159/000229917. [DOI] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOENIG M. G., MELLY M. A., ROGERS D. E. Factors relating to the virulence of Staphylococci. II. Observations on four mouse-pathogenic strains. J Exp Med. 1962 Nov 1;116:589–599. doi: 10.1084/jem.116.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Friend P. S., Dresner I. G., Yunis E. J., Michael A. F. Inherited deficiency of the second component of complement (C2) with membranoproliferative glomerulonephritis. Am J Med. 1977 May;62(5):765–771. doi: 10.1016/0002-9343(77)90881-6. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., NILSSON U., ARONSSON T. Isolation and characterization of two beta1-glycoproteins of human serum. J Exp Med. 1960 Feb 1;111:201–215. doi: 10.1084/jem.111.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. H., Geiger H., Burke B., Simmons R., Najarian J., Vernier R. L., Michael A. F. Recurrence of membranoproliferative glomerulonephritis following kidney transplantation. Serum complement component studies. Am J Med. 1976 Jan;60(1):60–72. doi: 10.1016/0002-9343(76)90534-9. [DOI] [PubMed] [Google Scholar]
- McLean R. H., Michael A. F. Properdin anc C3 proactivator: alternate pathway components in human glomerulonephritis. J Clin Invest. 1973 Mar;52(3):634–644. doi: 10.1172/JCI107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly M. A., Duke L. J., Liau D. F., Hash J. H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974 Aug;10(2):389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E., MELLY M. A. Observations on the immunology of pathogenic staphylococci. Yale J Biol Med. 1962 Jun;34:560–581. [PMC free article] [PubMed] [Google Scholar]
- Scott A. C. A capsulate Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):253–260. doi: 10.1099/00222615-2-3-253. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Wiley B. B., Maverakis N. H. Virulent and avirulent encapsulated variants of Staphyococcus aureus. J Bacteriol. 1968 Mar;95(3):998–1002. doi: 10.1128/jb.95.3.998-1002.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Smith M. R., Naito Y. Biological and Immunological Properties of Encapsulated Strains of Staphylococcus aureus from Human Sources. Infect Immun. 1970 Nov;2(5):528–532. doi: 10.1128/iai.2.5.528-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Takeuchi Y. Comparison of Compact and Diffuse Variants of Strains of Staphylococcus aureus. Infect Immun. 1970 Nov;2(5):523–527. doi: 10.1128/iai.2.5.523-527.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]




