Abstract
Background
MicroRNA (miRNA) expression in atrial tissue has been implicated in pathologic susceptibility to atrial fibrillation (AF). Nevertheless, data are limited on how circulating levels relate to AF.
Objective
To test the hypothesis that circulating miRNAs would be associated with AF.
Methods
Among 2445 Framingham Heart Study Offspring participants, we measured the expression of 385 circulating whole blood miRNAs by high-throughput quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). We related miRNA levels with prevalent and new-onset AF.
Results
The mean age in the cohort was 66.3 ± 8.9 years and 56% were women; 153 participants had clinically apparent AF at baseline and 107 developed AF during a median of 5.4 years of follow-up. miRNA-328 (miR-328) expression was lower among participants with prevalent AF [8.76 cycle threshold (Ct)] compared to individuals with no AF (7.75 Ct, p <0.001). The association between miR-328 and prevalent AF persisted after adjustment for age, sex, and technical covariates (OR=1.21, P = 1.8 × 10−4) but was attenuated in analyses adjusting for clinical AF risk factors (OR=1.14, P = 0.017). In contrast to the associations between miR-328 and prevalent AF, none of the circulating miRNAs were associated with incident AF.
Conclusions
Circulating levels of miR-328, a miRNA known to promote atrial electrical remodeling by reducing L-type Ca2+ channel density, were associated with prevalent AF. Adjustment for risk factors that promote atrial remodeling, including hypertension, attenuated the association between miR-328 and AF, potentially implicating miR-328 as a potential mediator of atrial remodeling and AF vulnerability.
Keywords: atrial fibrillation; epidemiology, circulation; microRNA; risk factors
Introduction
Atrial fibrillation (AF) is an important clinical and public health problem.1 The prevalence of AF is expected to rise from 3 to 6 million Americans today to about 12 million by 2050.2 AF is associated with an increased risk of stroke,3 heart failure,4 and all-cause mortality.5 The estimated excess annual national cost from AF treatment is $26 billion.6 Despite a strong need for biomarkers of diagnostic and/or prognostic value in AF,7 few robust biomarkers exist.
MicroRNAs (miRNAs), a class of short endogenous non-coding RNA species, are key regulators of gene expression in cardiovascular development and disease (CVD).8 miRNAs have been associated with different forms of heart disease, including atrial and ventricular arrhythmias (miR-1, miRs-26 and 29, miR-133, miR-328),9-11 cardiac hypertrophy (miR-208), and myocardial fibrosis (miR-21, miR-29).12-14 Data indicate that miRNAs can provide insights into gene regulatory events in vivo.14 miRNAs have some useful characteristics as biomarkers, since they are stable and detectable in the peripheral circulation. Despite reports suggesting that miRNAs are involved in regulating pathological atrial remodeling and AF under experimental conditions, few studies have examined the relations between circulating miRNAs and prevalent or incident AF.15 Therefore, we conducted the present investigation to determine if circulating miRNAs are associated with AF in Framingham Heart Study Offspring participants.
Methods
Study Sample
The design and methods of the Framingham Heart Study (FHS) Offspring study have been published previously.16 The Offspring Study is a prospective, community-based observational study of CVD and its risk factors. Study participants included individuals who were children of the Original FHS cohort, and their spouses.16 Beginning in 1971, investigators enrolled 5,124 participants and evaluated these individuals about every 4 to 8 years.
For the present investigation, we focused on the 2467 attendees of examination cycle 8 (2005-2008) who consented to genomic research and had miRNA expression quantified. Participants who experienced AF within 30 days after coronary artery bypass graft surgery (n=22) were excluded. For incident analyses, the follow up ended at diagnosis of AF, last contact, or death, whichever came first. All FHS blood samples were obtained and stored using methods proven to maintain miRNA stability (http://www.preanalytix.com/product-catalog/blood/rna/products/paxgeneblood-rna-tube/. All participants gave informed consent. The FHS protocol was approved by the Boston University Medical Center Institutional Review Board and University of Massachusetts Medical School Review Board.
Atrial Fibrillation
FHS Offspring study participants are asked about AF at all study examinations and at biennial health updates.3 If AF or any CVD or neurological disease is reported, records are sought. Presence of AF is determined from multiple sources including the 12-lead ECGs obtained at each FHS examination and from all CVD-related hospitalizations and clinician visits (follow-up ended in 2011). Cases of suspected AF undergo rigorous adjudication by at least 2 expert study reviewers.16
Risk Factor Definitions
At each FHS examination, Offspring participants undergo a history, laboratory evaluation of CVD risk factors, anthropometry, and physician-administered physical examination. FHS Offspring participants with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or receiving treatment for hypertension were categorized to have hypertension.5 Body mass index was calculated as weight in kilograms divided by the square of his/her height in meters (kg/m2).16 Diabetes was defined as fasting plasma glucose ≥126 mg/dL or treatment with diabetes medications. Criteria for defining myocardial infarction and heart failure in the FHS Offspring Study have been described elsewhere.16 Participants were considered to be current smokers if they smoked 1 or more cigarettes on a daily basis during the year prior to their study examination.
MiRNA expression profiling
Whole blood was used for RNA isolation. The high-throughput Gene Expression Core Laboratory at the University of Massachusetts Medical School profiled 385 miRNAs isolated from whole blood (RNA isolation was performed by Asuragen, Inc) in 2445 FHS Offspring Cohort participants using TaqMan chemistry-based assays (PAXgene) as part of the FHS Systems Approach to Biomarker Research Project. MiRNAs were measured from venous blood samples obtained from participants after an overnight fast. Detailed methods used for cDNA conversion, preamplification, and quantitative real-time polymerase chain reaction (QRT-PCR) are included in the Supplemental Data. The miRNA list encompassed all commercially available TaqMan miRNA assays available at the start of the study. Two hundred fifty-three miRNAs were present in at least 30% of samples.
QRT-PCR reactions were performed with a high-throughput instrument (BioMark; Fluidigm, San Francisco, CA). The Framingham Heart Study (FHS) Systems Approach to Biomarker Research in Cardiovascular Disease Initiative Steering Committee reviewed all quality control measures and praised the assay’s excellent reproducibility, noting that for replicates, > 95% of the data points had coefficients of variation <10% (mean ~4%). As described in the published literature, miRNA expression is quantified using cycle threshold (Ct)17 We adjusted for batch effects in our regression model, but did not perform normalization on the raw Ct values.
Statistical analyses
Descriptive statistics are reported as count (percentage) for dichotomous variables and mean ± SD for continuous variables. We separated the analysis for prevalent AF and incident AF. We used generalized estimating equation to examine the associations between miRNAs with prevalent AF, in which AF status was used as the dependent measure, and the miRNA was used as the exposure. We assessed Cox proportional hazards models with robust sandwich estimators for time-to-AF diagnosis analyses, with death before new-onset AF as a censoring factor (the miRNA was used as the exposure and time-to-AF was used as the dependent measure).
We also applied linear mixed models to test the association of 9 AF susceptibility loci [rs10821415 (C9orf3), rs10824026 (SYNPO2L), rs1152591 (SYNE2), rs2106261 (ZFHX3), rs3807989 (CAV1), rs3903239 (PRRX1), rs6666258 (KCNN3), rs6817105 (PITX2), rs7164883 (HCN4)] with circulating miRNAs.18
We limited our primary analysis to include only the 253 miRNAs present in at least 70% of participant samples (Supplementary Figure 1) but examined the associations between less abundant miRNAs and AF in secondary analyses. MiRNAs that were not detected using high-throughput PCR in >70% of samples could have been due to failed chemical reactions or expression below the minimum detection level. Since we could not determine the cause of missingness, we excluded these miRNAs from our analysis. In contrast to conventional Real-Time PCR instruments, which can detect a single copy in a well at 35-36 Ct values, the BioMark System used for our experiments detects single copies at 26-27 Ct. Therefore, for these analyses we performed the PCR reactions to 28 Ct (Supplementary Figure 2). The assay is still quantitative for low abundant miRNAs.17 To account for factors related to RNA processing, we adjusted analyses for age, sex, isolation batch, RNA quality, concentration, and 260/280 ratio (defined as the ratio of the absorbance at 260 and 280nm; measured using a spectrophotometer).
We then adjusted for known factors associated with pathological atrial remodeling and AF, including height, weight, systolic blood pressure, diastolic blood pressure, current smoking, antihypertensive medication use, diabetes mellitus, and prevalent heart failure, and myocardial infarction.19 Based on Bonferroni corrections for multiple testing, we used a p value cutoff of 2.0×10−4 (0.05/253 miRNAs) to define the level of statistical significance.
In exploratory analyses, due to the fact that miR-328 is known to be expressed in platelets and to further explore the possibilities that differential medication use or levels of an inflammatory biomarker associated with AF (e.g., interleukin-6) accounted for our findings, we also conducted an exploratory analysis examining the association between miR-328 and AF adjusting for medications listed in Table 1 and interleukin-6. In light of prior work linking circulating miRNAs to acute coronary syndromes8 and heart failure and the close relations between CVD and AF, we also examined the relations between miR-328 and AF excluding participants with prior myocardial infarction or heart failure.14
Table 1.
Framingham Heart Study Offspring Study Participant Characteristics
| Variable | No AF* (n=2185) |
Prevalent AF (n=153) |
Incident AF (n=107) |
|---|---|---|---|
| Age, y | 65.6 ± 8.7 | 72.7± 8.2 | 71.6 ± 8.5 |
| Men, n (%) | 932 (43) | 93 (61) | 60 (56) |
| Body mass index, kg/m2 | 28.2 ± 5.4 | 29.5± 5.2 | 28.8 ± 5.4 |
| Systolic blood pressure, mm Hg | 128 ± 17 | 129 ± 20 | 135 ± 17 |
| Diastolic blood pressure, mm Hg | 73 ± 10 | 70 ± 10 | 71 ± 10 |
| Current smoking, n (%) | 192 (8.8) | 6 (3.9) | 10 (9.4) |
| Prevalent myocardial infarction, n (%) | 70 (3.2) | 33 (22) | 6 (5.6) |
| Prevalent heart failure, n (%) | 21 (1.0) | 33 (22) | 5 (4.7) |
| Prevalent diabetes mellitus, n (%) | 273 (13) | 38 (25) | 21 (20) |
| Antihypertensive treatment, n (%) | 995 (46) | 103 (68) | 72 (68) |
| Aspirin, n (%) | 537 (25) | 34 (22) | 33 (31) |
| Warfarin, n (%) | 24 (1) | 83 (54) | 2 (2) |
| Beta-blocker, n (%) | 497 (23) | 117 (76) | 48 (45) |
| Digoxin, n (%) | 12 (1) | 32 (21) | 2 (2) |
| Calcium-channel blocker, n (%) | 296 (14) | 40 (26) | 26 (24) |
Data are presented as means standard deviation or number (percentage). AF = atrial fibrillation. Values reported were measured at enrollment.
No AF indicates absence of diagnosed AF at baseline or in follow-up
Results
The demographic, clinical, treatment and laboratory characteristics of the 2445 FHS Offspring Cohort participants included in our analysis are shown in Table 1. Our cohort included middle-age to older adults with a modest burden of CVD risk factors. Prevalent AF was present in 153 participants; new-onset AF developed in an additional 107 individuals during a median follow-up of 5.4 years. Participants with prevalent AF were, on average older, more likely to be men and receive antihypertensive, anticoagulant, and rate-controlling agents, have a higher mean height and weight, as well as a history of myocardial infarction, heart failure, and diabetes mellitus.
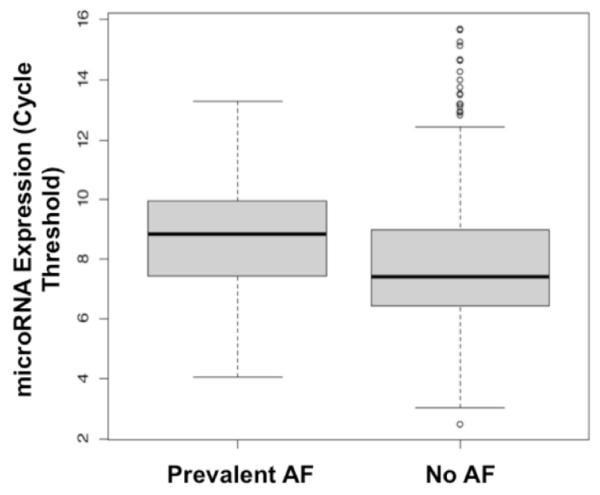
A complete list of quantified miRNAs and their mean expression (Ct) values are shown in Supplemental Table 1. miRNAs with the most significant associations with prevalent and incident AF are shown in Tables 2 and 3. Expression of several miRNAs, including miR-328, miR-150-5p, miR-331-3p and miR-28-5p were negatively nominally associated with prevalent AF (Table 2). Only the association between miR-328 [odds ratio (OR), 1.21; 95% confidence interval (CI), 1.09-1.33; P=0.00018] and prevalent AF remained statistically significant after adjustment for age, sex, isolation batch, RNA quality, concentration, and 260/280 ratio (Figure 1). miR-328 was also the only miRNA with a false discovery rate (FDR) of < 0.05. The second most significant miRNA, miR-150-5p, had an FDR of 0.13. MiR-328 was relatively abundant in most participants (Table 2). Further adjustment for AF risk factors linked to atrial size and/or pathological atrial remodeling, including height, weight, systolic blood pressure, diastolic blood pressure, current smoking, antihypertensive medication (including beta-blocker) use, prevalent heart failure, myocardial infarction, and diabetes mellitus in secondary analyses attenuated the observed association between circulating miR-328 and AF (these findings are shown in Tables 2 and 3).
Table 2.
Most significant miRNAs in Association with Prevalent Atrial Fibrillation in Framingham Offspring Participants
| N | Average Expression | Adjustment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| miRN A |
Age-, Sex- and miR processing |
Multivariable*** | |||||||||
| Total | AF Case s |
Prevalen t AF (n=153) |
No AF (n=2185 ) |
Fold Change** |
OR**** | 95 % CI |
P- value* |
OR**** | 95 % CI |
P-value* | |
| miR- 328 |
2407 | 150 | 8.76 | 7.75 | 1.13 | 1.21 | 1.09 - 1.33 |
<0.001 | 1.14 | 1.02 - 1.28 |
0.017 |
| miR- 150-5p |
2415 | 151 | 4.07 | 3.70 | 1.10 | 1.29 | 1.11 - 1.50 |
0.001 | 1.24 | 1.04 - 1.49 |
0.018 |
| miR- 331-3p |
2396 | 150 | 6.54 | 5.98 | 1.09 | 1.18 | 1.06 - 1.31 |
0.002 | 1.14 | 1.01 - 1.28 |
0.035 |
| miR- 28-5p |
2410 | 150 | 13.13 | 12.10 | 1.09 | 1.15 | 1.05 - 1.26 |
0.002 | 1.08 | 0.98 - 1.19 |
0.14 |
| miR- 99b-5p |
2420 | 151 | 13.18 | 12.32 | 1.07 | 1.12 | 1.04 - 1.20 |
0.003 | 1.05 | 0.96 - 1.16 |
0.27 |
| miR- 182-5p |
1946 | 126 | 20.56 | 21.80 | 0.94 | 0.91 | 0.85 - 0.97 |
0.003 | 0.93 | 0.87 - 1.00 |
0.04 |
| miR- 31-3p |
747 | 39 | 24.26 | 24.85 | 0.98 | 0.69 | 0.54 - 0.89 |
0.004 | 0.69 | 0.54 - 0.90 |
0.005 |
| miR- 196b- 5p |
1829 | 116 | 22.92 | 23.56 | 0.97 | 0.84 | 0.75 - 0.95 |
0.005 | 0.83 | 0.73 - 0.94 |
0.004 |
| RNU48 -a2 |
2409 | 151 | 3.81 | 3.46 | 1.10 | 1.29 | 1.08 - 1.53 |
0.005 | 1.28 | 1.03 - 1.59 |
0.027 |
| miR- 339-5p |
2403 | 151 | 11.30 | 10.82 | 1.04 | 1.13 | 1.04 - 1.23 |
0.006 | 1.10 | 1.00 - 1.21 |
0.05 |
Bonferroni p value cutoff = 0.05/253 miRNAs = 0.0002
Fold-change is the change in miRNA expression associated with one cycle-threshold increase. For example, if the Ct increase by 1, the fold-change in expression would be = 0.5.
Multivariable adjusted models included age, sex, miR-processing, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, antihypertensive medication use, prevalent heart failure, myocardial infarction, and diabetes mellitus
The OR reported represents the ratio of odds resulting from a 1-cycle threshold increase.
AF=atrial fibrillation; OR = odds ratio; miR = miRNA; CI = Confidence Interval
Table 3.
Most significant miRNAs in Association with Incident Atrial Fibrillation in Framingham Offspring Participants
| N | Average expression | Adjustment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age-, Sex- and miR processing |
Multivariable*** | ||||||||||
| miRN A |
Total | AF Case s |
Inciden t AF (n=107) |
No AF (n=2185 ) |
Fold Change** |
OR**** | 95 % CI |
P- value* |
OR**** | 95 % CI |
P- value* |
| miR- 152 |
2394 | 106 | 16.37 | 16.88 | 0.97 | 0.87 | 0.79 - 0.95 |
0.001 | 0.89 | 0.81 - 0.98 |
0.015 |
| miR- 375 |
2315 | 102 | 20.74 | 20.97 | 0.99 | 0.83 | 0.72 - 0.96 |
0.001 | 0.82 | 0.71 - 0.95 |
0.007 |
| miR- 221-3p |
2358 | 103 | 14.38 | 14.47 | 0.99 | 0.86 | 0.76 - 0.96 |
0.01 | 0.85 | 0.76 - 0.97 |
0.012 |
| miR- 1274b |
2416 | 107 | 7.14 | 7.60 | 0.94 | 0.81 | 0.69 - 0.96 |
0.013 | 0.83 | 0.71 - 0.99 |
0.033 |
| miR- 134 |
1337 | 61 | 23.01 | 23.63 | 0.97 | 0.86 | 0.76 - 0.97 |
0.013 | 0.85 | 0.75 - 0.96 |
0.009 |
| miR- 200c- 3p |
1831 | 83 | 22.07 | 22.95 | 0.96 | 0.88 | 0.80 - 0.98 |
0.017 | 0.89 | 0.80 - 0.98 |
0.022 |
| miR- 193a- 5p |
2405 | 106 | 16.72 | 17.05 | 0.98 | 0.87 | 0.77 - 0.98 |
0.018 | 0.87 | 0.77 - 0.98 |
0.024 |
| miR- 720 |
2420 | 107 | 5.47 | 5.83 | 0.94 | 0.80 | 0.66 - 0.96 |
0.018 | 0.83 | 0.68 - 1.00 |
0.051 |
| miR- 151a- 3p |
2403 | 105 | 12.44 | 13.10 | 0.95 | 0.87 | 0.78 - 0.98 |
0.019 | 0.88 | 0.78 - 0.99 |
0.031 |
| miR- 29b-2- p |
2330 | 102 | 20.84 | 21.05 | 0.99 | 0.84 | 0.73 - 0.97 |
0.02 | 0.83 | 0.72 - 0.97 |
0.016 |
Bonferroni p value cutoff = 0.05/253 miRNAs = 0.0002
Fold-change is the change in miRNA expression associated with one cycle-threshold increase. For example, if the Ct increase by 1, the fold-change in expression would be = 0.5.
Multivariable adjusted models included age, sex, miR-processing, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, antihypertensive medication use, prevalent heart failure, myocardial infarction, and diabetes mellitus
The OR reported represents the ratio of odds resulting from a 1-cycle threshold increase.
AF=atrial fibrillation; OR = odds ratio; miR = miRNA; CI = Confidence Interval
Figure 1.
MicroRNA-328 Expression in Prevalent Atrial Fibrillation (AF) vs. those with No Atrial Fibrillation
As shown in Supplemental Table 2, several of the top AF-related miRNAs, including miR-328, were associated in unadjusted analyses with common medications used in participants with AF (Table 1). As shown in Supplemental Table 2, in primary models, use of a beta-blocker was associated with lower circulating miR-328 levels. In further exploratory analyses, we also noted that circulating levels of interleukin-6 were also related to several of the top AF-related miRNAs (Supplemental Table 3). However, adjustment for antiplatelet agents did not significantly alter study findings (OR, 1.14; 95% CI, 1.02-1.28; P=0.0017) nor did exclusion of participants with myocardial infarction or heart failure (OR, 1.12; 95% CI, 0.99-1.27; P=0.075).
Notably, there was little overlap between miRNAs associated with prevalent and incident AF. No miRNAs associated with incident AF met the predefined cutoff for statistical significance after adjustment for multiple testing.
We did not observe any statistically significant relations between circulating miRNAs with any of the 9 genetic loci [rs10821415 (C9orf3), rs10824026 (SYNPO2L), rs1152591 (SYNE2), rs2106261 (ZFHX3), rs3807989 (CAV1), rs3903239 (PRRX1), rs6666258 (KCNN3), rs6817105 (PITX2), rs7164883 (HCN4)], which were associated with AF susceptibility in a recent genome-wide association study (Table 4).18
Table 4.
Most significant miRNAs in Association with Gene Loci previously implicated in Risk for Atrial Fibrillation
| AF locus | Closest Gene |
# Samples with miRNA |
miRNA | Fold- Difference |
SE | P- value* |
|---|---|---|---|---|---|---|
| rs10824026 | SYNPO2L | 1494 | miR-362-5p | -0.44 | 0.11 | 8.5×10−5 |
| rs10824026 | SYNPO2L | 1030 | miR-191-3p | -0.28 | 0.07 | 2.3×10−4 |
| rs1152591 | SYNE2 | 2181 | miR-769-5p | -0.25 | 0.07 | 6.5×10−4 |
| rs1152591 | SYNE2 | 2118 | miR-576-3p | -0.14 | 0.04 | 7.3×10−4 |
| rs10824026 | SYNPO2L | 1799 | miR-301a-3p | -0.39 | 0.12 | 1.2×10−3 |
| rs3903239 | PRRX1 | 847 | miR-1282 | -0.24 | 0.07 | 1.2×10−3 |
| rs1152591 | SYNE2 | 2218 | miR-1260a | -0.15 | 0.05 | 1.2×10−3 |
| rs1152591 | SYNE2 | 1760 | miR-142-5p | -0.31 | 0.11 | 1.3×10−3 |
| rs10824026 | SYNPO2L | 1775 | miR126-5p | -0.20 | 0.06 | 1.7×10−3 |
| rs1152591 | SYNE2 | 2225 | miR-1274b | -0.31 | 0.10 | 1.3×10−3 |
Bonferroni p value cutoff = 0.05/(253 miRNAs x 9 SNP loci)= 2.2 ×10−5
AF=atrial fibrillation; SE = standard error; miR = miRNA; CI = Confidence Interval.
Discussion
In our study including 2445 FHS Offspring study participants, we observed that circulating levels of miRNA-328 was lower among individuals with pre-existing AF as compared to individuals with no known prior AF. This association persisted after adjustment for age, sex, and technical factors. Adjustment for AF risk factors related to degree of pathological atrial remodeling, including hypertension, heart failure, and myocardial infarction, attenuated the association between miR-328 and AF, implicating miR-328 as a potential mediator of atrial remodeling and AF vulnerability. We did not observe study-wide significant associations between miRNAs and incident AF or with any of the 9 genetic loci associated with AF in genome-wide association studies.
Circulating miRNAs have been associated with CVD, including coronary artery disease, myocardial infarction and heart failure.8,14 MiRNA are released or secreted by cardiomyocytes in stress states, and the cellular secretion of unique miRNA suggests process specificity. However, we do not know the extent to which atrial pathological events are reflected in the circulating miRNA pool or the time course(s) involved in miRNA release. Nevertheless, miRNAs regulate genes important to cardiac conduction and atrial structure, some of which have been implicated in the pathogenesis of AF. For example, miR-208 transgenic mice have prolonged PR intervals (an AF intermediate phenotype related to pathological atrial remodeling and incident AF) and miR208a-/- mice develop AF.8 Moreover, recent studies have shown that plasma levels of miRNAs, including miR-150, are associated with paroxysmal or persistent AF.15 Notably, and in contrast to prior work, we did not observe a significant association between circulating miR-150 and prevalent AF.15 These discrepant findings may be due to the fact that we profiled miRNA expression in whole blood as opposed to plasma, as was used in this study or due to differences in the two study populations (e.g., prior study excluded patients with structural heart disease and included only individuals of Chinese descent).15
miR-328 regulates a number of genes (predicted number of gene targets=207)20 involved in inflammation and cell-cell signaling (CD44), myocyte depolarization (CACNA1C and CACNB1), vascular function (ABCG2), and cellular aging (H2AFX), processes implicated in the pathogenesis of AF.21-24 Notably, expression of miR-328 is 2-fold higher in atrial samples from patients with rheumatic heart disease and AF than among controls.25 Expression of miR-328 through adenovirus infection in canine atria has been shown to diminish L-type Ca2+ current, shorten the atrial action potential duration, and enhance vulnerability to AF.25 In contrast, normalization of miR-328 expression using antagomiRs reverses this phenotype and genetic knockdown of endogenous miR-328 activity reduces AF vulnerability in canine models.25
Although pathogenesis and directionality cannot be established from our observational study, when viewed in light of prior work, our findings further implicate miR-328 as a gene regulator of importance in AF.25 It has previously been shown that miRNAs may be secreted by cardiomyocytes under stress (e.g., AF) and therefore circulating miRNAs may not directly regulate myocardial gene expression but may instead correlate with levels inside atrial myocytes. Although one might have expected that higher levels of intracellular miR-328 would relate to higher circulating miR-328 levels, it is also possible that cardiomyocytes down-regulate secretion of miR-328 or induce degradation of circulating miR-328 in the context of AF. Alternative hypotheses that may explain our findings include reverse causality, differential use of medications affecting miR-328 expression (e.g., miR-328 is associated with treated hypertension), intermediate mechanisms, residual confounding, or chance. The observed association between circulating miR-328 levels and beta-blocker use in minimally-adjusted analyses raises the possibility that the higher rates of beta-blocker use among participants with AF may have mediated or confounded the observed association between miR-328 and AF (Supplemental Table 2). When antihypertensive medications, including beta-blockers and angiotensin-converting enzyme inhibitors, were included in multivariable models, the association between miR-328 and AF was attenuated (Table 2). When the association between circulating miRNAs and interleukin-6 are viewed in light of our prior work showing that circulating interleukin-6 influences platelet gene expression, these results suggest a possible role for miRNAs as platelet-derived inflammatory gene regulators.26
Several circulating miRNAs, including miR-328, are platelet-derived or enriched.8 Moreover, antiplatelet therapy reduces key platelet-derived miRNA levels.27 These findings are notable in the context of our observation that miR-328 was lower in patients with AF and in light of the association between AF, altered platelet function, and stroke.
Despite the lack of association noted between miR-328 and incident AF, in light of the association observed between miR-328 and prevalent AF and prior work implicating miR-328 as key regulator of atrial electrical remodeling, the potential role for miR-328 as a biomarker of diagnostic or prognostic importance in AF warrants further exploration. The lack of association between miR-328 and incident AF may be explained by the limited number of new-onset AF cases developing in our sample. We note that biomarkers related to the prevalence of AF have proven useful for the prediction of incident AF.16 Further studies examining the association between miR-328 and AF including greater numbers of patients with new-onset AF are needed.
We did not observe an association between miRNA levels in whole blood and any of the pre-specified loci associated with AF in genome-wide association studies. Our findings may be explained by the fact that miRNA levels may regulate gene expression not only by inhibiting translation, but also post-transcriptionally by degrading specific RNAs.8 Moreover, since certain miRNAs are expressed cell-type specifically, or are up regulated in the setting of physiological disturbances,14 miRNAs may not be expected to be associated with AF loci per se. Finally, single nucleotide polymorphisms associated with AF are sometimes located outside of intronic gene regions and may not relate to gene expression.
Study Limitations
RNA signatures may vary by cell type and patterns of miRNA expression are known to differ between blood components. Utilization of whole blood preparations for the miRNA analyses in our study does not provide the specific source of miRNA. Adjustment could not be performed for cell composition because whole blood cell composition was not determined contemporaneously with miRNA quantification. Due to the retrospective nature of the present study and limited amount of whole blood provided per FHS participant, we did not perform internal replication of our main study findings using conventional PCR techniques. Further studies using PCR platforms to quantify circulating miRNA levels in other community-based cohorts are needed to validate the observed associations between miR-328 and AF. In a community-based cohort it is not realistic to obtain atrial tissue, which is most relevant for AF but unfeasible for clinical biomarker discovery and screening work. Due to the nature of the present community-based investigation, it was not possible to determine the directionality of the associations observed or source of the miRNAs quantified in the circulation. Low abundance miRNAs were excluded from the present analysis if they were not present in at least 30% of samples but such miRNAs may have relevance in pathologic disease states. In addition, we cannot exclude residual confounding. The methods used to define incident and prevalent cases of AF in FHS are well validated.16 They do not, however, enable subclassification of AF (paroxysmal, persistent, permanent) nor do they distinguish between atrial flutter vs. AF. Finally, the FHS Offspring cohort is composed largely of white, middle-age to older adults of European ancestry. Generalizability to other races and ethnicities or younger individuals is uncertain.
Conclusion
In summary, we found an association between AF and a circulating miR-328, a miRNA that is upregulated in the atria of human subjects with AF, regulates L-type Ca2+ channel density, shortens the atrial effective refractory period, and enhances AF vulnerability.
Supplementary Material
Acknowledgments
This work was supported by N01-HC 25195, 6R01-NS 17950; RFA-HL-12-008 (JEF, EM), RO1 HL087201A (JEF, KT) RFA-HL-12-008 (JEF) and 1RO1 HL64753; R01 HL076784; 1 R01 AG028321 (EJB) from the National Heart, Lung and Blood Institute of the National Institutes of Health, and the Division of Intramural Research, National Heart, Lung, and Blood Institute of the National Institutes of Health, Bethesda, MA. Partial salary support is additionally provided by National Institute of Health grants 1U01HL105268-01 (DDM) and KL2RR031981 (DDM).
Abbreviations
- (ECG)
electrocardiogram
- (AF)
atrial fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the united states. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: Report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small EM, Olson EN. Pervasive roles of micrornas in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW., 2nd Microrna-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo X, Pan Z, Shan H, Xiao J, et al. Microrna-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi Y, Tardif JC, Comtois P, Nattel S. Role for microrna-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5:1027–1035. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 12.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of micrornas after myocardial infarction reveals a role of mir-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rooij E, Olson EN. Searching for mir-acles in cardiac fibrosis. Circ Res. 2009;104:138–140. doi: 10.1161/CIRCRESAHA.108.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive micrornas that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Zhou C, Liu Y, Wang S, Ye P, Miao X, Xia J. The expression levels of plasma micornas in atrial fibrillation patients. PLoS One. 2012;7:e44906. doi: 10.1371/journal.pone.0044906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JS, Simon VA, Feddersen RM, Rakhshan F, Schultz DA, Zschunke MA, Lingle WL, Kolbert CP, Jen J. Quantitative mirna expression analysis using fluidigm microfluidics dynamic arrays. BMC Genomics. 2011;12:144. doi: 10.1186/1471-2164-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nature genetics. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The charge-af consortium. Journal of the American Heart Association. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. http://Www.Targetscan.Com.
- 21.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related micrornas in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 22.Ronkainen JJ, Hanninen SL, Korhonen T, Koivumaki JT, Skoumal R, Rautio S, Ronkainen VP, Tavi P. Ca2+-calmodulin-dependent protein kinase ii represses cardiac transcription of the l-type calcium channel alpha(1c)-subunit gene (cacna1c) by dream translocation. The Journal of physiology. 2011;589:2669–2686. doi: 10.1113/jphysiol.2010.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashikuni Y, Sainz J, Nakamura K, Takaoka M, Enomoto S, Iwata H, Tanaka K, Sahara M, Hirata Y, Nagai R, Sata M. The atp-binding cassette transporter abcg2 protects against pressure overload-induced cardiac hypertrophy and heart failure by promoting angiogenesis and antioxidant response. Arterioscler Thromb Vasc Biol. 2012;32:654–661. doi: 10.1161/ATVBAHA.111.240341. [DOI] [PubMed] [Google Scholar]
- 24.Novik KL, Spinelli JJ, Macarthur AC, Shumansky K, Sipahimalani P, Leach S, Lai A, Connors JM, Gascoyne RD, Gallagher RP, Brooks-Wilson AR. Genetic variation in h2afx contributes to risk of non-hodgkin lymphoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1098–1106. doi: 10.1158/1055-9965.EPI-06-0639. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Zhang Y, Wang N, Pan Z, Gao X, Zhang F, Shan H, Luo X, Bai Y, Sun L, Song W, Xu C, Wang Z, Yang B. Microrna-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 2010;122:2378–2387. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- 26.McManus DD, Beaulieu LM, Mick E, Tanriverdi K, Larson MG, Keaney JF, Benjamin EJ, Freedman JE. Relationship Among Circulating Inflammatory Proteins, Platelet Gene Expression, and Cardiovascular Risk. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.112.301112. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer HC, van Solingen C, Prins J, Duijs JM, Huisman MV, Rabelink TJ, van Zonneveld AJ. Aspirin treatment hampers the use of plasma microrna-126 as a biomarker for the progression of vascular disease. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.