Abstract
Background
While pulmonary vein isolation (PVI) has become a mainstream therapy for selected patients with atrial fibrillation (AF), late recurrent AF is common and its risk factors remain poorly defined. The purpose of our study was to test the hypothesis that reduced left atrial passive emptying function (LAPEF) as determined by cardiac magnetic resonance (CMR) has a strong association with late recurrent AF following PVI.
Methods and Results
346 AF patients referred for CMR PV mapping prior to PVI were included. Maximum LA volumes (VOLmax) and volumes before atrial contraction (VOLbac) were measured; LAPEF was calculated as (VOLmax − VOLbac)/VOLmax × 100. Kaplan-Meier curves were constructed to determine late recurrent AF stratified by LAPEF quintile. Cox proportional hazards regression was used to adjust for known markers of recurrence. Over a median follow-up of 27 months, 124 patients (35.8%) experienced late recurrent AF. Patients with recurrence were more likely to have non-paroxysmal AF (75.8% vs. 51.4%, P<0.01), higher mean VOLmax (60.2 ml/m2 vs. 52.8 ml/m2, P<0.01), and lower mean LAPEF (19.1% vs. 26.0%, P<0.01). Patients in the lowest LAPEF quintile were at highest risk of developing recurrent AF (two-year recurrence lowest vs. highest: 60.5% vs. 17.3%, P<0.01). After adjusting for known predictors of recurrence, patients with low LAPEF remained significantly more likely to recur (HR lowest vs. highest quintile = 3.92, 95% CI 2.01–7.65).
Conclusion
We found a strong association between LAPEF and recurrent AF after PVI that persisted after multivariable adjustment.
Keywords: Magnetic resonance imaging, fibrillation, ablation, atrium
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia and leads to substantial morbidity and mortality1. For patients with symptomatic AF, pulmonary vein isolation (PVI) with catheter ablation may restore normal sinus rhythm and significantly improve symptoms and quality of life2. However, a significant number of patients undergoing PVI experience late recurrent AF, with an estimated recurrence rate of up to 40% at five years despite repeated attempts at PVI3.
It remains difficult to predict which patients will experience late recurrent AF after PVI2, 4. While several patient-level risk factors have been identified, including hypertension5, diabetes6, left atrial volume5, and non-paroxysmal AF3, 6, the associations are generally weak and are not routinely incorporated into clinical decision-making. In addition, the mechanisms behind late recurrence of AF remain unclear. Identifying novel markers that predict recurrent AF after PVI may help physicians and patients better make informed decisions about the expected success rate of a procedure that carries an expected complication rate of 5%2, 7.
It has been hypothesized that one of the key underlying mechanisms involved in the occurrence of AF involves a reduction in passive LA emptying during early diastole (left atrial passive emptying function, or LAPEF) and a subsequent increase in LA pressures, leading to pulmonary vein dilatation and electrical remodeling8. However, traditional imaging modalities have been limited in determining LAPEF and its association with recurrent AF after PVI. Cardiovascular magnetic resonance (CMR), with its high in-plane spatial resolution, represents a potentially novel technique to quantify LAPEF. The purpose of our study was therefore to analyze the association of CMR-determined LAPEF with recurrent AF after PVI at a large tertiary referral center. We hypothesized that poor LAPEF would be independently associated with an increased rate of late recurrent AF following PVI.
METHODS
Patients
For derivation of our study cohort we initially included all patients who underwent CMR at Brigham and Women’s Hospital for definition of pulmonary vein anatomy prior to PVI from September 2005 through June 2011 (N=721). At our institution CMR is the standard technique for imaging pulmonary veins prior to PVI in all patients without any absolute contraindications to CMR scanning (permanent pacemaker or defibrillator, severe claustrophobia, glomerular filtration rate <30 ml/min/1.73 m2). We then further restricted our sample to patients in sinus rhythm at the time of CMR in order to separate left atrial passive function from active emptying function; 375 patients (52.0%) were in AF during CMR and were therefore excluded. The final study sample consisted of 346 patients. Patient demographics and comorbidities were ascertained by a physician at the time of CMR. Paroxysmal AF was defined as AF that terminated spontaneously less than 7 days after onset, while non-paroxysmal AF was defined as AF extending beyond 7 days.
CMR protocol
CMR was performed with a 1.5 or 3.0-Tesla CMR system (Signa CV/i HDXt platform, General Electric Healthcare, Waukesha, Wisconsin; Tim Trio, Siemens, Erlangen, Germany, respectively). All images were ECG-gated and acquired with breath holding, whenever possible, with the patient in a supine position. The CMR protocol consisted of standard techniques including 2D cine steady-state free precession imaging (SSFP) in radial long axis and a stack of parallel short-axis for cardiac function (temporal resolution 45–55 msec, in-plane resolution 2–3 mm), 3D magnetic resonance angiography (MRA) to determine pulmonary vein anatomy, as previously described by others9, 10, and late gadolinium enhancement (LGE) imaging. Imaging for pulmonary vein anatomy and LGE imaging was performed after a single bolus of 0.15-mmol/kg of gadopentetate dimeglumine (Magnevist®, Bayer HealthCare). In a recent subset of patients, a free-breathing 3D navigator-guided MRA during slow infusion of 0.2-mmol/Kg of gadobenate dimeglumine (Multihance®, Bracco Diagnostics, Inc) was used to determine pulmonary vein anatomy.
Left Atrium Analysis
We used a commercial software package (CMR42, Circle Cardiovascular Imaging, Canada) to analyze LA emptying function. LA volumes were measured at (1) the beginning of LV diastole (defined as the frame immediately prior to opening of the mitral leaflets, VOLmax), (2) the end of passive LV filling (defined as the frame immediately prior to LA contraction, VOLbac), and (3) the end of LA contraction (VOLmin). The inferior LA border was defined as the plane of the mitral annulus based on prior convention11, 12. To calculate LA volumes, we measured atrial length (from the midpoint of the mitral annulus plane) and border (excluding atrial appendage and pulmonary veins) in the two- and four-chamber views (Figure 1). We then applied the biplane area-length method: LA volume = (4-chamber area) × (2-chamber area) × 0.85/atrial length11, 13. LAPEF was calculated as (VOLmax − VOLbac)/VOLmax × 100. We also calculated left atrial active emptying function (LAAEF, VOLbac − VOLmin)/VOLmax × 100, and left atrial total emptying function (LATEF, VOLmax − VOLmin), consistent with a previous methodology12.
Figure 1. Measurement of left atrial function by CMR.
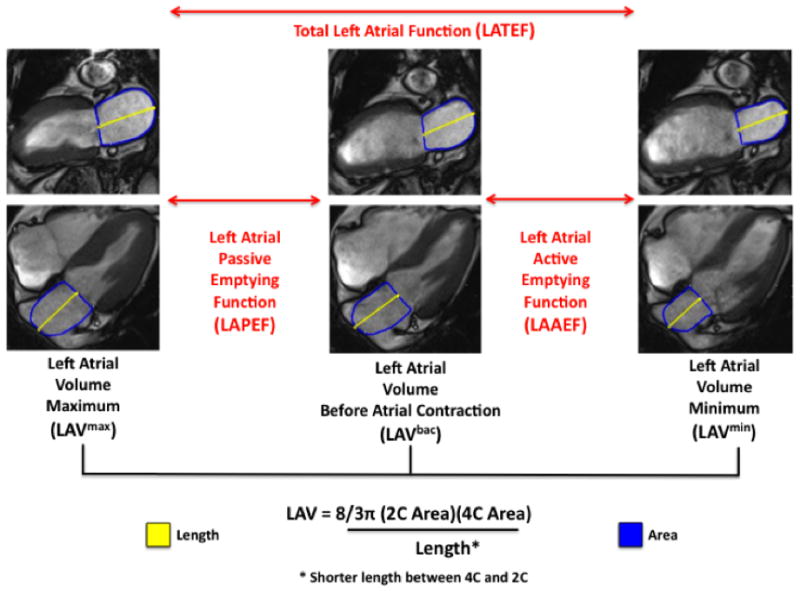
Left atrial passive emptying function (LAPEF) was determined as the difference between volumes at maximum LA size (LAVmax) and LA size before atrial contraction (LAVbac). LA volumes were determined according to the area-length method.
Pulmonary Vein Isolation Protocol
For patients with paroxysmal AF, PVI consisted of point-by-point radiofrequency ablation to encircle the left and right pulmonary veins or by the use of a cryo-ballon catheter (Arctic Front, Medtronic inc., Minneapolis, Mn). In all cases, PVI was confirmed by recording within the veins using a circular multipolar catheter to confirm entrance block into the veins. For patients with persistent AF, additional linear left atrial ablations were performed in addition to PVI. Often, this consisted of linear ablations to create conduction block across the left atrial roof, and along the region between the lateral mitral annulus and left inferior pulmonary vein14. Areas of complex fractionated electrograms during AF were also targeted for ablation. If sinus rhythm could not be restored with ablation alone, administration of ibutilide or external cardioversion was performed to restore sinus rhythm.
Outcomes
The primary outcome of interest was late recurrence of AF. Patients were interviewed during regular clinic visits as a part of post-PVI care or via telephone using standard checklists and a follow-up questionnaire. Late recurrent AF (termed “recurrent AF”) was defined as AF occurring >3 months after pulmonary vein isolation and confirmed by either EKG or cardiac monitoring. Patients underwent electrocardiography at all clinical visits, otherwise routine outpatient cardiac monitoring was performed only in patients based on symptoms. Patients were followed post procedure at 3- to 6-month intervals via clinic visits. The duration of follow-up was determined from the CMR study date (time zero) to the occurrence of an end-point. If no end-point occurred, patients were censored at the date of the last clinical follow-up.
Statistical Analysis
We described continuous variables as means (± standard deviation) and categorical variables as percentages. Bivariate comparisons of patients with and without the primary outcome (late recurrent AF) were performed using the t-test (continuous variables) and the chi-square test (categorical variables).
To further examine the association of LAPEF with late recurrent AF, we stratified LAPEF into quintiles and examined their relationship with the outcome by constructing Kaplan-Meier curves showing survival free from late recurrent AF. We then performed an adjusted time-to-event analysis using Cox proportional hazards regression including age, sex, and covariates that were found to predict recurrent AF in prior studies which included hypertension5, left atrial volume (VOLmax) adjusted for body surface area (BSA)5, 15, non-paroxysmal AF3, 6, diabetes6, left ventricular systolic function16, and >1 ablation procedure3. We also pre-specified that covariates would be included in the model would if they had a P value of <0.15 on bivariate analyses. The group of patients in the highest (“best”) quintile of LAPEF served as the reference group. The proportional hazards assumption was tested in each multivariable model and was found to be valid.
Given the prior relationship described between LAPEF and left ventricular systolic function12, we tested whether the association of LAPEF and late recurrent AF was dependent on left ventricular ejection fraction (LVEF) by adding an interaction term (LAPEF*LVEF) to our multivariable model. We then stratified subjects based on preserved (≥50%) versus reduced (<50%) ejection fraction and analyzed the association of LAPEF with late recurrent AF in these strata.
For purposes of clinical applicability we then analyzed the rate of AF recurrence at 2 years and created a logistic regression model with the previously mentioned clinical characteristics (hypertension, BSA-adjusted VOLmax, non-paroxysmal AF, diabetes, left ventricular systolic function, >1 ablation procedure). We used this model to generate a receiver operator characteristic (ROC) curve and C statistic to evaluate model discrimination for prediction of 2 year recurrence. We subsequently added LAPEF to the clinical model to assess whether discrimination improved. In addition, we generated an ROC curve using the logistic model to evaluate the sensitivity and specificity of LAPEF alone, using LAPEF (continuous) as a predictor of late recurrent AF in the model. Based on this curve, we generated sensitivity and specificity values for three selected cutoffs (LAPEF <20%, LAPEF <25%, LAPEF <30%).
A two-sided P value of <0.05 was considered statistically significant for all tests. Analyses were performed with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
Patient Characteristics
The mean age of the study population was 56 years, and 75.1% were male. The most common comorbidities were hypertension (52.0%), sleep apnea (27.5%), and heart failure (21.1%). Approximately one out of five patients (20.5%) had undergone prior PVI. The mean LAPEF among the study sample was 23.5% ± 11.6%, and the mean body surface area (BSA)-adjusted LA volume was 55.5 ± 17.8 ml/m2.
Over a median follow-up period of 27 months, 124 patients (35.8%) experienced late recurrent AF (>3 months after ablation). The mortality rate during the follow-up period was 11.3% and did not differ significantly between patients with and without recurrent AF (12.9% vs. 10.4%, P=0.47).
Univariable Association of LAPEF with Late Recurrent AF
Characteristics of patients with and without late recurrent AF are shown in Table 1. On average, patients with AF recurrence were more likely to have a history of non-paroxysmal AF (75.8% vs. 51.4%, P<0.01) and larger BSA-adjusted LA volumes (mean VOLmax 60.2 ml/m2 vs. 52.8 ml/m2, P<0.01) (Table 1). Mean LAPEF was lower in patients with recurrent AF than in patients without recurrent AF (19.1% vs. 26.0%, P<0.01). There were no significant differences in age, sex, or other comorbidities between the two groups.
Table 1.
Characteristics of patients with and without late recurrent AF
| Late Recurrent AF* | P-value | ||
|---|---|---|---|
| Yes (N=124) | No (N=222) | ||
| Demographics | |||
| Age, mean ± SD | 56.2 ± 11.3 | 55.9 ± 10.6 | 0.79 |
| Male, N (%) | 96 (77.4%) | 164 (73.9%) | 0.46 |
| Medical History | |||
| Hypertension, N (%) | 71 (57.3%) | 109 (49.1%) | 0.15 |
| Diabetes, N (%) | 24 (19.4%) | 39 (17.6%) | 0.68 |
| Hyperlipidemia, N (%) | 50 (40.3%) | 82 (36.9%) | 0.53 |
| Coronary artery disease, N (%) | 13 (10.5%) | 23 (10.4%) | 0.97 |
| Heart failure, N (%) | 26 (21.0%) | 47 (21.2%) | 0.96 |
| Chronic kidney disease†, N (%) | 8 (6.5%) | 20 (9.0%) | 0.40 |
| Valvular heart disease§, N (%) | 9 (7.3%) | 21 (9.5%) | 0.49 |
| Prior AF ablation, N (%) | 26 (21.0%) | 45 (20.3%) | 0.88 |
| Non-paroxysmal AF, N (%) | 94 (75.8%) | 114 (51.4%) | <0.01 |
| Beta blocker use, N (%) | 77 (62.1%) | 148 (66.7%) | 0.39 |
| Ace inhibitor use, N (%) | 51 (41.1%) | 76 (34.2%) | 0.20 |
| Statin use, N (%) | 43 (34.7%) | 82 (36.9%) | 0.67 |
| Antiarrhythmic drug use**, N (%) | 93 (75.0%) | 151 (68.0%) | 0.17 |
| Diagnostics | |||
| Ejection fraction, mean ± SD | 57.9 ± 9.3 | 58.2 ± 9.9 | 0.81 |
| LA volume (BSA-adjusted)‡, mean ± SD | 60.2 ± 19.3 | 52.8 ± 16.4 | <0.01 |
| LAPEF, mean ± SD | 19.1% ± 10.5% | 26.0% ± 11.4% | <0.01 |
| LATEF, mean ± SD | 37.3% ± 15.8% | 45.4% ± 14.7% | <0.01 |
| Body mass index, mean ± SD | 30.2 ± 5.2 | 29.3 ± 5.2 | 0.11 |
| Late gadolinium enhancement (any), N (%) | 39 (17.6%) | 16 (12.9%) | 0.26 |
Recurrent AF >3 months after ablation procedure
GFR = glomerular filtration rate (patients with GFR <30 ml/min/1.73 m2 were excluded from scan)
Includes ≥moderate aortic stenosis, aortic regurgitation, mitral stenosis, or mitral regurgitation
Volume in ml/m2
Class I or Class III antiarrhythmic drug, prescribed at hospital discharge
As shown in Table 2, the number of late recurrent AF cases increased in lower quintiles of LAPEF over the entire study period. After two-years of follow-up, the AF recurrence rate for patients in the lowest LAPEF quintile (“worst function”) was 60.5%, while for patients in the highest LAPEF quintile (“best function”) it was 17.3%. The relationship between LAPEF and recurrent AF by quintile is shown in Figure 2; the trend was roughly linear, with lower LAPEF associated with a significantly higher rate of late recurrent AF (P <0.001 for linear trend across quintiles). Figure 3 shows the Kaplan-Meier curves for the five LAPEF quintiles (log-rank P<0.0001).
Table 2.
Description of late recurrent AF stratified by LAPEF quintile
| LAPEF Quintile | Mean LAPEF (%) | # Recurrences within quintile | Recurrence rate (%) |
|---|---|---|---|
| 5 (best function) | 40.4 | 12/69 | 17.4% |
| 4 | 29.7 | 20/69 | 29.0% |
| 3 | 23.4 | 21/70 | 30.0% |
| 2 | 16.0 | 29/69 | 42.0% |
| 1 (worst function) | 8.2 | 42/69 | 60.9% |
Figure 2. Rate of late recurrent AF 2 years following PVI stratified by quintile of left atrial passive emptying function (LAPEF).
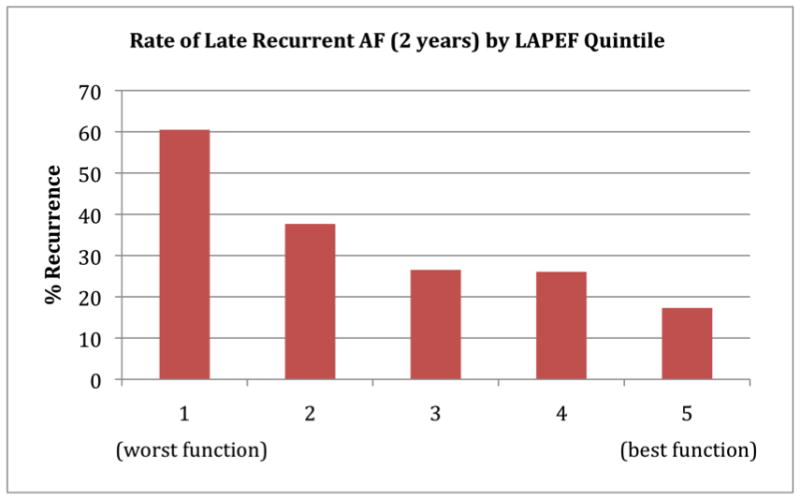
There was a decrease in the AF recurrence rate from lowest to highest LAPEF (P for linear trend <0.001). Patients in the lowest quintile of LAPEF (worst function) had a 2-year recurrence rate of 60.5%, while those in the highest quintile (best function) had a 2-year recurrence rate 17.3%.
Figure 3. Kaplan-Meier survival curves for late recurrent AF after PVI stratified by LAPEF showing event-free survival time among five quintiles of function.
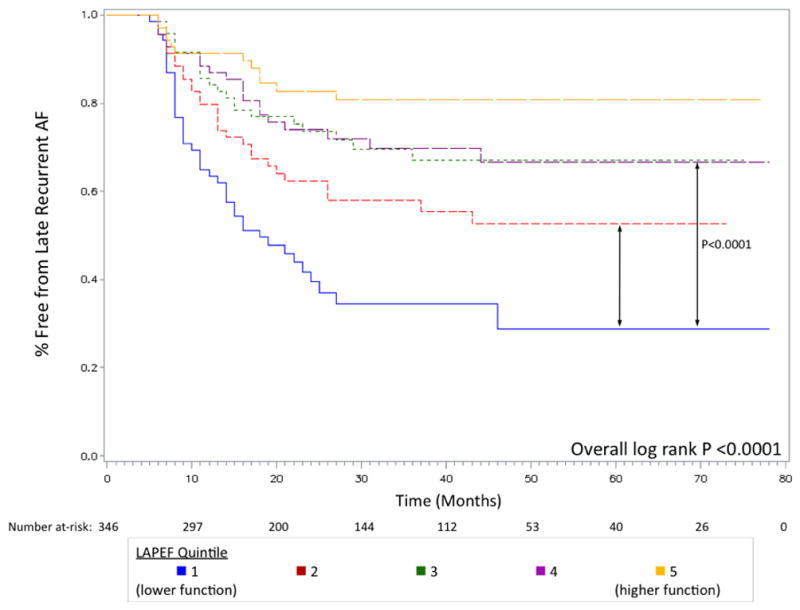
Patients with the highest LAPEF (“best function”, shown in orange) had the lowest recurrence rate over a median follow-up of 27 months. Selected P values for comparisons between groups are shown (quintile 1 vs. quintile 2, P=0.0113; quintile 1 vs. quintile 3, P<0.0001).
Multivariable Association with Recurrent AF
Results of the adjusted model are shown in Table 3. After adjusting for age, sex, and known clinical characteristics associated with AF recurrence (history of hypertension, history of diabetes, BSA-adjusted VOLmax, non-paroxysmal AF, LVEF, history of prior left atrial ablation), LAPEF maintained a strong association with AF recurrence. Compared with patients in the highest LAPEF quintile (“best function”), patients in the lowest LAPEF quintile (“worst function”) were nearly four times more likely to experience recurrent AF (hazard ratio [HR] for lowest vs. highest quintile = 3.92, 95% CI 2.01–7.65, P<0.0001). Presence of LGE in the left ventricular myocardium did not modify the robust association of LAPEF with AF recurrence in the multivariable model (HR for lowest vs. highest quintile = 3.88, P<0.0001).
Table 3.
Likelihood of late recurrent AF stratified by LAPEF quintile
| Hazard Ratio (95% Confidence Interval) for AF Recurrence | ||
|---|---|---|
|
| ||
| LAPEF Quintile | Unadjusted model | Adjusted model* |
| 5 (best function) | 1.00 (Ref) | 1.00 (Ref) |
| 4 | 1.73 (0.85–3.55) | 1.42 (0.68–2.94) |
| 3 | 1.77 (0.87–3.59) | 1.47 (0.72–3.03) |
| 2 | 2.80 (1.43–5.49) | 2.26 (1.13–4.51) |
| 1 (worst function) | 5.04 (2.65–9.60) | 3.92 (2.01–7.65) |
Wald Chi-square (unadjusted model) = 35.4; Wald Chi-square (adjusted model) = 53.7
Adjusted model: includes age, sex, hypertension, BSA-adjusted left atrial volume, non-paroxysmal AF, diabetes, left ventricular systolic function, >1 prior ablation procedure
We then evaluated whether the association of LAPEF with late recurrent AF was dependent on LVEF by including an interaction term (LAPEF*LVEF) in our multivariable model. We found that a significant interaction was present (Wald Chi Square = 12.84, P=0.012) which suggested that the association of LAPEF on late recurrent AF varied depending on LVEF. We subsequently stratified patients into categories of preserved (LVEF ≥50%) versus reduced (LVEF <50%) systolic function and ran adjusted multivariable models for the two categories. For patients with LVEF ≥50%, there was a strong association between LAPEF and late recurrent AF (unadjusted HR for lowest vs. highest quintile = 7.93, 95% CI 3.54–17.76, P<0.0001) while for patients with LVEF <50% the association was not significant (unadjusted HR for lowest vs. highest quintile = 0.98, 95% CI 0.23–4.15, P=0.976). Full results of these models are presented in Electronic Tables 1 and 2.
Model Discrimination for Recurrent AF at Two Years
For purposes of clinical applicability, we evaluated the incremental ability of LAPEF to predict late recurrent AF two years after PCI. The overall two-year rate of late recurrent AF was 33.4%. Model discrimination was relatively weak for the clinical model alone (C statistic = 0.674). With the addition of LAPEF, model discrimination improved significantly (C statistic = 0.724, P<0.0001 compared with clinical model alone). The ROC curves corresponding to these models are shown in Figure 4.
Figure 4. Receiver operating characteristic (ROC) curve for prediction of 2-year late recurrent AF after PVI with clinical model (blue) and clinical model plus LAPEF (orange).
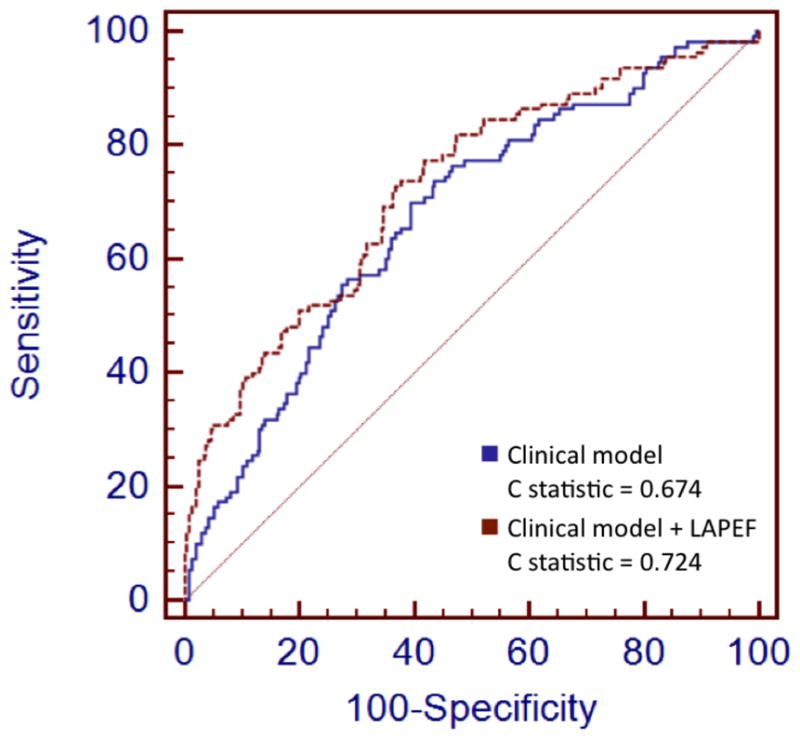
Clincial model included variables associated with late recurrent AF in prior studies (hypertension, left atrial volume adjusted for body surface area, non-paroxysmal AF, diabetes, left ventricular systolic function, and >1 ablation procedure). Addition of LAPEF to clinical model improved discrimination compared with clinical model alone (C statistics = 0.724 and 0.674 respectively, P<0.0001).
Sensitivity and Specificity of LAPEF Cutoff Values
We generated an ROC curve representing the model with the outcome of late recurrent AF with LAPEF (continuous) as the lone predictor (Figure 5). Sensitivity and specificity for three selected LAPEF cutpoints are displayed. With lower LAPEF, sensitivity for predicting recurrence decreased while specificity increased (LAPEF <20%: sensitivity=0.58, specificity=0.68; LAPEF <25%: sensitivity=0.70, specificity=0.54; LAPEF<30%: sensitivity=0.83, specificity=0.36. The C statistic for the logistic model with LAPEF alone was 0.676.
Figure 5. Receiver operating characteristic (ROC) curve for the prediction of late recurrent AF in model using LAPEF alone.
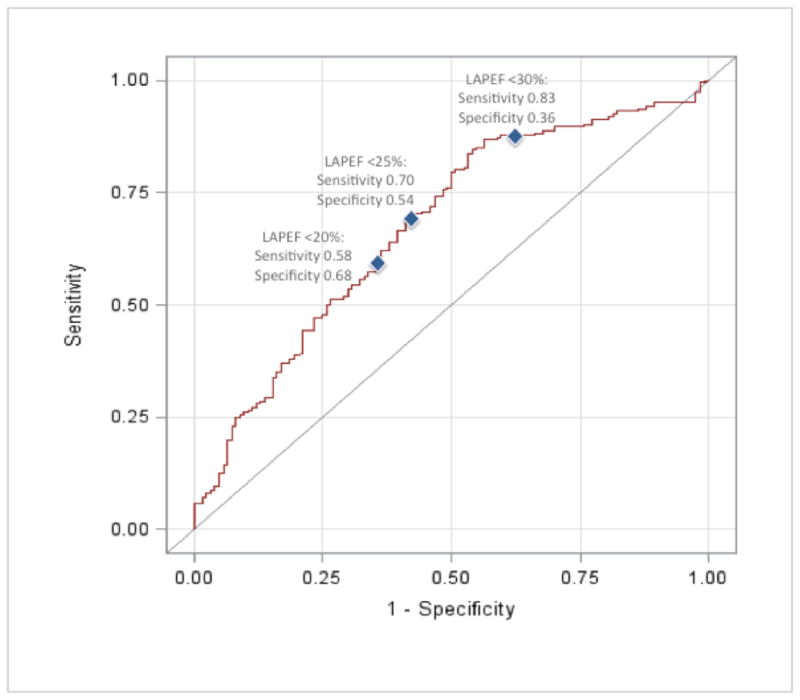
Sensitivity and specificity for three selected cutoffs (LAPEF <20%, LAPEF <25%, LAPEF <30%) are shown. With lower LAPEF values, sensitivity for predicting late recurrent AF decreased while specificity increased. Model C statistic = 0.676.
DISCUSSION
We found a strong association between CMR-determined left atrial passive emptying function (LAPEF) and late recurrent AF in patients undergoing PVI. This association was robust even after adjusting for other factors previously shown to be associated with recurrence, indicating that LAPEF represents an important predictor of outcomes in these individuals.
It has been recognized that impaired left atrial function independently contributes to adverse cardiac outcomes including new-onset heart failure17, heart failure hospitalization18, and AF after cardiac surgery19. Left atrial passive function occurs in early diastole and represents the “conduit” phase of left atrial function as blood is transferred from the pulmonary veins into the left ventricle. In patients with impaired left ventricular relaxation, filling pressures increase and left atrial passive function declines11. It has been hypothesized that this leads to the sequelae of left atrial stretch and pulmonary vein dilatation which places patients at increased risk for AF8. LAPEF can be precisely measured by CMR, which allowed us to investigate the association of passive ventricular filling (reflected in changes in left atrial volumes) with outcomes.
We chose to focus our study on patients undergoing PVI for AF as late recurrence of AF is a common clinical problem with an incidence of 25% at one year and 40% at five years, even with repeated PVI procedures2, 3, 5. Prior studies have demonstrated several predictors of late recurrent AF after ablation, including non-paroxysmal AF3, 6, left atrial volume5, 15, and hypertension15, although the associations have been generally weak and not universally observed. Currently there is no accepted way of individualizing risk stratification for recurrence, and patients are generally provided with an expected success rate of 60–80% after one or possibly two procedures2.
Our cohort showed a marked difference in the rate of late recurrent AF between quintiles; patients in the lowest quintile of LAPEF (worst left atrial passive function) had a recurrence rate of 60.5% at 2 years; conversely, those in the highest quintile of LAPEF (best left atrial passive function) had a recurrence rate of 17.3% over the same time period. Our findings support a mechanistic link between elevated filling pressures (as reflected by LAPEF) and late recurrent AF. Given the cost and potential complications associated with PVI, we believe that LAPEF may represent a physiologic marker that can assist physicians and patients in having more individualized discussions about the potential benefits of the procedure. While there is no universally accepted cutoff for rate of recurrence that would prohibit consideration for PVI, as multiple factors such as symptom burden and quality of life enter into the decision-making process, over half of patients with LAPEF <10% in our sample experienced late recurrent AF and these individuals should be considered at high risk for recurrence. This is in stark contrast to patients with LAPEF >40%, where fewer than one in five experienced recurrence. As CMR is routinely performed in many centers prior to PVI, the assessment of LAPEF can be done with little added cost.
Our findings must be interpreted in the context of our study design. Patients who were unable to undergo CMR prior to PVI were excluded, and certain comorbidities were therefore not available in our multivariable model (most notably, advanced renal insufficiency). As we were interested in the physiology of early (passive) diastolic filling, we also excluded patients who were in AF at the time of scan, since determination of LAPEF was not possible in these individuals. The use of LAPEF as a risk factor for late recurrent AF is therefore only applicable to patients in sinus rhythm at the time of CMR. In addition, since all patients did not undergo routine ambulatory ECG monitoring following PVI, some individuals may have experienced asymptomatic recurrent AF that was not counted in the primary outcome; this may have led to an under-estimate of the risk of recurrence. However, our methods reflect the care of post-PVI patients (and determination of recurrence) in routine clinical practice during the study period. In addition, our results represent a single-institution experience, although our reported recurrence rate is similar to those published from other experienced centers3, 5. Finally, our study sample was predominantly male, although this reflects PVI practice patterns described elsewhere20.
In conclusion, we found a strong association between CMR-determined LAPEF and recurrent AF after PVI that persisted after multivariable adjustment. We believe that LAPEF may be used as a potential marker for risk stratification and to inform patients about the potential success rate of PVI in a more individualized manner.
Supplementary Material
Acknowledgments
FUNDING SOURCES:
Dr. Dodson performed this work while on a T32 training grant from the NIH/NIA (T32 AG000158) and is currently supported by a grant from the NIH/NIA (RO3 AG045067), a T. Franklin Williams Scholarship Award (funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American College of Cardiology), and is the recipient of a Clinical Research Loan Repayment award from the NHLBI. Dr. Neilan is supported by an American Heart Association Fellow to Faculty Grant (12FTF12060588).
Abbreviations
- AF
atrial fibrillation
- LA
left atrium
- LAPEF
left atrial passive emptying function
- LV
left ventricle
- PVI
pulmonary vein isolation
Footnotes
CONFLICT OF INTEREST DISCLOSURES: None
References
- 1.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries: 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S, Crijns HJG, Damiano RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim Y, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao H, Wilber D, Calkins H, Kuck KH, Cappato R, Chen S, Prystowsky EN, Kuck KH, Natale A, Haines DE, Marchlinski FE, Calkins H, Davies DW, Lindsay BD, Damiano R, Packer DL, Brugada J, Camm AJ, Crijns HJG, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haissaguerre M, Hindricks G, Iesaka Y, Jackman WM, Jais P, Jalife J, Kalman J, Keane D, Kim Y, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Mansour M, Marchlinski F, McCarthy P, Mont JL, Morady F, Nademanee K, Nakagawa H, Nattel S, Pappone C, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao H, Wilber D, Ad N, Cummings J, Gillinov AM, Heidbuchel H, January C, Lip G, Markowitz S, Nair M, Ovsyshcher IE, Pak H, Tsuchiya T, Shah D, Siong TW, Vardas PE. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- 3.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 4.Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. doi: 10.1111/j.1540-8167.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 5.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Méndez F, Matiello M, Molina I, Brugada J. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 6.Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM, Cha Y, Shen W, Brady PA, Bluhm CM, Haroldson JM, Hammill SC, Packer DL. Long-term outcome of atrial fibrillation ablation: Impact and predictors of very late recurrence. J Cardiovasc Electrophysiol. 2010;21:1071–1078. doi: 10.1111/j.1540-8167.2010.01786.x. [DOI] [PubMed] [Google Scholar]
- 7.Cappato R, Calkins H, Chen S, Davies W, Iesaka Y, Kalman J, Kim Y, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 8.Tsang TSM, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 9.Allgayer C, Zellweger MJ, Sticherling C, Haller S, Weber O, Buser PT, Bremerich J. Optimization of imaging before pulmonary vein isolation by radiofrequency ablation: breath-held ungated versus ECG/breath-gated MRA. Eur Radiol. 2008;18:2879–2884. doi: 10.1007/s00330-008-1070-2. [DOI] [PubMed] [Google Scholar]
- 10.Syed MA, Peters DC, Rashid H, Arai AE. Pulmonary vein imaging: Comparison of 3D magnetic resonance angiography with 2D cine MRI for characterizing anatomy and size. J Cardiovasc Magn Reson. 2005;7:355–360. doi: 10.1081/jcmr-200053458. [DOI] [PubMed] [Google Scholar]
- 11.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TSM. Left atrial size: Physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Farzaneh-Far A, Ariyarajah V, Shenoy C, Dorval J, Kaminski M, Curillova Z, Wu H, Brown KB, Kwong RY. Left atrial passive emptying function during dobutamine stress MR imaging is a predictor of cardiac events in patients with suspected myocardial ischemia. JACC Cardiovasc Imaging. 2011;4:378–388. doi: 10.1016/j.jcmg.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Michaud GF, John R. Percutaneous pulmonary vein isolation for atrial fibrillation ablation. Circulation. 2011;123:e596–e601. doi: 10.1161/CIRCULATIONAHA.110.990028. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Tai C, Hsieh M, Tsai C, Lin Y, Tsao H, Yu W, Huang J, Ueng K, Cheng J, Ding Y, Chen S. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004;10:221–226. doi: 10.1023/B:JICE.0000026915.02503.92. [DOI] [PubMed] [Google Scholar]
- 16.Cha Y, Wokhlu A, Asirvatham SJ, Shen WK, Friedman PA, Munger TM, Oh JK, Monahan KH, Haroldson JM, Hodge DO, Herges RM, Hammill SC, Packer DL. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: A comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011;4:724–32. doi: 10.1161/CIRCEP.110.960690. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TSM. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–836. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease. Longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. 2012;59:673–680. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haffajee JA, Lee Y, Alsheikh-Ali AA, Kuvin JT, Pandian NG, Patel AR. Pre-operative left atrial mechanical function predicts risk of atrial fibrillation following cardiac surgery. JACC Cardiovasc Imaging. 2011;4:833–840. doi: 10.1016/j.jcmg.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Roten L, Rimoldi SF, Schwick N, Sakata T, Heimgartner C, Fuhrer J, Delacretaz E, Tanner H. Gender differences in patients referred for atrial fibrillation management to a tertiary center. Pacing Clin Electrophysiol. 2009;32:622–626. doi: 10.1111/j.1540-8159.2009.02335.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


