Abstract
Cultured heart cells have long been valuable for characterizing biological mechanism and disease pathogenesis. However, these preparations have limitations, relating to immaturity in key properties like excitation-contraction coupling and β-adrenergic stimulation. Progressive attenuation of the latter is intimately related to pathogenesis and therapy in heart failure. Highly valuable would be a long-term culture system that emulates the structural and functional changes that accompany disease and development, while concurrently permitting ready access to underlying molecular events. Accordingly, we here produce functional monolayers of adult guinea-pig ventricular myocytes (aGPVMs) that can be maintained in long-term culture for several weeks. At baseline, these monolayers exhibit considerable myofibrillar organization and a significant contribution of sarcoplasmic reticular (SR) Ca2+ release to global Ca2+ transients. In terms of electrical signaling, these monolayers support propagated electrical activity and manifest monophasic restitution of action-potential duration and conduction velocity. Intriguingly, β-adrenergic stimulation increases chronotropy but not inotropy, indicating selective maintenance of β-adrenergic signaling. It is interesting that this overall phenotypic profile is not fixed, but can be readily enhanced by chronic electrical stimulation of cultures. This simple environmental cue significantly enhances myofibrillar organization as well as β-adrenergic sensitivity. In particular, the chronotropic response increased, and an inotropic effect now emerges, mimicking a reversal of the progression seen in heart failure. Thus, these aGPVM monolayer cultures offer a valuable platform for clarifying long elusive features of β-adrenergic signaling and its plasticity.
Keywords: adult cardiomyocyte, cell culture, β-adrenergic response, electrical stimulation, cardiac electrophysiology, optical mapping
1. Introduction
Cultured heart cell preparations have proven to be an invaluable platform for the in-depth examination of biological mechanism, pathogenesis, and proof-of-principle therapy [1–6]. These models allow convenient observation and manipulation of numerous physiological mechanisms. Among the most important properties rendered accessible for scrutiny are sarcomeric myofibrillar architecture and excitation-contraction (E-C) coupling, action-potential propagation and morphology, and β-adrenergic signaling. These dimensions bear respectively on Ca2+ cycling and dysfunction, arrhythmogenesis, and the evolution of heart failure [7–9]. The latter malady is intimately mirrored by gradual desensitization of such signaling, while pharmacological blockade of the β-adrenergic cascade helps counter disease progression [10].
Immature myocytes have been the most popular starting source for cultures, because these cells are plentiful and typically yield preparations with lifespans of weeks or longer, permitting convenient genetic manipulability by viral transduction. In particular, commonly used cells include ventricular myocytes from neonatal/embryonic rat [11–15] and mouse [16, 17], and more recently from human stem-cell-derived cardiomyocytes [18–20]. The comparative ease of engineering transgenic mice adds to the popularity of the murine source [16], and immature cells from all of these origins can readily form planar monolayers or even three-dimensional configurations, allowing examination of higher-order network properties. Thus, phenomena like electrical wave propagation relating to arrhythmogenesis may be deconstructed with considerable resolution.
At the same time, cultures from these cell sources present significant limitations. Firstly, myofibrillar organization in these cultures can be incomplete, and related E-C coupling properties rather immature [19, 21, 22]. Secondly, cardiac action potential (AP) morphologies in rats and mice differ considerably from humans, in that the phase-2 plateau is largely lacking in these rodents [23], hampering their use as models for short- and long-QT-related human phenomena such as the genesis of early afterdepolarizations. This difference may reflect a predominance of rapidly activating K+ currents in the cardiomyocytes of these small rodents [24]. Thirdly, β-adrenergic signaling in these preparations is incomplete compared with adult cardiomyocytes, often featuring only chronotropic but not inotropic actions [19, 25, 26], in the absence of elaborate tissue engineering cues [20, 27]. In a sense, these cultures may reflect the immaturity of the source cells themselves. Finally, although preparations derived from human stem cells feature somewhat longer APs reminiscent of adult humans; they nonetheless lack mature β-adrenergic signaling and E-C coupling [19, 26, 28]. They are also comprised of a pleiotropic mixture of immature precursors to various cardiac cell types [29, 30].
Alternatives of considerable promise are cultures derived from adult cardiac myocytes, specifically from species featuring prominent phase-2 morphology, as well as robust chronotropic and inotropic responses to β-adrenergic stimulation [31] (e.g., guinea pigs, rabbits, and dogs). Acutely isolated myocytes from these sources are a mainstay of biophysical inquiry, but cultures derived from these cells have generally been associated with limited longevity, lasting less than a week [32, 33]. Yet, there have been reports of long-term cultures of adult cardiomyocytes [34], particularly from guinea pig [35, 36], rat [1, 37], and rabbit [38–40]. Indeed, guinea-pig preparations can even form confluent monolayers [35, 41]. Because of the adult origin of these cultured myocytes, there is hope that these cultures may recapitulate a more mature phenotype. To be specific, adult cells in culture initially undergo partial dematuration and subsequent rematuration [1, 5, 33, 41]. After rematuration, however, some cultures do exhibit clearly visualized striations, fueling expectations that a mature profile has been partially restored [1, 41]. Despite such potential, the extent to which these adult-derived, long-term cultures maintain mature E-C coupling and organization, adult-like AP morphology, and robust β-adrenergic responsiveness has received comparatively little study. Moreover, the functional tissue properties of such adult-cardiomyocyte-derived preparations, when reassembled as monolayers, remain unknown. Finally, largely unexplored is the potential of these cultures to adopt a more mature phenotype, upon exposure to appropriate environmental cues.
Here, we therefore scrutinize the morphological and functional characteristics of long-term cultures derived from adult guinea-pig ventricular myocytes (aGPVMs). A guinea-pig source is chosen because of the robust AP plateau phases and β-adrenergic signaling in these animals [31]. We form these cultures into monolayers, permitting exploration of myofibrillar organization and E-C coupling, AP propagation and morphology, and β-adrenergic responsiveness. We also demonstrate straightforward genetic manipulability by viral-mediated expression of auxiliary beta subunits of voltage-gated calcium channels, which markedly alters AP morphology. Most notably, we provide evidence that long-term aGPVM cultures possess considerable potential for additional maturation by an appropriate external prompt. Simple application of repetitive electrical stimulation over one week in culture [42, 43] improves spatial organization of contractile proteins. More intriguing is the manner of enhancement of β-adrenergic stimulation. Whereas baseline cultures (without long-term electrical stimulation) demonstrate chronotropic but not inotropic responses, cultures exposed to prolonged electrical stimuli exhibit not only stronger chronotropic enhancement, but clear reemergence of inotropic response. This preparation therefore offers a powerful and convenient system for exploring the molecular underpinnings of specific limbs of the β-adrenergic response, whose selective loss and gain parallels heart failure progression and treatment [10, 44]. Applying other environmental cues drawn from a burgeoning tissue-engineering toolkit will likely yield further maturation of this promising preparation. A portion of this study was previously presented [45].
2. Methods
2.1. Isolation and long-term culture of aGPVMs
The perfusion and isolation of adult guinea-pig ventricular myocytes was performed according to published protocols [46]. In brief, single ventricular myocytes were isolated from whole hearts of adult male guinea pigs (Hartley strain, 3–4 wk old, weight 250–350 g). After excision, the heart was placed in cold (4°C) Tyrode's solution (Ca2+ free) to remove blood and was mounted onto a Langendorff apparatus, and perfused retrogradely with the following three oxygenated solutions in sequential order: 1) modified Tyrode's buffer pH 7.5 containing (in M) NaCl (0.14), MgCl2 (0.001), HEPES (0.01), KCl (0.005) and Glucose (0.01); 2) digestion enzyme solution in Tyrode buffer pH 7.5, containing collagenase type 2 (78.13 U/ml) (Worthington CLS-2, Cat no. LS004177) and protease XIV (0.37 U/ml) (Sigma P5147); 3) High K solution (pH 7.4) containing (in M) K-Glutamate (0.12), KCl (0.025), HEPES (0.01), MgCl2 (0.001) and EGTA (0.001). Following perfusion the heart is excised and placed in a sterile high K-buffer in a petri dish and transferred to a laminar flow hood. The tissue is minced into smaller pieces and suspended several times to dissociate the cells. The cell suspension is filtered through a mesh and allowed to sediment for 15 min. The pellet is suspended in 10% M199 media and observed under a microscope.
For long-term culture, we used a method modified from Horackova et al. [47]. Plastic or glass cover slips were coated with laminin (20 μg/ml overnight at 4°C) and placed in 12 well tissue culture dishes (Becton Dickinson, NJ, USA). The dissociated adult guinea-pig cardiomyocytes were suspended in M199 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 2 mM L-Glutamine (Gibco), and MEM non-essential amino acids (Gibco). We found that the key to making a monolayer was to use a high density (105cells/ml) of rod-shaped cells during plating (60,000 cells/cm2 plating density). Cell-cell contact affects the hypertrophic response of the cells and the differentiated phenotype, and aids in cell survival [2]. Accordingly, guinea pig cardiomyocytes were plated at a cell density of 2×105 cells/ml on 20 mm coverslips placed in tissue culture dish. The cells were allowed to settle and attach at 37°C overnight in an atmosphere of 5% CO2-95% air. 24 hr post incubation, fresh M199 plus 10% FBS was added, and cells were cultured for 48hr. The culture medium was then changed to M199 plus 5% FBS for the next 72 hr. Following that, the medium was changed every other day to M199 plus 1% FBS, for the next three weeks. The inclusion of FBS was necessary for maintenance of monolayer cultures beyond ~1.5 weeks [1]. Cultures were periodically monitored for viability and confluency for a period of up to 5 weeks.
2.2. Electrical stimulation of aGPVM monolayers
Electrical stimulation was initiated 10–14 days after plating, once confluency was attained in the monolayer. Electrical stimulation was then applied for up to 21 days. Trains of electrical stimuli (4 ms duration, 1.5 Hz, 10 V/cm) were delivered using a C-Pace EP Culture Pacer (IonOptix, LLC, MA, USA), which likely impart increased repetitive regularity in syncytial contraction during culture [42, 48]. aGPVM cultures without electrical stimulation under identical conditions were included as `nonstimulated' controls.
2.3. Lentivirus or adenovirus transduction of aGPVMs
Adenoviral vectors for the β2a subunit were generated as previously described [49]. The lentiviral vectors for the β2a subunit were generated by using third-generation constructs as previously reported [50]. The cDNA for rat β2a (GenBank accession no. M80545) was subcloned into the lentiviral plasmid, pRRLsin18.cPPT.CMV.eGFP.Wpre (designated as LV-eGFP). The resulting plasmid, pPPT.CMV.β2aGFP was designated as LV-β2aGFP. LV was produced by calcium-phosphate transfection of HEK293 cells, followed by column-based purification and titering via previously described protocols [50].
aGPVMs at 19 days post-plating were infected with Adv-β2aGFP, and 24 h post-infection the medium was aspirated and replaced with fresh M199 medium containing 1% serum. 24 h later, the expression of Adv-β2aGFP was ~90% in aGPVMs. Alternatively, aGPVMs at 10 days post-plating were infected with LV-β2aGFP. LV-β2aGFP stock stored at −80°C was thawed on ice, suspended in M199 medium containing 5% serum and 8 μg/ml polybrene, and added onto the target aGPVMs. After 24 h of incubation at 37°C, the medium was aspirated from transduced aGPVM cultures, and fresh M199 medium containing 5% serum was added. The medium was then changed every 3–4 days. ~30% of myocytes were seen to express LV-β2aGFP (as judged by green fluorescence) after 10 days post-infection, and both the percentage and intensity of green-fluorescent cells continued to increase over time, approaching about 50% by 3 weeks in culture.
2.4. Immunocytochemistry
Immunocytochemistry of cultured aGPVM monolayers was performed according to previously established protocols [51]. Primary antibodies were diluted in 10% non-fat milk plus PBS, incubated with secondary antibodies conjugated with Alexa fluorochromes (1:500) for 1 hour at room temperature, and washed with Ca2+-free PBS. DAPI (1:100, Invitrogen) was used as a nuclear counterstain. Cells were mounted in Vectashield (Vector Laboratories) and visualized using a confocal microscope (Zeiss Axiovert 200 with 510-Meta). Two-dimensional spatial Fourier transforms were computed from immunostained confocal images (512×512) using the fft2 function of MATLAB 7.11.0 (MathWorks, Inc., MA). Images were initially rotated to align the long major axis along a vertical orientation, and the mean component of intensity subtracted prior to Fourier analysis. Absolute values of the Fourier components were averaged across transforms derived from multiple images.
2.5. Ratiometric calcium imaging
Total intracellular calcium [Ca2+]i was determined using indo-1/AM (Molecular Probes Inc.) according to our protocol described in [52]. Briefly, the aGPVM monolayer was loaded with indo-1/AM (1 μM) for 30 min at 37°C. The monolayer was gently washed several times with Ca2+ free Tyrode buffer pH 7.4 (described above) to washout extracellular dye, and further incubated for 15 min at 37°C to allow de-esterification of indo-1/AM. The monolayer was washed several times with Tyrode's solution and placed on the stage of an inverted microscope. The cells were electrically stimulated at a frequency of 0.5 Hz, and the fluorescence was measured using 340-nm excitation and 405- and 485-nm emissions. The 405/485nm fluorescence ratio was used as an indicator of [Ca2+]i. The monolayer was treated with ryanodine (10 μM) to inhibit SR function. Ca2+ concentration ([Ca2+]) was calculated as in our previous work [51], where [Ca2+] = Kd-Indo · β · (RIndo – Rmin) / (Rmax – RIndo). RIndo is the ratio of fluorescence emission signals at 485 and 405 nm (S485 / S405), with excitation at 340 nm. Kd-Indo was determined as 800 nM [53]. Rmin was determined to be 0.545 in our cultures bathed in 0 mM Ca2+ containing Tyrode's solution. Rmax was determined to be 1.56 in our cultures bathed in 5 mM 2,3-butanedione monoxime and 10 mM caffeine, all in standard Tyrode's solution. β was determined to be 1.754 as defined by the ratio of fluorescence emission signals at 485 nm obtained in elevated Ca2+ (caffeine) and zero Ca2+ conditions, as defined above.
2.6. Electrophysiological measurements
Optical mapping studies were performed on aGPVM monolayers 21 days post-plating using 10 μ-M di-4-ANEPPS (voltage-sensitive dye) or 5 μ-M Rhod-2-AM (calcium-sensitive dye), mainly using published methods [54]. Monolayers on plastic coverslips were utilized for robust characterization of the isochrone maps displayed in Figures 2 and 5, while glass coverslips were used for all Ca2+ imaging and lentiviral experiments to avoid coverslip autofluorescence. For completeness, characterization of monolayers on glass is displayed in Supplementary Notes 1, 2. To determine APD at 80% repolarization (APD80) and conduction velocity (CV), cells were stimulated at various pacing frequencies delivered by a bipolar line electrode at twice the diastolic threshold. A 2000 ms recording was taken after a twenty beat drive train. Action potentials were recorded from 253 sites using a custom-built contact fluorescence imaging system [54]. Conduction velocity, APD values and their spatio-temporal characteristics were analyzed from the optical maps using MATLAB data analysis scripts developed in the laboratory. The action potentials recorded at each of the 253 sites were scrutinized for quality and removed if the recording contained a weak signal. An average train of action potentials was calculated for each monolayer. For each channel, the action potential train was shifted in time to align the first action potential to time zero. This allowed for direct averaging of all 253 channels. APD80 for the monolayer was then calculated from this average trace, by taking the mean duration of several action potentials across the average train. Supplementary Note 8 demonstrates that the electrical responses were reproducible across multiple cultures, and that AP waveforms at individual sites were closely similar to the spatially averaged AP waveforms. All experiments involving isoproterenol were performed by adding 0.5 μM isoproterenol to the monolayer, and paired recordings were obtained both before and after 5–10 mins of drug exposure. Monolayers which had no measurable spontaneous activity before the addition of isoproterenol (≤ 15% of monolayers) were not included in the calculation of spontaneous rate, to facilitate comparisons between normally distributed populations. Calculation of relaxation time constant of Ca2+ transients (tau) was done by fitting a single Ca2+ transient exponential decay to the descending portion of the recorded calcium transient according to Ca = A · exp(-t / tau) + B where the baseline B was allowed to vary. To facilitate robust measurement, the exponential was fit by linear regression on dCa/dt plotted versus Ca, such that B was derived from the y intercept and −-1 / tau by the slope. For visual comparison, the average traces were replotted with the estimated baseline, and normalized before averaging the traces for display.
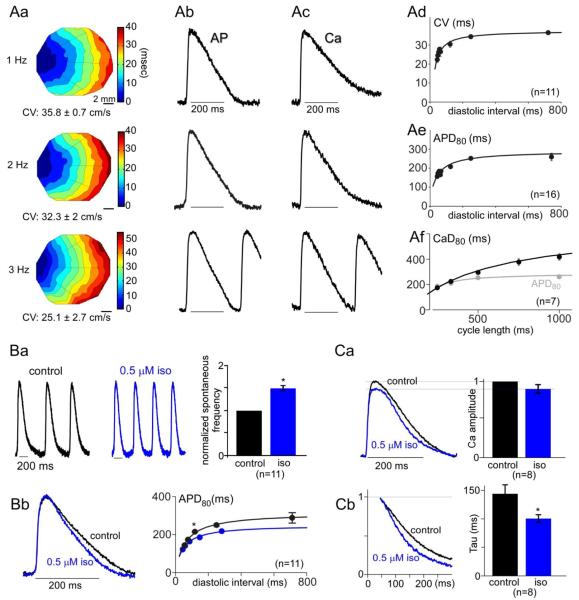
Fig. 2.
Interrelations between APs and cytosolic Ca2+ transients in syncytial aGPVM cultures; modulability by β-adrenergic stimulation. A: Baseline APs and Ca2+ transients in monolayer cultures without adrenergic stimulation. Aa: Exemplar isochrone maps for initial depolarization during steady stimulation at indicated frequencies, derived from optical mapping of di-4-ANEPPS fluorescence. CV, conduction velocity. CV was calculated along line trajectories, and averaged. Ab: AP morphology averaged across monolayer; same monolayer as in Aa. Ac: Ca2+ transient averaged across different monolayer, using Rhod-2. Ad: CV restitution curve, averaged from n = 11 monolayers. Error bars, SEM throughout. Ae: AP duration (APD80) restitution curve, averaged from n = 16 monolayers. Af: Ca2+ transient duration (Ca80, black) recovery curve, averaged from n = 7 monolayers. For reference, APD80 replotted from panel Ae as a function of cycle length (gray). B: Effect of isoproterenol on AP properties. Ba: Left and middle, spontaneous APs in exemplar monolayer, before and after exposure to isoproterenol (iso). This demonstrates a chronotropic effect. Right, average chronotropic effect across n = 11 monolayers (*, p < 0.01). Initial rate normalized to unity. Bb: Corresponding shortening of AP duration upon β-adrenergic stimulation. Left, exemplar APs averaged across another monolayer, ± iso. Steady 3 Hz pacing. Right, AP restitution curve ± 0.5 μM isoproterenol, averaged across n = 11 monolayers. Significant AP shortening by iso (*, p < 0.01). C: Effect of isoproterenol on Ca2+ transients, during 3 Hz stimulation. Ca: Lack of inotropic effect. Left, Ca2+ waveforms ± iso, averaged across n = 8 preparations. Right, corresponding bar graph summary. Cb: Isoproterenol nonetheless speeds Ca2+ transient relaxation. Left, normalized relaxation phase of Ca2+ transients, averaged across n = 8 preparations. Bar-graph summary of time constants for single-exponential fits to relaxation phase of Ca2+ transients (*, p < 0.01).
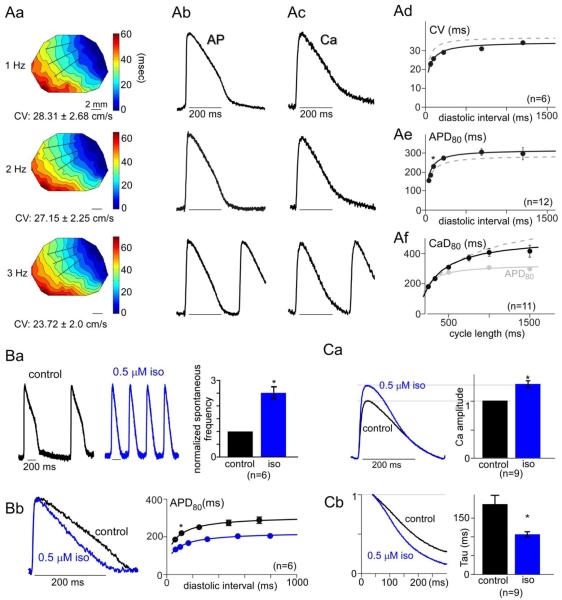
Fig. 5.
Effects of electrical stimulatory cue on syncytial aGPVM cultures, specifically in regards to AP properties, Ca2+ transients, and β-adrenergic responsiveness. Format identical to that in Fig. 2. A: AP and Ca2+ properties, demonstrating slight AP plateau enhancement and phase 3 acceleration in exemplar APs (Ab). Ae: Small prolongation of APD80 evident in electrical restitution curve (*, p = 0.01). B: Amplified chronotropic responsiveness (*, p < 0.01). C: Renaissance of inotropic effects (*, p < 0.01).
For cell-attached inside-out patch clamp studies, recordings were filtered at 2 kHz. To zero the membrane potential, the bath contained (in M): K glutamate, 0.132; KCl, 0.005; NaCl, 0.005; MgCl2, 0.003; EGTA, 0.002; glucose, 0.010; and HEPES (pH 7.4), 0.020, at 300 mOsm adjusted with glucose. The pipette solution contained (in M): TEA-MeSO3, 0.140; HEPES (pH 7.4), 0.010; and CaCl2 or BaCl2, 0.005; at 300 mOsm, adjusted with TEA-MeSO3. Data was fit with smooth leak functions before averaging, using published methods [49].
3. Results
3.1. Establishing long-term cultures of adult guinea-pig ventricular myocytes
As reported previously, successful long-term cultures required that freshly isolated adult guinea-pig ventricular myocytes be of high quality, as gauged by ~80–90% rod-shaped cells upon enzymatic dissociation. Myocytes that attached to laminin-coated substrate retained their striated patterns and rod-like shape for several days, but with increasing rounding at their ends. After this initial period, an intermediate phase was observed wherein cells remodeled substantially, as they flattened out, increased in surface area, and lost much of their visually apparent striation [5, 33, 34]. Following this intervening period, myocytes formed a confluent monolayer within ~2–3 weeks of isolation. During this `syncytial phase,' constituent cells regained sarcomeric structure, and monolayers demonstrated synchronous contractile activity. This configuration was maintained up to 5 weeks in culture, as observed previously [41]. Further characterization of these qualitative phases appears in Supplementary Note 3.
3.2. Myofibrillar organization and E-C coupling of long-term aGPVM cultures
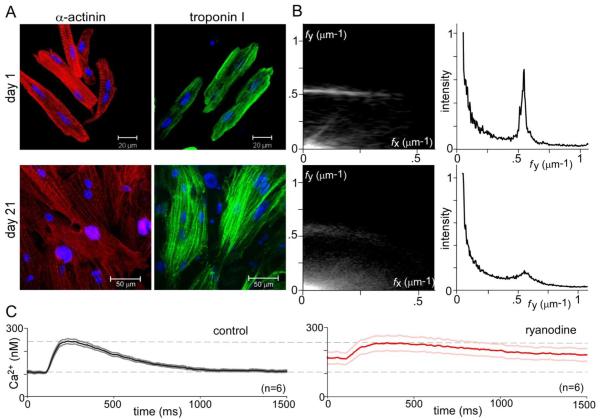
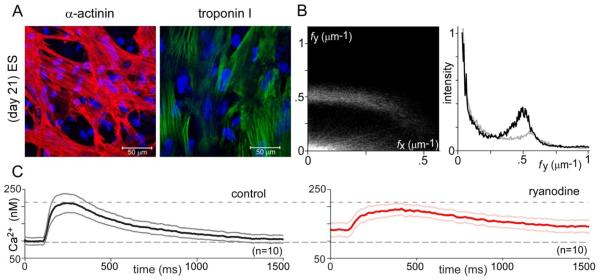
A key aspect of coordinated contraction of intact adult myocardium is myofibrillar organization and tight coupling between the sarcolemma and Ca2+ release sites [55]. We therefore compared representative attributes of these characteristics in acutely isolated (1 day) versus syncytial long-term cultures (21 day) of aGPVMs (Fig. 1). In confocal images of acute cardiomyocytes (Fig. 1A, top row), a high degree of myofibrillar organization is visually apparent from immunolabeling of α-actinin (red) and troponin I (green). The striated, sarcomeric organization of each of these proteins along the longer major axis of cells is obvious, with cellular orientation confirmed by the arrangement of DAPI-stained nuclei (blue). This ordered spatial configuration is quantitatively confirmed by the two-dimensional spatial Fourier transform of α-actinin images (Fig. 1B, left top), averaged over multiple cells and fields. Spatial frequencies along the major (fy) and minor (fx) axes are respectively displayed along the ordinate and abscissa, with Fourier magnitudes represented as plot intensity. Notably, a dominant spatial feature is the bright horizontal band at fy ~0.5 μm−1, corresponding to sarcomere repetition mainly along the major axis, with a dominant regular spacing of ~2 μm. The ordered sarcomeric organization along the major axis is further confirmed by the sharp peak at fy ~0.5 in the corresponding relative histogram representation (Fig. 1B, right top), obtained by summing Fourier amplitudes along fx, and plotting these sums as a function of fy. For reference, randomly oriented sarcomeres with ~2 μm spacing would appear as a bright semi-circle of radius ~0.5 μm−1 from the origin; and a lack of spatial organization would manifest as an overall diminution of specific Fourier features.
Fig. 1.
Myofibrillar organization and Ca2+ cycling in acute versus syncytial aGPVM cultures. A: Immunocytochemical images showing α-actinin (red) and troponin I (green) in acute aGPVMs (day 1, top row) and syncytial aGPVM cultures (21 days, bottom row). Nuclei of each cell stained by DAPI (blue). B: Two-dimensional spatial Fourier analysis of α-actinin images, averaged over multiple coverslips (top row, acute, n = 15; bottom row, syncytial cultures n = 16). Left, magnitude of Fourier components (brightness) as a function of spatial frequency along long major (fy) and orthogonal minor (fx) axes. Right, histogram representation of magnitude of Fourier components along fy, after summing over all fx. C: Cytosolic Ca2+ transients of syncytial aGPVM cultures obtained during 0.5 Hz field stimulation, as reported by Indo-1. Average response before (left, dark black) and after (right, dark red, n = 6) addition of ryanodine (10 μM), an inhibitor of RyRs. Gray (left) and pink (right) indicate ± SEM.
By comparison to acutely isolated cardiomyocytes, characterization of syncytial cultures (21 days) also indicated considerable myofibrillar organization (Figs. 1A, B, bottom row), but with diminished alignment along a major axis, and reduced observance of a strict ~2 μm spacing. These features are quantitatively evident in the Fourier analysis (Fig. 1B, bottom row), particularly in regards to the unmistakable existence of a peak (at fy ~0.5) in the histogram on the right, which is nonetheless broader and smaller. Still, no detectable t-tubular structure was evident as will be detailed later. Additional characterization of structural organization appears in Supplementary Note 4.
Beyond anatomical infrastructure, we also examined the origins of twitch Ca2+ transients in syncytial monolayer cultures of aGPVMs (Fig. 1C). The left panel displays the baseline Ca2+ transient during stimulation at 0.5 Hz. The waveform was derived from ratiometric Indo-1 signals acquired from a region encompassing several myocytes, and data represent the mean and standard error from several cultures. To test for the contribution of phasic Ca2+ release from SR in this overall response, we re-examined cytoplasmic Ca2+ transients after blockade of SR Ca2+ release by ryanodine (10 μM, Fig. 1C, right). This caused a significant reduction in the dynamic amplitude, and elevation of basal resting Ca2+ concentration. Both of these features demonstrate a substantial role of SR in maintaining Ca2+ dynamics, but not to the degree observed in acutely isolated myocytes [56]. In all, Ca2+ cycling in syncytial cultures retain important characteristics of the native adult phenotype, but fall short of a fully conserved profile.
3.3. Action-potential propagation and morphology
A second desired class of behaviors for cultures relates to AP propagation and morphology, both telling features for understanding electrical stability and arrhythmogenesis in the heart. Accordingly, we used optical mapping to characterize depolarizing waves in these monolayers, as visualized by staining preparations with the voltage-dependent fluorescent dye di-4-ANEPPS. We point-stimulated monolayers at frequencies exceeding their slow spontaneous rates, so as to achieve reproducible steady pacing under our command. Under these conditions, exemplar isochrone maps of AP depolarizing wave propagation could be readily obtained across a range of pacing frequencies (Fig. 2Aa), allowing measurement of conduction velocity (CV) across 18–20 mm2 recording areas. Radially-expanding waves were initiated and propagated across the monolayer, without wave breaks or arrhythmias. Moreover, we could routinely apply pacing cycle lengths of 1000, 500, 333, 250 and 200 ms. Overall population behavior could then be summarized by the CV restitution curve shown in Fig. 2Ad, which conforms to canonical expectations that CVs decrease with shortened intervals between depolarizations (Table 1).
Table 1.
Average action potential duration (APD80) and conduction velocities at different pacing cycle lengths in nonpaced and electrically paced aGPVMs
| Avg APD80 | Avg CV | |||
|---|---|---|---|---|
|
| ||||
| CL | Nonpaced | Electrically paced | Nonpaced | Electrically paced |
|
| ||||
| 1500 | Spont | 523 ± 21 (8) | Spont | 34.2 ± 0.6 (3) |
| 1000 | 281 ± 13 (10) | 430 ± 16 (14) | 32.5 ± 0.5 (2) | 31.0 ± 1.0 (5) |
| 500 | 250 ± 6 (11) | 323 ± 10 (12)** | 31.8 ± 1.3 (8) | 29.0 ± 1.0 (6) |
| 333 | 206 ± 4 (11) | 255 ± 6 (11)* | 30.3 ± 0.4 (8) | 25.7 ± 0.8 (6) |
| 250 | 182 ± 3 (11) | 197 ± 1 (7) | 26.8 ± 2.1 (10) | 22.9 ± 1.4 (5) |
| 200 | 145 ± 4 (10) | not captured | 19.9 ± 0.4 (6) | not captured |
p = 0.01 ;
p < 0.05
To assess AP morphology, optical action potentials were averaged across the monolayer, yielding the black waveforms in Fig. 2Ab. These exemplar responses approximated the duration of native adult action potentials. Indeed, this resemblance was confirmed over multiple monolayer preparations, as summarized by average AP duration expressed as a function of interstimulus interval (Fig. 2Ae). Durations were calculated at 80% depolarization (APD80), and further metrics are summarized in Table 1. Despite these similarities, APs in syncytial cultures did differ from the native profile by the lack of an overt plateau phase (Fig. 2Ab; Supplementary Note 5). The overall characteristics of APs were confirmed by current-clamp records, showing robust morphology and resting potentials near −80 mV (Supplementary Note 6).
Finally, the interrelation between AP waveforms and Ca2+ transients constitutes a remaining important coordinative feature of native adult myocytes; fully mature cardiomyocytes exhibit Ca2+ transients that outlast APs [57]. Such a comparison could be made for our syncytial monolayer cultures, by utilizing Rhod-2 Ca2+ imaging on the identical optical mapping configuration used for assessment of AP propagation and morphology. Fig. 2Ac displays exemplar Ca2+ transients obtained via this approach, where Ca2+ responses appear to outlast corresponding APs (Fig. 2Ab). Indeed, this impression was confirmed by averaging the corresponding duration of Ca2+ transients CaD80 (interval between transient onset and 80% return to resting levels) across multiple monolayers (Fig. 2Af, black). In particular, plotting CaD80 as a function of interstimulus interval reveals that while Ca2+ responses shorten with increasing stimulus rate, they persist beyond APs at nearly every frequency (Fig. 2Ae, gray). Overall, syncytial aGPVM cultures recapitulated many of the AP features of native adult tissue, as well as their interrelation to Ca2+ responses.
3.4. Beta-adrenergic modulation
A third major class of cardiomyocyte performance regards β-adrenergic responsiveness. Acutely isolated myocytes from guinea-pig, canine, and rabbit sources all exhibit strong responses mimicking those in humans, whereas those from diseased tissue exhibit a characteristic reduction in modulation [31]. We therefore wondered whether syncytial aGPVM monolayers would retain the favorable β-adrenergic sensitivity seen in acutely dissociated cells. A first indication of such responsiveness was a significant boost in the frequency of spontaneous action potentials seen upon application of 0.5 μM isoproterenol to an exemplar monolayer (Fig. 2Ba, left and middle). More specifically, before isoproterenol application, spontaneous APs occurred at a baseline frequency of 1.4 ± 0.2 Hz, as averaged across multiple (n = 11) monolayers. This spontaneous frequency increased to 2 ± 0.15 Hz with isoproterenol. These observations of a positive chronotropic response are summarized in the bar graph on the right (Fig. 2Ba).
To accommodate chronotropic effects, a related limb of the β-adrenergic response is the abbreviation of individual electrical responses even at fixed rates [58]. Indeed, APs shortened upon β-adrenergic stimulation, as visualized in exemplar APs elicited at a steady 3-Hz stimulation rate (Fig. 2Bb, left). Population data confirmed this trend, as demonstrated by an overall lowering of the average electrical restitution curve upon isoproterenol application (Fig. 2Bb, right).
Finally, we checked for an inotropic response, which would manifest as an increase in the peak amplitude of monolayer Ca2+ transients obtained with Rhod-2. Fig. 2Ca (left) shows Ca2+ transients averaged across multiple monolayers, before and after exposure to isoproterenol, with pacing held constant at 3 Hz. If anything, the Ca2+ transient was slightly diminished following β-adrenergic stimulation. This outcome was confirmed by representing the average effect across multiple monolayers in bar-graph format (Fig. 2Ca, right), where the response in isoproterenol was always normalized to a control obtained in the same monolayer. Thus, these syncytial aGPVM monolayers lacked the inotropic limb of the β-adrenergic response. Nonetheless, Ca2+ cycling was not entirely insensitive to β-adrenergic drive, as the relaxation rate of Ca2+ transients was accelerated by isoproterenol (lusitropic response), despite the lack of amplitude increase. This trend can be visually appreciated upon normalizing the average Ca2+ transients shortly after their peaks (Fig. 2Cb, left), and statistically confirmed by averaging across exponential fits performed in multiple monolayers (right). In all, these results substantiate that syncytial aGPVM monolayer cultures possess strong chronotropic and lusitropic responses to β-adrenergic drive, but lack an inotropic effect. This selective expression of adrenergic effects resembles that seen in many long-term culture preparations [25, 31], and suggests again that our syncytial aGPVM cultures only partially regain the native adult cardiac phenotype.
Having characterized baseline attributes of long-term aGPVM cultures, we next addressed two important and unexplored dimensions of this system: namely, genetic manipulability by viral transduction, and the potential for further maturation by appropriate environmental cues.
3.5. Genetic manipulability by Ca2+ channel subunits
Ca2+ channel auxiliary β subunits are required for trafficking channels to the surface membrane, and for regulating their inactivation properties [49, 59, 60]. In particular, the β2a subunit isoform is particularly adept at decreasing voltage-dependent inactivation (VDI) of cardiac L-type Ca2+ channels [49]. We therefore tested the capacity of cultured aGPVMs to be genetically manipulated by virally overexpressing β2a, and then checking for alterations of AP morphology and L-type Ca2+ channel activity. Should the transduction be successful, APs would be strongly prolonged by the reduced VDI.
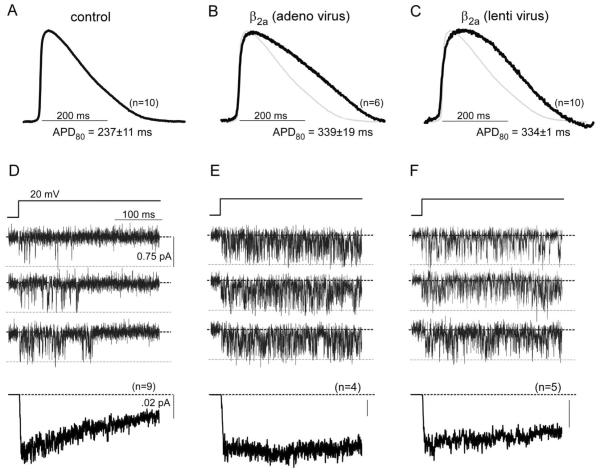
Viral transduction was undertaken in two ways, with similar outcomes. In the first, syncytial aGPVM monolayers (21 days) were exposed to adenoviral β2a-GFP for two days, after which preparations expressed green fluorescence. Corresponding APs, averaged across multiple monolayers, demonstrate marked prolongation (Fig. 3B, black trace) compared to those in parallel, uninfected cultures (Fig. 3A). As reference, the average control profile is reproduced in Fig. 3B as a gray waveform. This outcome demonstrated the ability of the culture to support intense transient expression of exogenous channel subunits. As an alternative approach, to test whether long-term expression of exogenous genes is also possible, we exposed aGPVM cultures within their first week to lentivirus bearing the same β2a-GFP construct. After establishment of syncytial cultures at 21 days, expressed green fluorescence, and APs similarly prolonged (Fig. 3C). Such AP prolongation was maintained up to the fifth week in culture (data not shown).
Fig. 3.
Genetic manipulability of aGPVM cultures by Ca2+ channel subunits. A: Control AP averaged across n = 10 syncytial aGPVM monolayers. Steady 2 Hz pacing. D: Corresponding single L-type Ca2+ channel activity from parallel cultures to those in A. Top, exemplar unitary Ba2+ currents evoked by +20-mV voltage step. Bottom, ensemble average current calculated from n = 9 such patches. Decay illustrates VDI. B, E: AP and Ca2+ channel activity following short-term expression of β2a-GFP construct via adenoviral transduction. Format as in A and D. Continued activity throughout voltage step indicates suppression of VDI. C, F: AP and Ca2+ channel activity following long-term expression of β2a-GFP construct via lentiviral transduction. Format as in A and D. Continued activity throughout voltage steps also indicates suppression of VDI.
To verify directly the effect of β2a subunits on L-type Ca2+ channels in the aGPVMs, we undertook cell-attached single-channel patch-clamp recordings of corresponding monolayers. Ba2+ was used as the charge carrier to focus observations on VDI effects, by eliminating the overlay of additional actions of Ca2+-dependent inactivation [61]. Exemplar single-channel records from parallel, uninfected control cells (Fig. 3D, upper three rows) demonstrated classic bursts of openings (downward deflections) that disappeared with maintained depolarization. The ensemble average current, drawn from multiple patches, clearly reveals a sharp decline indicating strong VDI (Fig. 3D, bottom row). When short-term expression of the β2a auxiliary subunit was achieved via adenovirus, channel openings were clearly enhanced across the duration of the step, and VDI was absent (Fig. 3E). Similarly, long-term lentiviral expression of β2a also achieved a reduction in VDI (Fig. 3F). These effects are consistent with those we observed previously upon β2a subunit expression in acutely dissociated aGPVMs [49]; rationalize the AP prolongation seen in these cultures.
3.6. Functional enhancement of syncytial aGPVM cultures by electrical stimulation
A remaining unexplored dimension of our experimental system concerns its potential for further maturation by application of appropriate external cues. One of simplest approaches in this regard has been the application of electrical stimulation to cardiomyocytes in culture, which has been reported to engender some enhancement of conduction and myofibrillar organization in other systems [34, 43, 48, 62, 63]. Here, we applied such stimulation to aGPVM cultures, commencing just after monolayer confluency was achieved (10–14 days), and continuing through 21 days in culture. We used a C-Pace stimulator to deliver 4-ms field stimuli at 1.5 Hz, with field strength of 10 V/cm. The resulting monolayer cultures were then characterized by the same screen used earlier for cultures obtained without the stimulatory cue (Figs. 1–2).
Following electrical stimulation of 1-week duration, myofibrillar organization was strikingly enhanced, as can be qualitatively appreciated from exemplar images of monolayers stained with α-actinin and troponin I (Fig. 4A). Myofibrillar organization is more uniformly arrayed along a major axis, and there is greater adherence to a periodic staining pattern across sarcomeres. These impressions were corroborated by two-dimensional Fourier analysis of α-actinin images, averaged across multiple preparations (Fig. 4B). The horizontal band at fy ~0.5 μm−1 is more prominent (Fig. 4B, left) than in controls (Fig. 1B, left), and the corresponding histogram (Fig. 4B, right, black trace) demonstrates a taller and sharper peak at fy ~0.5 μm−1 (gray curve from controls reproduced for reference). By contrast, this improvement in anatomical infrastructure was not matched by greater reliance upon SR Ca2+ release for overall Ca2+ cycling: the baseline Ca2+ transient and its alteration by ryanodine (Fig. 4C) is closely similar to that seen in controls (Fig. 1C). Likewise, no detectable t-tubule structure was evident even after chronic electrical stimulation (Supplementary Note 7).
Fig. 4.
Improved myofibrillar organization and maintained Ca2+ cycling of syncytial aGPVM cultures exposed to electrical stimulatory cue. Format identical to that in Fig. 1. A, B: Enhanced myofibrillar organization, most obviously apparent from larger and more distinct peak in B at fy ~ 0.5 μm−1 (black relation, averaged from n = 17 monolayers). For reference, gray relation is control histogram of culture without stimulatory cue, reproduced from Fig. 1B. C: Unchanged global Ca2+ cycling.
For AP propagation and morphology, the stimulatory cue also improved behavior, but to a lesser extent. To start, the spontaneous rate of cultures following the stimulatory cue was reduced to 0.83 ± 1.3 Hz (versus 1.4 Hz in controls, p < 0.05), facilitating capture of monolayers by external stimuli. Indeed, monolayer depolarization maps confirmed well-organized propagation (Fig. 5Aa) and conduction-velocity (CV) restitution curves (Fig. 5Ad; Table 1), which nonetheless were hardly different than control (cf., Fig. 2Aa, Ad). The control CV restitution relation is reproduced as the dashed gray curve in Fig. 5Ad, for reference. Regarding AP morphology, however, exemplar responses following the stimulatory cue (Fig. 5Ab, black) gave the impression of stronger repolarizing rates in phase 3, and a now almost-discernible phase 2. Fitting with this impression, the corresponding APD80 recovery curve, averaged over multiple monolayers (Fig. 5Ae, black; Table 1), confirmed longer AP durations than in control (Fig. 5Ae, * p < 0.05; Supplementary Fig. 2). Finally, the stimulatory cue did not alter the temporal interrelation between APs and Ca2+ transients. Exemplar Ca2+ transients (Fig. 5Ac, black) outlasted APs, and were nearly identical in time course to control waveforms (Fig. 2Ac). This impression was substantiated by the average restitution curve for Ca2+ transient durations (Fig. 5Af, CaD80 in black), which was identical to controls (gray dashed curve), and exceeded the electrical restitution curve throughout nearly all diastolic intervals (Fig. 5Af, gray symbols and solid fit).
Finally, the most remarkable effect of the electrical stimulatory cue relates to the profound increase in the sensitivity of cultures to β-adrenergic stimulation. Following this cue, the chronotropic response to 0.5 μM isoproterenol becomes enormous (Fig. 5Ba), and the corresponding shortening of individual APs was greatly amplified (Fig. 5Bb). Comparison with the behavior of cultures without the stimulatory cue (Fig. 2B) emphasizes just how large is the boost in chronotropy and related AP abbreviation. Still more striking was the effect on the inotropic response of monolayers (Fig. 5Ca). At 3-Hz stimulation, average Ca2+ transients (left) now demonstrate an outright boost in amplitude upon isoproterenol exposure, as confirmed statistically in bar-graph format (right). As well, the acceleration of Ca2+ transient relaxation rate by isoproterenol is markedly enhanced (Fig. 5Cb). Comparison with the baseline behavior in Fig. 2C highlights the stark change in the inotropic and related responses. The observed plasticity of β-adrenergic responses augurs well for the potential of aGPVM monolayers to undergo still further maturation upon exposure to additional environmental cues.
4. Discussion
Cultures drawn from adult cardiomyocytes have long served as a mainstay of cardiac mechanistic and biophysical inquiry [32, 64–66]. However, the bulk of work has focused on short-term versions of these cultures (< 1 week), comprised of separated individual cardiomyocytes that have not re-established network connectivity. Following this acute period, cultured adult myocytes undergo rapid and extensive dematuration, which may initially connote a downward spiral towards cell death [33]. Thus, questions relating to higher-order network behavior, or requiring longer-term stability, have usually fallen to apparently longer-lasting monolayer cultures derived from embryonic, neonatal, or stem-cell-derived sources [11–15, 18–20]. These cultures nonetheless lack many of the attributes relevant to adult myocytes. Another possible strategy arises from the pioneering work of Horackova and Claycomb, who established that following the extensive dematuration of adult ventricular myocytes in culture, these cardiomyocytes partially reverse course and rematurate to form spontaneously beating monolayers endowed with striations [1, 5, 35, 41, 67]. Their work raised the possibility that these `syncytial' aGPVM cultures might serve the requirements of longer-term stability and interconnectivity, while manifesting adult-like characteristics. Here, we deepen the characterization of this culture system, and reveal important new maturational plasticity that favorably positions this system for understanding cardiac mechanism and disease.
4.1. Baseline properties of long-term aGPVM cutures
Syncytial monolayer cultures of aGPVMs have received comparatively little characterization from the functional perspective, and have not been routinely evolved to the point of rivaling those that can be obtained from neonatal or embryonic cardiomyocytes. We find that these cultures exhibit substantial myofibrillar organization arrayed along a major longitudinal axis (Fig. 1A, B), and that the SR release contributes substantially to global Ca2+ transients (Fig. 1C). With regard to electrical signaling, aGPVM monolayers support propagated activity at multiple pacing rates with no conduction block; as well, they exhibit well behaved, monotonic restitution relations for CV, APD and Ca2+ transient duration. Unlike the abbreviated APDs seen in cultures from rat and murine sources [24, 68], APDs in the syncytial aGPVM cultures (Fig. 2A) are similar to those from acutely isolated aGPVMs and humans [69, 70], although a distinct phase 2 plateau is missing. Finally, β-adrenergic stimulation triggers a chronotropic and lusitropic, but not inotropic effect in these cultures (Fig. 2B, C). Overall, syncytial aGPVM monolayer cultures support stable, well-behaved properties reminiscent of a partially-retained, perhaps pre-adult functional profile.
4.2. Genetic manipulability of syncytial aGPVM cultures
Manipulation of protein levels by siRNA knockdown and recombinant construct expression, as conveniently mediated by viral techniques, has become an important tool for studying cardiac function [50, 71, 72]. However, the limited longevity of short-term cultures of acutely isolated adult myocytes restricts genetic manipulability to intense brief perturbations, as can be accommodated by adenoviral but not lentiviral strategies [71, 73]. Here, we have demonstrated that syncytial aGPVM cultures permit both acute and long-term manipulation of specific proteins, as respectively mediated by adenoviral and lentiviral approaches (Fig. 3). These results demonstrate a valuable capability for studying genetic abnormalities and signaling pathways in a syncytial setting, all with a unique functional profile.
4.3 Maturational plasticity of long-term aGPVM monolayer cultures
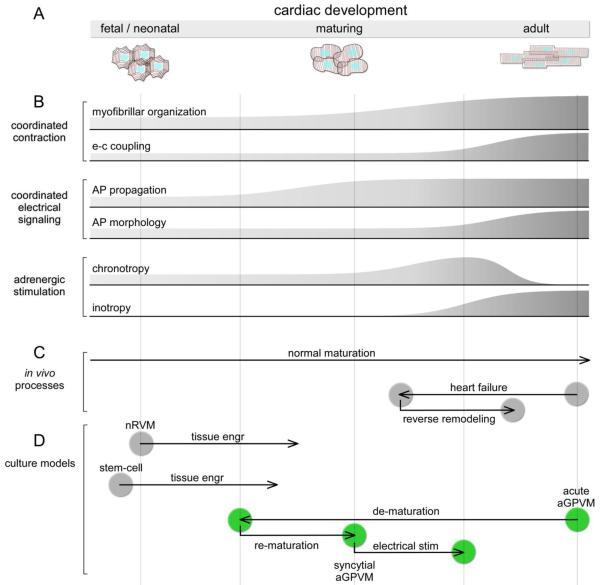
Perhaps, the most remarkable finding is the latent ability of long-term aGPVM monolayer cultures to readily undergo further maturation upon exposure to even simple environmental cues like electrical field stimulation. Impressive are the enhancements to myofibrillar organization and β-adrenergic signaling (Fig. 4–5). Certainly, the organization has not reached the level of native adult myocytes, as no definitive indications of t-tubules could be discerned following the stimulatory cue (Supplementary Note 7). Moreover, the magnitude of the inotropic effect in Fig. 5C still falls short of that observed in acutely isolated guinea-pig myocytes [31]. Nonetheless, given the growing sophistication of the tissue engineering toolkit for fostering syncytial maturation [20, 43, 62], significant additional improvements are likely forthcoming [74]. For the present, however, the maturational plasticity induced by simple stimulation is sufficiently impressive for us to consider where the long-term aGPVM culture might reside along the developmental spectrum portrayed in Fig. 6A.
Fig 6.
Spectrum of cardiac development and correspondence to various in vivo processes and culture models. A: Schematic and names of various epochs in cardiac development; cartoons of coarse cell-morphologic features for cardiac ventricular myocytes in various stages. B: Stylized graphical representation of various phenotypic limbs corresponding to development. C: Normal and heart-failure processes diagrammed with approximate registration to developmental spectrum (A) and phenotypic events (B). D: Approximate positions of various cultured cardiomyocyte systems. Green corresponds to aGPVM system, showing overlap with key events and positions of presumed heart-failure and reverse remodeling trajectories (C).
This spectrum is classically viewed according to the cell-morphologic progression from fetal/neonatal to adult phases, drawn here for ventricular myocytes (Fig. 6A). Myofibrillar and sarcomeric organization initially adopts reduced axial preference (fetal/neonatal), but gradually observes strict alignment with a major long axis (adult), culminating with the emergence of t-tubules late in this progression [21, 75–77]. Intimately related to these anatomical refinements is the maturation of E-C coupling, with increasing reliance upon SR Ca2+ release (versus L-type Ca2+ channel entry and Na+/Ca2+ exchange) for phasic contractions [77–79]. This sequence is represented in stylized graphical format below (Fig. 6B), under the grouping relating to coordinated contraction. Accompanying these classic hallmarks of development are multiple other phenotypic limbs, only two of which are shown. For coordinated electrical signaling, there is a progression of AP propagation, and refinement of AP morphology [80]. Finally, the schematized changes in chronotropic responsiveness mirrors the waxing and waning of HCN trancripts [81], and automaticity mostly disappears in fully mature ventricular myocardium [82]. In parallel, inotropy is initially absent, and emerges along the route to full maturity [83, 84].
Any such linear representation certainly oversimplifies development, but the overall scheme is nonetheless a useful heuristic construct, particularly for conceptualizing aspects of heart failure. While normal maturation proceeds simply from left to right (Fig. 6C), an insightful realization has been that heart failure represents in some sense a partial regression towards the left (Fig. 6C), with mimicry of a more neonatal-like profile [85, 86]. Likewise, ameliorative therapy (reverse remodeling) may represent a rightward return towards full maturity [10] (Fig. 6C). This view of heart failure is particularly apt with regard to β-adrenergic signaling, where a hallmark of heart failure is a loss of inotropy, coupled with the appearance of spontaneous ventricular contraction exacerbated by β-adrenergic drive [10, 87, 88]. These properties are just as predicted by progression along the right end of the developmental framework (Fig. 6B, β-adrenergic stimulation). Intriguingly, β-adrenergic blockade therapy for heart failure not only regularizes β-adrenergic signaling, but also many other phenotypic limbs of heart failure [10, 44]. Thus, progression along the developmental axis in regards to β-adrenergic signaling may be intimately intertwined with heart failure pathogenesis and therapy [44].
That said, the potential position of long-term aGPVM cultures along this developmental spectrum suggests a potentially unique and powerful role of this system for understanding heart disease. For orientation, neonatal and stem-cell-derived cultures begin towards the left end of the spectrum (Fig. 6D, gray), and there are notably few reports of inotropic responsiveness in these systems [20, 25]. Thus, even with environmental cues, their positions may often fall short of the rightward region traversed by adult heart disease. By contrast, for aGPVMs, the culture begins from the right end of the spectrum (Fig. 6D, green). Moreover, as exposure to the electrical stimulatory cue brings about the clear onset of inotropic responsiveness, it is tempting to speculate that our syncytial aGPVM cultures (before and after this cue) might straddle positions towards the rightward portion of the spectrum, overlapping the presumed trajectory of heart failure (Fig. 6C). Accordingly, the syncytial aGPVM culture offers a potentially special perspective on the β-adrenergic progression. The opportunity to reproducibly observe the onset of inotropy, especially when coupled with proteomic and transcriptome analysis [89, 90], may prove invaluable towards deducing underlying molecular mechanisms. In particular, the lack of inotropy before the stimulatory cue (Fig. 2Ca) likely corresponds to the inability of β-adrenergic stimulation to upregulate L-type channel Ca2+ channel opening (not shown), and the ability of such stimulation to enhance channel opening following chronic electrical stimulation. As the molecular mechanisms permitting such L-type channel upregulation have long remained contested [91, 92]. The long-term aGPVM culture may prove pivotal in shedding light on this and other long-standing questions.
Supplementary Material
Research Highlights JMCC7725R1.
Robust and convenient long-term cultures of adult guinea-pig ventricular myocytes.
Syncytial monolayers support radial wave propagation with enduring APs (200–300 ms).
Culture system readily permitting lasting viral expression of ion channel subunits.
Stable culture allowing selective mechanistic dissection of chronotropy and inotropy.
Reproducible plasticity of β-adrenergic inotropy induced by chronic pacing.
Acknowledgments
We thank Wanjun Yang for technical support and and Rajesh Sekar for his kind advice on lenti viral production. We also thank Manu Ben Johny for valuable advice and immunocytochemistry analysis. This work was supported by NIH R01 HL066239 (L.T.) and NIH 5R37HL076795 (D.T.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None.
References
- [1].Horackova M, Byczko Z. Differences in the structural characteristics of adult guinea pig and rat cardiomyocytes during their adaptation and maintenance in long-term cultures: confocal microscopy study. Exp Cell Res. 1997;237:158–75. doi: 10.1006/excr.1997.3775. [DOI] [PubMed] [Google Scholar]
- [2].Clark WA, Decker ML, Behnke-Barclay M, Janes DM, Decker RS. Cell contact as an independent factor modulating cardiac myocyte hypertrophy and survival in long-term primary culture. J Mol Cell Cardiol. 1998;30:139–55. doi: 10.1006/jmcc.1997.0580. [DOI] [PubMed] [Google Scholar]
- [3].Zhang Y, Sekar RB, McCulloch AD, Tung L. Cell cultures as models of cardiac mechanoelectric feedback. Prog Biophys Mol Biol. 2008;97:367–82. doi: 10.1016/j.pbiomolbio.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cho HC, Marban E. Biological therapies for cardiac arrhythmias: can genes and cells replace drugs and devices? Circ Res. 2010;106:674–85. doi: 10.1161/CIRCRESAHA.109.212936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kadota S, Minami I, Morone N, Heuser JE, Agladze K, Nakatsuji N. Development of a reentrant arrhythmia model in human pluripotent stem cell-derived cardiac cell sheets. Eur Heart J. 2013;34:1147–56. doi: 10.1093/eurheartj/ehs418. [DOI] [PubMed] [Google Scholar]
- [7].Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation. 2001;104:688–93. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- [8].Hobai IA, O'Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103:1577–84. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- [9].Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, et al. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- [10].Reinkober J, Tscheschner H, Pleger ST, Most P, Katus HA, Koch WJ, et al. Targeting GRK2 by gene therapy for heart failure: benefits above beta-blockade. Gene Ther. 2012;19:686–93. doi: 10.1038/gt.2012.9. [DOI] [PubMed] [Google Scholar]
- [11].Harary I, Farley B. In vitro studies of single isolated beating heart cells. Science. 1960;131:1674–5. doi: 10.1126/science.131.3414.1674. [DOI] [PubMed] [Google Scholar]
- [12].Jongsma HJ, van Rijn HE. Electronic spread of current in monolayer cultures of neonatal rat heart cells. J Membr Biol. 1972;9:341–60. [PubMed] [Google Scholar]
- [13].Sipido KR, Marban E. L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circ Res. 1991;69:1487–99. doi: 10.1161/01.res.69.6.1487. [DOI] [PubMed] [Google Scholar]
- [14].Price RL, Nakagawa M, Terracio L, Borg TK. Ultrastructural localization of laminin on in vivo embryonic, neonatal, and adult rat cardiac myocytes and in early rat embryos raised in whole-embryo culture. J Histochem Cytochem. 1992;40:1373–81. doi: 10.1177/40.9.1506674. [DOI] [PubMed] [Google Scholar]
- [15].Chlopcikova S, Psotova J, Miketova P. Neonatal rat cardiomyocytes--a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145:49–55. [PubMed] [Google Scholar]
- [16].Brand NJ, Lara-Pezzi E, Rosenthal N, Barton PJ. Analysis of cardiac myocyte biology in transgenic mice: a protocol for preparation of neonatal mouse cardiac myocyte cultures. Methods Mol Biol. 2010;633:113–24. doi: 10.1007/978-1-59745-019-5_9. [DOI] [PubMed] [Google Scholar]
- [17].Nuss HB, Marban E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J Physiol. 1994;479(Pt 2):265–79. doi: 10.1113/jphysiol.1994.sp020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee P, Klos M, Bollensdorff C, Hou L, Ewart P, Kamp TJ, et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res. 2012;110:1556–63. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cells Dev. 2013 doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–20. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Poindexter BJ, Smith JR, Buja LM, Bick RJ. Calcium signaling mechanisms in dedifferentiated cardiac myocytes: comparison with neonatal and adult cardiomyocytes. Cell Calcium. 2001;30:373–82. doi: 10.1054/ceca.2001.0249. [DOI] [PubMed] [Google Scholar]
- [22].Korhonen T, Hanninen SL, Tavi P. Model of excitation-contraction coupling of rat neonatal ventricular myocytes. Biophys J. 2009;96:1189–209. doi: 10.1016/j.bpj.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wetzel GT, Klitzner TS. Developmental cardiac electrophysiology recent advances in cellular physiology. Cardiovasc Res. 1996;31(Spec No):E52–60. [PubMed] [Google Scholar]
- [24].Knollmann BC, Schober T, Petersen AO, Sirenko SG, Franz MR. Action potential characterization in intact mouse heart: steady-state cycle length dependence and electrical restitution. Am J Physiol Heart Circ Physiol. 2007;292:H614–21. doi: 10.1152/ajpheart.01085.2005. [DOI] [PubMed] [Google Scholar]
- [25].Kuznetsov V, Pak E, Robinson RB, Steinberg SF. Beta 2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- [26].Pillekamp F, Haustein M, Khalil M, Emmelheinz M, Nazzal R, Adelmann R, et al. Contractile properties of early human embryonic stem cell-derived cardiomyocytes: beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy. Stem Cells Dev. 2012;21:2111–21. doi: 10.1089/scd.2011.0312. [DOI] [PubMed] [Google Scholar]
- [27].Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68:106–14. [PubMed] [Google Scholar]
- [28].Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–44. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- [29].Blazeski A, Zhu R, Hunter DW, Weinberg SH, Boheler KR, Zambidis ET, et al. Electrophysiological and contractile function of cardiomyocytes derived from human embryonic stem cells. Prog Biophys Mol Biol. 2012;110:178–95. doi: 10.1016/j.pbiomolbio.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blazeski A, Zhu R, Hunter DW, Weinberg SH, Zambidis ET, Tung L. Cardiomyocytes derived from human induced pluripotent stem cells as models for normal and diseased cardiac electrophysiology and contractility. Prog Biophys Mol Biol. 2012;110:166–77. doi: 10.1016/j.pbiomolbio.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ Res. 2008;102:e54–64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- [32].Mitcheson JS, Hancox JC, Levi AJ. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflugers Arch. 1996;431:814–27. doi: 10.1007/s004240050073. [DOI] [PubMed] [Google Scholar]
- [33].Bird SD, Doevendans PA, van Rooijen MA, Brutel de la Riviere A, Hassink RJ, Passier R, et al. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58:423–34. doi: 10.1016/s0008-6363(03)00253-0. [DOI] [PubMed] [Google Scholar]
- [34].Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- [35].Horackova M, Mapplebeck C. Electrical, contractile, and ultrastructural properties of adult rat and guinea-pig ventricular myocytes in long-term primary cultures. Can J Physiol Pharmacol. 1989;67:740–50. doi: 10.1139/y89-119. [DOI] [PubMed] [Google Scholar]
- [36].Huang XD, Horackova M, Pressler ML. Changes in the expression and distribution of connexin 43 in isolated cultured adult guinea pig cardiomyocytes. Exp Cell Res. 1996;228:254–61. doi: 10.1006/excr.1996.0324. [DOI] [PubMed] [Google Scholar]
- [37].Moses RL, Claycomb WC. Ultrastructure of terminally differentiated adult rat cardiac muscle cells in culture. The American journal of anatomy. 1982;164:113–31. doi: 10.1002/aja.1001640203. [DOI] [PubMed] [Google Scholar]
- [38].Haddad J, Decker ML, Hsieh LC, Lesch M, Samarel AM, Decker RS. Attachment and maintenance of adult rabbit cardiac myocytes in primary cell culture. Am J Physiol. 1988;255:C19–27. doi: 10.1152/ajpcell.1988.255.1.C19. [DOI] [PubMed] [Google Scholar]
- [39].Decker ML, Simpson DG, Behnke M, Cook MG, Decker RS. Morphological analysis of contracting and quiescent adult rabbit cardiac myocytes in long-term culture. Anat Rec. 1990;227:285–99. doi: 10.1002/ar.1092270303. [DOI] [PubMed] [Google Scholar]
- [40].Decker ML, Behnke-Barclay M, Cook MG, Lesch M, Decker RS. Morphometric evaluation of the contractile apparatus in primary cultures of rabbit cardiac myocytes. Circ Res. 1991;69:86–94. doi: 10.1161/01.res.69.1.86. [DOI] [PubMed] [Google Scholar]
- [41].Horackova M, Byzsko Z, Maillet-Frotten L. Immunohistochemical analysis of the adaptation of adult guinea-pig cardiomyocytes in long-term cultures and in cocultures with cardiac neurons: a novel model for studies of myocardial function. Mol Cell Biochem. 1997;172:227–38. [PubMed] [Google Scholar]
- [42].Sathaye A, Bursac N, Sheehy S, Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J Mol Cell Cardiol. 2006;41:633–41. doi: 10.1016/j.yjmcc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- [43].Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Blaxall BC, Spang R, Rockman HA, Koch WJ. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiological genomics. 2003;15:105–14. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- [45].Joshi-Mukherjee R, Dick IE, Liu T, O'Rourke B, Yue DT, Tung L. Manipulability of beta-adrenergic responsiveness in adult guinea-pig cardiomyocyte cultures (abstr.) Biophysical Journal. 2013;104:459a. [Google Scholar]
- [46].O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–6. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- [47].Horackova M, Morash B, Byczko Z. Altered transarcolemmal Ca transport modifies the myofibrillar ultrastructure and protein metabolism in cultured adult ventricular cardiomyocytes. Mol Cell Biochem. 2000;204:21–33. doi: 10.1023/a:1007080828602. [DOI] [PubMed] [Google Scholar]
- [48].Holt E, Lunde PK, Sejersted OM, Christensen G. Electrical stimulation of adult rat cardiomyocytes in culture improves contractile properties and is associated with altered calcium handling. Basic Res Cardiol. 1997;92:289–98. doi: 10.1007/BF00788941. [DOI] [PubMed] [Google Scholar]
- [49].Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, et al. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–52. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sekar RB, Kizana E, Smith RR, Barth AS, Zhang Y, Marban E, et al. Lentiviral vector-mediated expression of GFP or Kir2.1 alters the electrophysiology of neonatal rat ventricular myocytes without inducing cytotoxicity. Am J Physiol Heart Circ Physiol. 2007;293:H2757–70. doi: 10.1152/ajpheart.00477.2007. [DOI] [PubMed] [Google Scholar]
- [51].Joshi-Mukherjee R, Coombs W, Musa H, Oxford E, Taffet S, Delmar M. Characterization of the molecular phenotype of two arrhythmogenic right ventricular cardiomyopathy (ARVC)-related plakophilin-2 (PKP2) mutations. Heart Rhythm. 2008;5:1715–23. doi: 10.1016/j.hrthm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tay LH, Griesbeck O, Yue DT. Live-cell transforms between Ca2+ transients and FRET responses for a troponin-C-based Ca2+ sensor. Biophys J. 2007;93:4031–40. doi: 10.1529/biophysj.107.109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68:1453–60. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Weinberg S, Lipke EA, Tung L. In vitro electrophysiological mapping of stem cells. Methods Mol Biol. 2010;660:215–37. doi: 10.1007/978-1-60761-705-1_14. [DOI] [PubMed] [Google Scholar]
- [55].Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- [56].Sutko JL, Willerson JT. Ryanodine alteration of the contractile state of rat ventricular myocardium. Comparison with dog, cat, and rabbit ventricular tissues. Circ Res. 1980;46:332–43. doi: 10.1161/01.res.46.3.332. [DOI] [PubMed] [Google Scholar]
- [57].Yue DT. Intracellular [Ca2+] related to rate of force development in twitch contraction of heart. Am J Physiol. 1987;252:H760–70. doi: 10.1152/ajpheart.1987.252.4.H760. [DOI] [PubMed] [Google Scholar]
- [58].Munakata K, Dominic JA, Surawicz B. Variable effects of isoproterenol on action potential duration in guinea-pig papillary muscle: differences between nonsteady and steady state; role of extracellular calcium concentration. J Pharmacol Exp Ther. 1982;221:806–14. [PubMed] [Google Scholar]
- [59].Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J Biol Chem. 1996;271:26465–8. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- [60].Restituito S, Cens T, Barrere C, Geib S, Galas S, De Waard M, et al. The [beta]2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci. 2000;20:9046–52. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chao SH, Suzuki Y, Zysk JR, Cheung WY. Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol. 1984;26:75–82. [PubMed] [Google Scholar]
- [62].Radisic M, Park H, Gerecht S, Cannizzaro C, Langer R, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1357–68. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Heidi Au HT, Cui B, Chu ZE, Veres T, Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009;9:564–75. doi: 10.1039/b810034a. [DOI] [PubMed] [Google Scholar]
- [64].Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, et al. Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol. 2008;93:370–82. doi: 10.1113/expphysiol.2007.040659. [DOI] [PubMed] [Google Scholar]
- [65].Berger HJ, Prasad SK, Davidoff AJ, Pimental D, Ellingsen O, Marsh JD, et al. Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol. 1994;266:H341–9. doi: 10.1152/ajpheart.1994.266.1.H341. [DOI] [PubMed] [Google Scholar]
- [66].Ikeda U, Briggs GM, Allen PD, Sen L, Medford RM, Smith TW. Properties of adult rat ventricular cells in long-term culture. Cardioscience. 1990;1:225–33. [PubMed] [Google Scholar]
- [67].Claycomb WC, Moses RL. Culture of atrial and ventricular cardiac muscle cells from the adult squirrel monkey Saimiri sciureus. Exp Cell Res. 1985;161:95–100. doi: 10.1016/0014-4827(85)90493-8. [DOI] [PubMed] [Google Scholar]
- [68].Demir SS. Computational modeling of cardiac ventricular action potentials in rat and mouse: review. Jpn J Physiol. 2004;54:523–30. doi: 10.2170/jjphysiol.54.523. [DOI] [PubMed] [Google Scholar]
- [69].Rocchetti M, Besana A, Gurrola GB, Possani LD, Zaza A. Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J Physiol. 2001;534:721–32. doi: 10.1111/j.1469-7793.2001.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol. 2010;48:112–21. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kirshenbaum LA, MacLellan WR, Mazur W, French BA, Schneider MD. Highly efficient gene transfer into adult ventricular myocytes by recombinant adenovirus. J Clin Invest. 1993;92:381–7. doi: 10.1172/JCI116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sekar RB, Kizana E, Cho HC, Molitoris JM, Hesketh GG, Eaton BP, et al. IK1 heterogeneity affects genesis and stability of spiral waves in cardiac myocyte monolayers. Circ Res. 2009;104:355–64. doi: 10.1161/CIRCRESAHA.108.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kabaeva Z, Zhao M, Michele DE. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1667–74. doi: 10.1152/ajpheart.01144.2007. [DOI] [PubMed] [Google Scholar]
- [74].Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci USA. 2010;107:565–70. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, et al. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res. 1999;85:415–27. doi: 10.1161/01.res.85.5.415. [DOI] [PubMed] [Google Scholar]
- [76].Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 2007;22:167–73. doi: 10.1152/physiol.00005.2007. [DOI] [PubMed] [Google Scholar]
- [77].Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, et al. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res. 2003;58:535–48. doi: 10.1016/s0008-6363(03)00255-4. [DOI] [PubMed] [Google Scholar]
- [78].Orchard C, Brette F. t-Tubules and sarcoplasmic reticulum function in cardiac ventricular myocytes. Cardiovasc Res. 2008;77:237–44. doi: 10.1093/cvr/cvm002. [DOI] [PubMed] [Google Scholar]
- [79].Tanaka H, Shigenobu K. Effect of ryanodine on neonatal and adult rat heart: developmental increase in sarcoplasmic reticulum function. Journal of molecular and cellular cardiology. 1989;21:1305–13. doi: 10.1016/0022-2828(89)90676-7. [DOI] [PubMed] [Google Scholar]
- [80].Sheridan DJ. Postnatal developmental changes in the electrophysiological properties of cat right ventricular papillary muscles. Cardiovascular research. 1980;14:700–9. doi: 10.1093/cvr/14.12.700. [DOI] [PubMed] [Google Scholar]
- [81].Schweizer PA, Yampolsky P, Malik R, Thomas D, Zehelein J, Katus HA, et al. Transcription profiling of HCN-channel isotypes throughout mouse cardiac development. Basic research in cardiology. 2009;104:621–9. doi: 10.1007/s00395-009-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132–3. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- [83].Artman M, Kithas PA, Wike JS, Strada SJ. Inotropic responses change during postnatal maturation in rabbit. The American journal of physiology. 1988;255:H335–42. doi: 10.1152/ajpheart.1988.255.2.H335. [DOI] [PubMed] [Google Scholar]
- [84].Park MK, Sheridan PH, Morgan WW, Beck N. Comparative inotropic response of newborn and adult rabbit papillary muscles to isoproterenol and calcium. Developmental pharmacology and therapeutics. 1980;1:70–82. [PubMed] [Google Scholar]
- [85].Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–43. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- [86].Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–67. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- [87].Sridhar A, Dech SJ, Lacombe VA, Elton TS, McCune SA, Altschuld RA, et al. Abnormal diastolic currents in ventricular myocytes from spontaneous hypertensive heart failure rats. American journal of physiology Heart and circulatory physiology. 2006;291:H2192–8. doi: 10.1152/ajpheart.01146.2005. [DOI] [PubMed] [Google Scholar]
- [88].Cerbai E, Barbieri M, Mugelli A. Occurrence and properties of the hyperpolarization-activated current If in ventricular myocytes from normotensive and hypertensive rats during aging. Circulation. 1996;94:1674–81. doi: 10.1161/01.cir.94.7.1674. [DOI] [PubMed] [Google Scholar]
- [89].Aye TT, Soni S, van Veen TA, van der Heyden MA, Cappadona S, Varro A, et al. Reorganized PKA-AKAP associations in the failing human heart. J Mol Cell Cardiol. 2012;52:511–8. doi: 10.1016/j.yjmcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [90].Wayengera M. Searching for new clues about the molecular cause of endomyocardial fibrosis by way of in silico proteomics and analytical chemistry. PLoS One. 2009;4:e7420. doi: 10.1371/journal.pone.0007420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–44. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yang L, Katchman AN, Samad T, Morrow JP, Weinberg R, Marx SO. beta-Adrenergic Regulation of the L-type Ca2+ Channel Does Not Require Phosphorylation of alpha1C Ser1700. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.