Abstract
The frequent observation of both acute and chronic inflammation of unknown stimulus in the adult prostate has motivated a large body of research aimed at identifying potential infectious agents that may elicit prostatic inflammation. The overarching hypothesis is that infection-induced inflammation may be associated with prostate cancer development or progression, as inflammation is known to serve as an “enabling characteristic” of cancer. With recent advances in molecular techniques for microorganism identification, a panoply of microorganisms has been scrutinized in prostate tissues and in relation to prostate carcinogenesis. The aim of this review is to summarize the current literature on the evidence for infectious agents as a contributing factor to prostatic inflammation and prostate cancer, and to highlight recent literature suggesting an infectious etiology to the biogenesis of prostatic corpora amylacea and on the development of mouse models of prostatic infections.
Keywords: Infections, prostatitis, prostate cancer, acute inflammation, chronic inflammation, animal models
Introduction
The prevalence of both prostate cancer and prostatic inflammation are at nearly epidemic levels in the United States and in “Westernized” countries [1]. Prostate cancer development is thought to be mediated in part by genetics, but also by environmental exposures, as is evidenced by the apparent increase in prostate cancer risk when men from geographic areas with low prostate cancer incidence immigrate to Western countries [2]. Some of the environmental exposures that may confer this increase in prostate cancer risk likely include the same exposures that may contribute to the development of prostatic inflammation, including prostatic infections [1,3,4]. There is a well-known association between infections, infection-induced chronic inflammation, and the development of cancer. This is exemplified by the association between gastric infection by the bacterium Helicobacter pylori, the induction of chronic gastritis, peptic ulcers and gastric atrophy, and the subsequent development of gastric cancer. This is strikingly similar to prostate cancer where, in response to stimuli that are unknown but may potentially include prostatic infections, regions of prostatic atrophy, which are generally associated with inflammatory cell infiltrates, develop at a very high frequency to encompass large regions of the prostate. This inflammation-associated atrophy, or proliferative inflammatory atrophy (PIA), is hypothesized to serve at times as the direct precursor lesion to prostatic intraepithelial neoplasia (PIN) and/or prostate cancer [5,6].
The identification of infectious agents in the prostate of prostate cancer patients has been earnestly sought for many years. To date, there is no single microorganism (bacterial, viral, or otherwise) that is recognized as the stimulus for the asymptomatic inflammation observed in the prostate of cancer patients or that is known to directly contribute to prostate carcinogenesis, despite numerous reports on the presence of bacterial, protozoal, and/or viral species in the prostate of prostate cancer patients (see [4,7] for recent reviews on the topic). Microbial species postulated to contribute to prostate carcinogenesis include sexually transmitted infection (STI)-related microorganisms such as Chlamydia trachomatis and Trichomonas vaginalis, prostatitis-related microbes such as Escherichia coli (E. coli) and Pseudomonas spp., and viruses known to contribute to other cancer types, such as human papilloma virus (HPV). In this regard, STIs have probably received the most attention in relation to a potential contributory role in prostate carcinogenesis (reviewed in [8]). Trichomonas vaginalis (T. vaginalis) serostatus, for example, has been shown to be positively associated with prostate cancer risk [9], high grade disease [9], and measures of advanced disease [10]; although negative serological studies exist [11] as well as negative DNA-based studies performed on radical prostatectomy tissue specimens [12,13]. HPV is an STI that has also been widely examined in relation to prostate cancer, as it is known to infect the male genitals and genitourinary tract [14] and is associated with several types of cancer including cervical, vulvar, vaginal, penile, and oropharyngeal. Efforts to detect HPV DNA in prostate tissue samples have yielded both positive [15-17] and negative [12,13,18] results. Associations between prostate cancer risk and HPV subtype serostatus have generally yielded null results (studied and reviewed in [19]); however, it should be noted that HPV type-specific seroprevalence may not fully estimate cumulative HPV exposure, as not all HPV exposures in men lead to seroconversion [20]. Another study in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial found a slightly higher risk for prostate cancer (odds ratio, 1.3; 95% CI, 1.0-1.6) in men with any of 7 STIs (Chlamydia trachomatis, HPV-16 and -18, herpes simplex virus-2, cytomegalovirus, human herpesvirus-8, syphilis and gonorrhea) versus none [21]. Furthermore, an interesting series of studies have shown that men with certain STIs such as chlamydia, gonorrhea, and trichomonosis are more likely to have higher serum prostate-specific antigen (PSA) levels. These findings are of interest as they largely implicate prostate involvement of the STI [22,23]. In other words, the most straightforward mechanism by which PSA levels would be elevated with an STI would be that infection of the prostate by the STI leads to prostatic inflammation and damage to prostate epithelial cells, resulting in release of PSA extracellularly that, in turn, is released into the circulation.
An overall consensus on the contribution of infectious agents to prostate carcinogenesis is not yet established, and remains a field of active research. This has been somewhat confounded by the fact that many positive associations between microorganisms and prostate cancer that have been reported in the literature have failed to be confirmed in follow up studies, or have been debunked as assay contaminants as opposed to true infections [4,24-26]. Despite the fact that a definitive causative infectious agent or agents has yet to be identified, accumulating evidence both in human studies and in animal models continue to indicate that infections may contribute to potentially tumor-promoting chronic prostatic inflammation. The following sections will cover this recent literature, and provide hypotheses as to why the “H. pylori of prostate cancer” has been so difficult to find.
Evidence for prostatic infections in the adult prostate
In younger men, infection-induced chronic inflammation of the prostate is known to occur as a disease entity called chronic bacterial prostatitis. The clinical syndrome of prostatitis is heterogeneous and is categorized by the National Institutes of Health (NIH) consensus classification as chronic prostatitis⁄chronic pelvic pain syndrome (CPPS). CPPS is divided into four categories, only the first three of which relate to men with symptoms: (I) acute bacterial prostatitis; (II) chronic bacterial prostatitis; (III) chronic prostatitis⁄CPPS. Category (IV) is asymptomatic inflammatory prostatitis; meaning there are inflammatory infiltrates in prostatic tissue that are not recognizably associated with clinical symptoms [27]. Whereas there is some epidemiological evidence associating symptomatic prostatitis with prostate cancer risk [28,29], concerns with prostatitis case-control studies include biases such as detection bias. That is, since prostatitis is associated with increased PSA levels, if a man visits his doctor for prostatitis symptoms and is found to have an elevated PSA, he is more likely to be screened for prostate cancer and therefore more likely to be diagnosed with prostate cancer. Likewise, a comprehensive analysis of the influence of prostatic inflammation on prostate cancer initiation and/or progression is difficult to perform due to the very high prevalence of prostatic inflammation that occurs in many patients in which there is an absence of symptoms or in which inflammation is not recognizably related to symptoms. In other words, it is likely that only a small fraction of the inflammation that occurs in the adult prostate is either symptomatic or prompts a doctor’s visit. Evidence that asymptomatic prostatic inflammation (i.e., “histological prostatitis”) is very common in the prostate of the adult male includes histological analysis of biopsies from men tested for prostate cancer due to elevated PSA levels, radical prostatectomy specimens from men being treated for prostate cancer, transurethral resection of the prostate (TURP) specimens from men treated for benign prostatic hyperplasia (BPH), and autopsy specimens [3,30-37].
The majority of the asymptomatic inflammation that is observed in the prostate is classified as chronic inflammation (i.e., as evidenced by the presence of monocytic and/or lymphocytic inflammatory cell infiltrates), however acute inflammation is also observed to a lesser degree [3] (see Figure 1). Acute inflammation, as is typically evidenced by the infiltration of neutrophils, is classically an indicator of an infectious process; although this is not always the case. It could be argued that the presence of neutrophils in prostatectomy specimens from men undergoing treatment for prostate cancer is due to a response against colonic bacteria introduced into the prostate when the man underwent a prior diagnostic transrectal biopsy. This cannot account for all incidence of acute inflammation in prostatectomy specimens, however, as acute inflammation is also seen on prostate biopsies, and was therefore pre-existing and could not have been induced by the biopsy procedure [38,39]. Likewise, acute inflammation is also observed on autopsy prostate specimens [34]. Another theory is that since prostatic fluid contains chemokines, such as IL-8 [40] which is a potent neutrophil chemoattractant, injury to the prostatic epithelium or disruption of epithelial architecture and subsequent “leakage” of prostatic fluid could be potently pro-inflammatory. Addition-al evidence for a high prevalence of acute inflammatory events in the adult prostate, potentially due to bacterial infections, has come out of recent literature regarding tiny concretions called corpora amylacea that occur frequently in the adult prostate.
Figure 1.
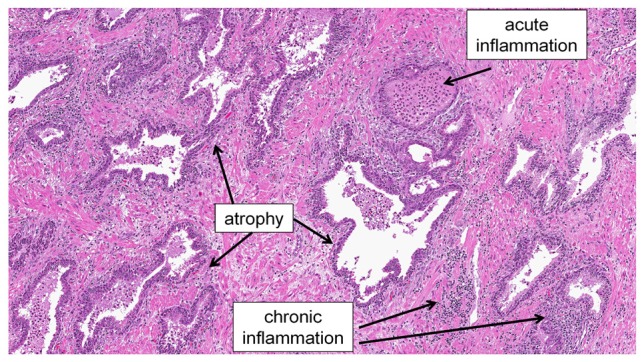
An example of the peripheral zone of the prostate from a radical prostatectomy specimen showing both acute and chronic inflammation in the setting of inflammatory atrophy.
Prostatic corpora amylacea as remnants of previous prostatic infections
Prostatic corpora amylacea are tiny laminated bodies that are observed histologically numbering from few to several thousands in the prostate of the adult male (Figure 2). Although prostatic corpora amylacea have been known to exist since the 1770’s [41,42], until recently, very little has been known about their composition or process of biogenesis. Corpora amylacea are presumably precursors to calcified “stones” called prostatic calculi that occur in the prostate at a much lower frequency. In 2009, a study that utilized high-performance liquid chromatography combined with tandem mass spectrometry (LC/MS/MS) to comprehensively analyze the protein components of prostatic corpora amylacea and calculi reported that the predominant proteins comprising these concretions are proteins involved in acute inflammation, and in particular proteins contained in neutrophil granules [43]. Speci-fically, the most prevalent protein identified was a protein called lactoferrin, which is a member of the transferrin family of proteins and is found in common bodily secretions such as milk, saliva, and tears. Lactoferrin is an iron-binding protein traditionally recognized for its role in innate immunity as a bacteriostatic molecule. Other proteins identified in this study included S100 calcium-binding proteins A8 and A9 (which complex to form human calprotectin), myeloperoxidase, and α-defensins, all of which (along with lactoferrin) are acute inflammatory proteins contained in neutrophil granules and play a critical role in defense against bacterial infections. The authors conclude that corpora amylacea may represent the remnants of past inflammatory events in the prostate, possibly due to bacterial infections [43].
Figure 2.
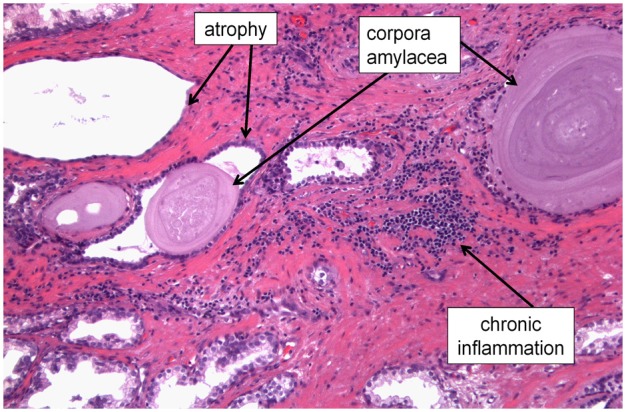
Corpora amylacea in a prostatectomy specimen shown along with areas of prostatic atrophy and chronic inflammation. We hypothesize that the presence of corpora amylacea in the adult prostate serves as an indicator of past acute inflammatory events, possibly due to previous prostatic infections.
In support of this, multiple studies have reported on the ability to culture bacteria such as E. coli and Pseudomonas spp. from prostatic calculi [44-47]. A study published subsequent to the Sfanos et al. study also reported the presence of S100A8 and S100A9 in corpora amylacea and additionally identified both DNA and proteins from E. coli in corpora amylacea samples [48]. Another recent study demonstrated the presence of bacterial “imprints” in prostatic calculi using scanning electron microscopy (SEM) [49]. Bacterial imprints are akin to fossil “footprints” that have been left imprinted into the chemical makeup of a stone, as is known to occur in kidney stones that form as a result of bacterial infections [50]. Due to the spherical shape and clustering pattern of the observed bacterial imprints in prostatic calculi, Dessombz et al. propose that the infecting organisms may be staphylococci [49]. Interestingly, Staphylococcus spp. have been previously implicated in bacterial prostatitis and have also been previously isolated from prostatectomy specimens [12,51-54]. Finally, structures resembling the early formation of corpora amylacea were reported to occur following neutrophil infiltration into the dorsal prostate in a rat model of infection-induced prostatitis [55]. Collectively, these studies indicate a potential infectious etiology to the development of corpora amylacea and prostatic calculi. Since corpora amylacea are so prevalent in the adult prostate, their presence may also indicate that asymptomatic acute inflammation, possibly due to bacterial infections, occurs far more frequently in the prostate than previously understood.
Corpora amylacea themselves may also serve as a stimulus for inflammation in the prostate, as they are often observed eliciting physical trauma to glandular epithelium and with associated surrounding focal chronic inflammation (Figure 2). Whether the biogenesis of or inflammation associated with corpora amylacea contributes to prostate carcinogenesis is unclear however. Previous studies on this topic have yielded disparate results [45,56-58], however, many of these studies are compromised by methods that would not necessarily identify corpora amylacea in the peripheral zone of the prostate where prostate cancer arises (reviewed in [43]). Intriguingly, ACI/Seg rats, which are among the few known rodent models of spontaneous prostate cancer, are also one of the only known rodent models to develop a large number of corpora amylacea in the prostate that precedes the development of adenocarcinoma [59]. Taken as a whole, whereas there is currently strong evidence that corpora amylacea are associated with inflammatory events in the prostate, it remains unclear as to whether the biogenesis or presence of corpora amylacea is directly related to the carcinogenic process.
Mouse models of infection-driven, long-term prostatic inflammation
One of the challenges to establishing a definitive role for inflammation and/or infection as a causative factor in prostate cancer may be evidenced by what is known about other recognized associations between infections, chronic inflammation and cancer. That is, there is often an extended period of time (many years) between initial infection with the microbial agent, the induction of chronic inflammation, and the development of cancer. This scenario would imply that chronic inflammation could persist in the prostate of men for many years without symptoms - and indeed, as discussed in detail above, the prevalence of asymptomatic prostatic inflammation in men (i.e. “histological prostatitis”) appears to be quite high in adult men. Whereas in other known associations between infections and cancer, the causative infectious agent is typically present and detectable at the time when cancer develops, we hypothesize that for prostate cancer this may not be the case. We propose that prostatic infections that occur early in life may induce chronic inflammation that persists for months or even years after the initial infection, and possibly independent of persistent presence of the infectious agent. Evidence in support of this hypothesis has come from animal models of prostate infection that have been recently described in the literature that use the bacterium Propionibacterium acnes (P. acnes) to elicit prostatic inflammation.
P. acnes is a pro-inflammatory bacterium that is considered to be the etiological agent in the skin condition acne (acne vulgaris), as well as several other inflammatory conditions including endocarditis and post-surgical infections [60]. This species was first reported to be correlated with prostate inflammation and cancer in 2005 [51], and several subsequent studies have also reported on the presence of P. acnes in prostate specimens [12,52,61,62]. Although not all studies have shown a positive association, the correlation between acne and/or plasma antibodies to P. acnes and prostate cancer incidence and outcomes has also been examined in multiple epidemiological studies [63-65]. In addition, in vitro studies have demonstrated that P. acnes is capable of inducing a strong inflammatory response in prostate cell lines [62,66,67]. A recent multi-locus sequence typing (MLST) study of prostatectomy-derived strains of P. acnes found that the strains of P. acnes that are isolated from prostate cancer patients group with the strains of P. acnes that are associated with urethral flora and/or opportunistic infections as opposed to isolates that are associated with normal skin flora [52]. This particular finding is of interest, as it supports the supposition that the route of entry for infectious agents that may contribute to prostate cancer etiology is via the prostatic urethra.
To date, two rodent models of P. acnes-induced prostatic inflammation using human prostate-derived isolates of P. acnes have been described. The first was a study in Sprague Dawley rats, where the ventral and dorso-lateral prostate lobes of 3-4 month old animals were inoculated with P. acnes via abdominal incision and direct prostate injection [55]. In this model, prostatic injection of P. acnes cells caused a strong acute response followed by chronic inflammation that persisted particularly in the dorso-lateral lobe in a subset of animals at 3 and 6 months post-infection. The presence of live bacteria was monitored by performing colony forming unit (CFU) counts on prostate tissue homogenates. Whereas high numbers of bacteria were recovered at 3 weeks post-infection, very low numbers of cells could be cultured at 3 months post-infection. By quantitative PCR (qPCR), low levels of P. acnes could still be detected by 6 months post-infection; however CFU counts were not performed at this time point.
A second study of P. acnes infection of the rodent prostate was performed in C57BL/6J mice where a human prostatectomy-derived isolate of P. acnes was introduced into the prostate of 8-10 week old animals via urethral catheterization [68]. In this model, a strong acute response was observed at 1 week post-infection that was restricted to the dorsal lobe of the prostate followed by chronic inflammation that persisted in a subset of animals up to 8 weeks post-infection. Although the extent and severity of chronic inflammation decreases somewhat over time, to date, we have observed that the chronic inflammation in this model can persist for at least a year post-infection (D. Biswal Shinohara, K. S. Sfanos, unpublished data). Intact P. acnes cells could be detected using immunohistochemistry (IHC) with a P. acnes-specific antibody at both one week and 2 weeks post-inoculation. Interestingly, an intracellular presence of the bacterium in prostate epithelial cells was described for this model [68]. By 8 weeks post-inoculation, P. acnes could no longer be detected using IHC, however chronic inflammation was observed in 60% of animals.
Collectively, these studies have important implications in regards to bacterial infections in the prostate. First, a single bacterial infection clearly has the potential to induce long-term chronic inflammation in the rodent prostate that persists for months or even up to a year post-infection. Second, although complete bacterial clearance is not certain, the persistence of chronic inflammation in the prostate does not appear to be dependent on the persistence of high levels of the infecting bacterium. It is possible that P. acnes could persist at cryptic levels in the prostate and cause recurrent infections over time that drive chronic inflammation, and this will undoubtedly be a focus of future studies in rodent models.
Conclusions and future directions
Although the “H. pylori of prostate cancer” has been sought for many years, there is no single infectious agent for which a definitive link to prostate cancer has been established. Despite this fact, evidence continues to mount for a high frequency of asymptomatic infections in the prostate that may contribute to the carcinogenic process via induction of long-term chronic inflammation. It is entirely possible that an as yet un-described pathogen may be identified in the prostate of cancer patients. We also present an alternative hypothesis in that the chronic inflammation observed in the prostate of cancer patients may have been initiated many years prior to the development of cancer and may have persisted independent of a high prevalence of the infecting agent. This is supported by recent studies describing prostatic infections with P. acnes in rodent models, and should remain a focus of future studies.
Acknowledgements
K.S.S. is supported as the Beth and A. Ross Meyers Scholar in which she is supported through the Patrick C. Walsh Prostate Cancer Research Fund and as the Chris and Felicia Evensen Prostate Cancer Foundation Young Investigator.
References
- 1.Nelson W, Sfanos K, DeMarzo A, Yegnasubramanian S. Prostate Inflammation and Prostate Cancer. In: Klein EA, Jones JS, editors. Management of Prostate Cancer. Humana Press; 2013. pp. 103–115. [Google Scholar]
- 2.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. New Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 7.Hrbacek J, Urban M, Hamsikova E, Tachezy R, Heracek J. Thirty years of research on infection and prostate cancer: No conclusive evidence for a link. A systematic review. Urol Oncol. 2013;31:951–965. doi: 10.1016/j.urolonc.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe S. Sexually transmitted infections and risk of prostate cancer: review of historical and emerging hypotheses. Future Oncol. 2010;6:1289–1311. doi: 10.2217/fon.10.95. [DOI] [PubMed] [Google Scholar]
- 9.Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, De Marzo AM, Willett WC, Platz EA. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- 10.Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, Platz EA, Sutcliffe S, Fall K, Kurth T, Ma J, Stampfer MJ, Mucci LA. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J Natl Cancer Inst. 2009;101:1406–1411. doi: 10.1093/jnci/djp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutcliffe S, Alderete JF, Till C, Goodman PJ, Hsing AW, Zenilman JM, De Marzo AM, Platz EA. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int J Cancer. 2009;124:2082–2087. doi: 10.1002/ijc.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sfanos KS, Sauvageot J, Fedor HL, Dick JD, De Marzo AM, Isaacs WB. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate. 2008;68:306–320. doi: 10.1002/pros.20680. [DOI] [PubMed] [Google Scholar]
- 13.Groom HC, Warren AY, Neal DE, Bishop KN. No evidence for infection of UK prostate cancer patients with XMRV, BK virus, Trichomonas vaginalis or human papilloma viruses. PLoS One. 2012;7:e34221. doi: 10.1371/journal.pone.0034221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigehara K, Sasagawa T, Kawaguchi S, Kobori Y, Nakashima T, Shimamura M, Taya T, Furubayashi K, Namiki M. Prevalence of human papillomavirus infection in the urinary tract of men with urethritis. Int J Urol. 2010;17:563–568. doi: 10.1111/j.1442-2042.2010.02521.x. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Fierro M, Leach R, Gomez-Guerra L, Garza-Guajardo R, Johnson-Pais T, Beuten J, Morales-Rodriguez I, Hernandez-Ordonez M, Calderon-Cardenas G, Ortiz-Lopez R, Rivas-Estilla A, Ancer-Rodriguez J, Rojas-Martinez A. Identification of viral infections in the prostate and evaluation of their association with cancer. BMC Cancer. 2010;10:326. doi: 10.1186/1471-2407-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascale M, Pracella D, Barbazza R, Marongiu B, Roggero E, Bonin S, Stanta G. Is human papillomavirus associated with prostate cancer survival? Dis Markers. 2013;35:7. doi: 10.1155/2013/735843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W, Lawson JS. Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate. 2013;73:236–241. doi: 10.1002/pros.22562. [DOI] [PubMed] [Google Scholar]
- 18.Rogler A, Rogenhofer M, Borchardt A, Lunz JC, Knoell A, Hofstaedter F, Tannapfel A, Wieland W, Hartmann A, Stoehr R. P53 codon 72 (Arg72Pro) polymorphism and prostate cancer risk: Association between disease onset and proline genotype. Pathobiology. 2011;78:193–200. doi: 10.1159/000326767. [DOI] [PubMed] [Google Scholar]
- 19.Sutcliffe S, Viscidi RP, Till C, Goodman PJ, Hoque AM, Hsing AW, Thompson IM, Zenilman JM, De Marzo AM, Platz EA. Human papillomavirus types 16, 18, and 31 serostatus and prostate cancer risk in the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2010;19:614–618. doi: 10.1158/1055-9965.EPI-09-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delany-Moretlwe S, Chikandiwa A, Gibbs J. Human papillomavirus infection and disease in men: Impact of HIV. Southern African Journal of HIV Medicine. 2013;14:183–188. [Google Scholar]
- 21.Huang WY, Hayes R, Pfeiffer R, Viscidi RP, Lee FK, Wang YF, Reding D, Whitby D, Papp JR, Rabkin CS. Sexually transmissible infections and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2374–2381. doi: 10.1158/1055-9965.EPI-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutcliffe S, Zenilman JM, Ghanem KG, Jadack RA, Sokoll LJ, Elliott DJ, Nelson WG, De Marzo AM, Cole SR, Isaacs WB, Platz EA. Sexually transmitted infections and prostatic inflammation/cell damage as measured by serum prostate specific antigen concentration. J Urol. 2006;175:1937–1942. doi: 10.1016/S0022-5347(05)00892-X. [DOI] [PubMed] [Google Scholar]
- 23.Sutcliffe S, Nevin RL, Pakpahan R, Elliott DJ, Cole SR, De Marzo AM, Gaydos CA, Isaacs WB, Nelson WG, Sokoll LJ, Zenilman JM, Cersovsky SB, Platz EA. Prostate involvement during sexually transmitted infections as measured by prostate-specific antigen concentration. Br J Cancer. 2011;105:602–605. doi: 10.1038/bjc.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sfanos KS, Aloia AL, De Marzo AM, Rein A. XMRV and prostate cancer-a ‘final’ perspective. Nat Rev Urol. 2012;9:111–118. doi: 10.1038/nrurol.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sfanos KS, Isaacs JT. The “infectious” nature of human prostate cancer: A cautionary note. Oncotarget. 2011;2:281–283. doi: 10.18632/oncotarget.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hempel HA, Burns K, De Marzo A, Sfanos K. Infection of xenotransplanted human cell lines by murine retroviruses: A lesson brought back to light by XMRV. Front Oncol. 2013;3:156. doi: 10.3389/fonc.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger J, Nyberg L, Nickel J. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 28.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 29.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 30.Gui-zhong LI, Libo M, Guanglin H, Jianwei W. The correlation of extent and grade of inflammation with serum PSA levels in patients with IV prostatitis. Int Urol Nephrol. 2011;43:295–301. doi: 10.1007/s11255-010-9825-5. [DOI] [PubMed] [Google Scholar]
- 31.Ugurlu O, Yaris M, Oztekin CV, Kosan TM, Adsan O, Cetinkaya M. Impacts of antibiotic and anti-inflammatory therapies on serum prostate-specific antigen levels in the presence of prostatic inflammation: a prospective randomized controlled trial. Urol Int. 2010;84:185–190. doi: 10.1159/000277596. [DOI] [PubMed] [Google Scholar]
- 32.Fujita K, Hosomi M, Tanigawa G, Okumi M, Fushimi H, Yamaguchi S. Prostatic inflammation detected in initial biopsy specimens and urinary pyuria are predictors of negative repeat prostate biopsy. J Urol. 2011;185:1722–1727. doi: 10.1016/j.juro.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 34.Delongchamps N, de la Roza G, Chandan V, Jones R, Sunheimer R, Threatte G, Jumbelic M, Haas G. Evaluation of prostatitis in autopsied prostates--is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179:1736–1740. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidsson S, Fiorentino M, Andrén O, Fang F, Mucci LA, Varenhorst E, Fall K, Rider JR. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2280–2287. doi: 10.1158/1055-9965.EPI-11-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornstein D, Smith D, Humphrey P, Catalona W. The effect of prostate volume, age, total prostate specific antigen level and acute inflammation on the percentage of free serum prostate specific antigen levels in men without clinically detectable prostate cancer. J Urol. 1998;159:1234–1237. [PubMed] [Google Scholar]
- 37.Stimac G, Reljic A, Spajic B, Dimanovski J, Ruzic B, Ulamec M, Sonicki Z, Kraus O. Aggressiveness of inflammation in histological prostatitis - Correlation with total and free prostate specific antigen levels in men with biochemical criteria for prostate biopsy. Scott Med J. 2009;54:8–12. doi: 10.1258/RSMSMJ.54.3.8. [DOI] [PubMed] [Google Scholar]
- 38.Okada K, Kojima M, Naya Y, Kamoi K, Yokoyama K, Takamatsu T, Miki T. Correlation of histological inflammation in needle biopsy specimens with serum prostate- specific antigen levels in men with negative biopsy for prostate cancer. Urology. 2000;55:892–898. doi: 10.1016/s0090-4295(00)00519-7. [DOI] [PubMed] [Google Scholar]
- 39.Rowe E, Laniado M, Walker M, Anup P. Incidental acute prostatic inflammation is associated with a lower percentage of free prostate-specific antigen than other benign conditions of the prostate: a prospective screening study. BJU Int. 2006;97:1039–1042. doi: 10.1111/j.1464-410X.2006.06132.x. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Meng S, Li R. Clinical evaluation of four cytokines in serum and prostatic fluid in chronic abacterial prostatitis. Zhonghua Nan Ke Xue. 2006;12:25–27. [PubMed] [Google Scholar]
- 41.Thompson S. The Diseases of the Prostate: Their Pathology and Treatment. London: J. & A. Churchill; 1883. [Google Scholar]
- 42.Cross PA, Bartley CJ, McClure J. Amyloid in prostatic corpora amylacea. J Clin Pathol. 1992;45:894–897. doi: 10.1136/jcp.45.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009;106:3443–3448. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas B, Robert J. Prostatic calculi. J Urol. 1927;18:470–493. [Google Scholar]
- 45.Finkle A. The relationship of antecedent genito-urinary infections to the development of prostatic calculi and carcinoma. Bull N Y Acad Med. 1953;29:585–586. [PMC free article] [PubMed] [Google Scholar]
- 46.Eykyn S, Bultitude MI, Mayo ME, Lloyd-Davies RW. Prostatic calculi as a source of recurrent bacteriuria in the male. Br J Urol. 1974;46:527–532. doi: 10.1111/j.1464-410x.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 47.Meares EM Jr. Infection stones of prostate gland: Laboratory diagnosis and clinical management. Urology. 1974;4:560–566. doi: 10.1016/0090-4295(74)90490-7. [DOI] [PubMed] [Google Scholar]
- 48.Yanamandra K, Alexeyev O, Zamotin V, Srivastava V, Shchukarev A, Brorsson AC, Tartaglia GG, Vogl T, Kayed R, Wingsle G, Olsson J, Dobson CM, Bergh A, Elgh F, Morozova-Roche LA. Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PLoS One. 2009;4:e5562. doi: 10.1371/journal.pone.0005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dessombz A, Méria P, Bazin D, Daudon M. Prostatic stones: Evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012;7:e51691. doi: 10.1371/journal.pone.0051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazin D, André G, Weil R, Matzen G, Emmanuel V, Carpentier X, Daudon M. Absence of bacterial imprints on struvite-containing kidney stones: A structural investigation at the mesoscopic and atomic scale. Urology. 2012;79:786–790. doi: 10.1016/j.urology.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 51.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 52.Mak TN, Yu SH, De Marzo AM, Brüggemann H, Sfanos KS. Multilocus sequence typing (MLST) analysis of Propionibacterium acnes isolates from radical prostatectomy specimens. Prostate. 2013;73:770–777. doi: 10.1002/pros.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kloos WE, Bannerman TL. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nickel J, Costerton J. Coagulase-negative staphylococcus in chronic prostatitis. J Urol. 1992;147:398–400. doi: 10.1016/s0022-5347(17)37247-6. discussion 400-391. [DOI] [PubMed] [Google Scholar]
- 55.Olsson J, Drott JB, Laurantzon L, Laurantzon O, Bergh A, Elgh F. Chronic prostatic infection and inflammation by Propionibacterium acnes in a rat prostate infection model. PLoS One. 2012;7:e51434. doi: 10.1371/journal.pone.0051434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golden M, Abeshouse BS. Prostatic calculi and neoplasm. Sinai Hosp J (Balt) 1952;1:20–28. [PubMed] [Google Scholar]
- 57.Cristol DS, Emmett JL. The incidence of coincident prostatic calculi, prostatic hyperplasia and carcinoma of the prostate gland. JAMA. 1944;124:646. [Google Scholar]
- 58.Kovi J, Rao MS, Heshmat MY, Akberzie ME, Jackson MA, Ogunmuyiwa TA. Incidence of prostatic calcification in blacks in Washington, D. C., and selected african cities Correlation of specimen roentgenographs and pathologic findings. Urology. 1979;14:363–369. doi: 10.1016/0090-4295(79)90081-5. [DOI] [PubMed] [Google Scholar]
- 59.Isaacs JT. The aging ACI/Seg versus copenhagen male rat as a model system for the study of prostatic carcinogenesis. Cancer Res. 1984;44:5785–5796. [PubMed] [Google Scholar]
- 60.Jakab E, Zbinden R, Gubler J, Ruef C, von Graevenitz A, Krause M. Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. Yale J Biol Med. 1996;69:477–482. [PMC free article] [PubMed] [Google Scholar]
- 61.Alexeyev O, Bergh J, Marklund I, Thellenberg-Karls C, Wiklund F, Gronberg H, Bergh A, Elgh F. Association between the presence of bacterial 16S RNA in prostate specimens taken during transurethral resection of prostate and subsequent risk of prostate cancer (Sweden) Cancer Causes Control. 2006;17:1127–1133. doi: 10.1007/s10552-006-0054-2. [DOI] [PubMed] [Google Scholar]
- 62.Fassi Fehri L, Mak TN, Laube B, Brinkmann V, Ogilvie LA, Mollenkopf H, Lein M, Schmidt T, Meyer TF, Brüggemann H. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med Microbiol. 2011;301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 63.Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer. 2007;121:2688–2692. doi: 10.1002/ijc.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Severi G, Shannon BA, Hoang HN, Baglietto L, English DR, Hopper JL, Pedersen J, Southey MC, Sinclair R, Cohen RJ, Giles GG. Plasma concentration of Propionibacterium acnes antibodies and prostate cancer risk: results from an Australian population-based case-control study. Br J Cancer. 2010;103:411–415. doi: 10.1038/sj.bjc.6605757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galobardes B, Smith GD, Jeffreys M, Kinra S, McCarron P. Acne in adolescence and cause-specific mortality: Lower coronary heart disease but higher prostate cancer mortality. Am J Epidemiol. 2005;161:1094–1101. doi: 10.1093/aje/kwi147. [DOI] [PubMed] [Google Scholar]
- 66.Drott J, Alexeyev O, Bergstrom P, Elgh F, Olsson J. Propionibacterium acnes infection induces upregulation of inflammatory genes and cytokine secretion in prostate epithelial cells. BMC Microbiol. 2010;10:126. doi: 10.1186/1471-2180-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mak TN, Fischer N, Laube B, Brinkmann V, Metruccio MM, Sfanos KS, Mollenkopf HJ, Meyer TF, Brüggemann H. Propionibacterium acnes host cell tropism contributes to vimentin-mediated invasion and induction of inflammation. Cell Microbiol. 2012;14:1720–1733. doi: 10.1111/j.1462-5822.2012.01833.x. [DOI] [PubMed] [Google Scholar]
- 68.Shinohara DB, Vaghasia AM, Yu SH, Mak TN, Brüggemann H, Nelson WG, De Marzo AM, Yegnasubramanian S, Sfanos KS. A mouse model of chronic prostatic inflammation using a human prostate cancer-derived isolate of Propionibacterium acnes. Prostate. 2013;73:1007–1015. doi: 10.1002/pros.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]


