Abstract
Background: It is now widely recognized that there is a strong correlation between oxidative stress and the risk of benign and malignant diseases of the prostate. Prostate-associated gene 4 (PAGE4) is a Cancer/Testis Antigen (CTA) that was previously shown to be up-regulated in prostate cancer (PCa) and symptomatic as opposed to histologic benign prostatic hyperplasia (BPH). However, its functional role in these diseases is not fully understood. Methods: The mRNA level of PAGE4 was detected in isolated cell types in PCa tissues that were obtained from 8 men with PCa. PAGE4 protein expression profile was analyzed in a prostate disease tissue microarray. PAGE4 was overexpressed by pCMV-PAGE4-GFP transfection and cell viability was determined using the WST-1 assay. Results: PAGE4 expression is highly dynamic; while its expression is very high in fetal prostate it is drastically decreased in the normal adult prostate but is up-regulated both in symptomatic BPH and PCa. However, in the diseased prostate, PAGE4 is highly expressed in the epithelial cells of Proliferative Inflammatory Atrophy (PIA) lesions alluding to a potential stress response function of PAGE4. Consistent with such a role, PAGE4 protein levels are up-regulated when prostate cancer (PCa) cell lines are treated with various stress factors including the proinflammatory cytokine TNFα. Interestingly, in cells challenged with stress there is increased translocation of the PAGE4 protein to the mitochondrion and production of reactive oxygen species is suppressed . Furthermore, p21 is elevated in a p53-independent manner in PAGE4-overexpressing cells which results in impeded cell cycle progression, attenuated stress-induced DNA damage, and decreased cell death. Conclusions: PAGE4 may be contributing to the development of PCa by playing a stress-protective and anti-apoptotic role.
Keywords: PAGE4, prostate cancer, Cancer/Testis Antigen, stress-response, proliferative infl ammatory atrophy
Introduction
Stress existing in tissue microenvironment has been shown to play a critical role in the development of prostate diseases such as benign prostatic hyperplasia (BPH), prostatitis, lower urinary tract symptoms (LUTS) and prostate cancer (PCa) [1-4]. A stressed microenvironment is thought to be linked to many of the factors that are associated with these symptoms/diseases, such as chronic inflammation and imbalance of androgens and estrogens. Microenvironmental stress factors such as prolonged hypoxia and glucose deprivation are capable of inducing mitochondrial reactive oxygen species (ROS) [5]. ROS is believed to promote oxidative DNA damage, defective DNA repair, and genomic instability, leading to increased mutations some of which may favor cellular transformation and accelerated proliferation [6-8]. Mild oxidative stress may also promote tumorigenesis by inducing premature senescence that is believed to be involved in aging and cancer [9]. Thus, it is speculated that microenvironmental stress may drive cancer initiation by promoting the selection of cells that are resistant to senescence and oxidative damage-mediated cell death.
On the other hand, chronic or recurrent inflammation that is a response of the tissue to stress caused by infectious agents, chemical irritants ischemia (hypoxia and/or glucose deprivation), hormones or chronic irritation [10-13], has been implicated in the development of both BPH and PCa [14,15]. Indeed, an inflammation-associated lesion in the prostate called, proliferative inflammatory atrophy (PIA), was proposed to be a precursor to prostatic intraepithelial neoplasia (PIN) and PCa [16]. PIA cells display high levels of glutathione S-transferase P1 (GSTP1) and cyclooxygenase-2 (COX-2) indicating a high level of cellular stress [16-18]. Like in the case of PCa, onset of BPH has also been linked to chronic inflammation. Many inflammatory cytokines such as interleukin (IL)-1α, IL-2, IL-4, IL-6, IL-8, and IL-17 are overexpressed in BPH, suggesting the involvement of chronic inflammation in BPH pathogenesis [19,20].
The Cancer/Testis Antigens (CTAs) are an important group of heterogeneous proteins that are typically restricted to the male germ cells with little or no expression in somatic cells; however, they are aberrantly expressed in several types of cancer [21]. More than half of the CTAs are located on the X chromosome and referred to as the CT-X antigens. The CT-X antigens appear to represent the bona fide CTAs; other than the testis, they are only detected in reproductive organs such as placenta and uterus [21]. Prostate-associated gene 4 (PAGE4) belongs to a family of CT-X antigens (PAGE1-5) that appears to have evolved recently and lacks orthologous in most lower mammals other than primates [21]. However, unlike most members of this group, PAGE4 is expressed not only in the testis but is also expressed in some fetal and normal reproductive organs such as the placenta, the prostate and the uterus albeit, at a basal level [22,23]. Furthermore, in addition to its reported upregulation in primary PCa samples in which cancer cells were not separated from other types of cells [23], we previously demonstrated that PAGE4 was highly expressed in stromal cells of symptomatic but not asymptomatic BPH [24-26], alluding to a potential stress-related regulation of PAGE4.
More recently, we reported that PAGE4 is a highly intrinsically disordered protein (IDP) which lacks stable 3D structure under physiological conditions in vitro [27]. By binding to multiple partners via high-specificity/low-affinity interactions, IDPs play a crucial role in signaling and transcriptional regulation, and are therefore, frequently associated with several diseases [28]. Indeed, we have demonstrated that PAGE4 has an anti-apoptotic function in PCa cells [27]. In this study, we show that PAGE4 expression is up-regulated in the inflammation-related PIA lesions in PCa. Overexpression of PAGE4 suppressed ROS production, attenuated DNA damage and rendered cells more resistant to various stress stimulants. Thus, the upregulation of PAGE4 in PIA lesions represents a novel stress-protective function of PAGE4 that plays a critical role in the development of PCa.
Materials and methods
This study complies with the Declaration of Helsinki and was approved by the local ethics committee. Written informed consent was obtained from all patients and the identities of the patients donating the samples were anonymous.
Cell culture
The PCa cell lines CWR22rv1 and LNCaP were obtained from the American Type Culture Collection. Cells were cultured at 37°C under routine conditions in RPMI (Invitrogen) media supplemented with 10% fetal bovine serum (FBS) with humidified air and 5% carbon dioxide. Normal prostate epithelial cells (PrECs, Lonza) were cultured in Prostate Epithelial Cell Basal Medium (PrEGM, Lonza) supplemented with BulletKit (Lonza). For the stress-response experiments, cells were cultured in 6-well plates overnight and then treated with either adriamycin (ADM) or TFN-α at the indicated concentration for 24-48 hr. Alternatively, cells were cultured in RPMI media without supplemental glucose to determine the effects of glucose deprivation.
Patients and prostate samples
PCa samples were obtained from 38 patients undergoing radical prostatectomy. Details of the samples are described in Supplemental Table 1. For control samples, 5 normal prostate tissue samples with no evidence of the disease were obtained from organ donors. The median age among these groups was not significantly different from the PCa patients. In another experiment, prostate tissue samples from 5 patients with bladder cancer and 8 patients with localized PCa were collected. The clinical characteristics of the patients have been described previously [29]. Epithelial and stromal cells were separately collected from each sample using laser-capture microdissection (LCM) as described previously [29].
RNA isolation and quantitative reverse transcription qPCR
Total RNA was isolated using the RNeasy mini kit (Qiagen) following the supplier’s protocol. RNA samples were treated with DNase I (Invitrogen), and cDNA was synthesized using the iScript cDNA synthesis kit (BioRad). cDNA from normal human tissues obtained from healthy adult donors and from human fetal tissues purchased from BioChain. Real-time qPCR was done in triplicate using an iCycler iQ Multicolor Real-time PCR Detection system (BioRad). Target gene expression was compared to TATA box binding protein (TBP) mRNA for normalization. PCR primer sequences were the same as described previously [27].
Tissue microarrays (TMAs)
A prostate tissue microarray (TMA) slide (PR8011) was purchased from US Biomax. The slide contains 78 cases/cores, including 29 cases of adenocarcinoma, 26 of hyperplasia, 6 of chronic inflammation, 9 of adjacent normal tissue, and 8 of normal tissue. The PAGE4 antibody WER2 was generated using the following peptide N-CKTPPNPKHAKTKEAGDGQP-C (Sigma-Gneosys). Immunohistochemical (IHC) staining was conducted using an EnVision™ FLEX System (Dako) according to the manufacturer’s protocol with minor modifications. Briefly, the section was blocked in 5% skim milk for 1 hr and then treated with WER2 rabbit polyclonal antibody that was diluted at 1:1000 for 30 min at room temperature. The grade of staining intensity was scored as strong (++), weak (+), or absent (-). The specificity of WER2 was shown in the previous study using PAGE4 specific siRNA [27]. PAGE4 immunizing peptide blocking was also performed on WER2 to confirm its specificity. Briefly, WER2 were blocked in TBST plus 5% skim milk with or without adding 1 μg/mL immunizing peptide described earlier at 4°C overnight, and then used for IHC staining.
Immunoblotting
Twenty five micrograms of protein were separated on 4-15% SDS-PAGE and transferred onto PVDF filters (Millipore, Billerica, MA). The membranes were then incubated with primary antibodies overnight at 4°C followed by horseradish peroxidase-conjugated secondary antibody and developed with the Super Signal West Dura Extended Duration Substrate kit (Pierce). Antibodies, with the exception of WER2, were all purchased from Cell Signaling. In some experiments, the membranes were incubated with secondary antibodies labeled with IRDye™ 800 or Alexa Fluor® 680, and the two-color images were collected using Odyssey Infrared Imaging System (LI-COR Biosciences).
PAGE4 overexpression
The pCMV6-PAGE4-GFP construct was generated from a pCMV6-PAGE4-Entry vector and a pCMV6-AC-GFP destination vector as described previously [27]. These constructs were expressed in CWR22rv1 cells by transfection with FuGENE HD (Roche) and clones that expressed GFP or PAGE4-GFP were selected. The pcDNA3.1-PAGE4-v5 plasmid was constructed using pENTR Directional TOPO Cloning kits (Invitrogen) following manufacturer’s protocol.
Cell viability and inhibition assay
Cell viability and inhibition under glucose-deprivation stress was evaluated using WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1, 3-benzene disulfonate) assay as described previously [30].
Clonogenic assay
Cell survival following cytotoxic stress was analyzed by clonogenic assay as previously described [30]. Briefly, cells were seeded in a 10-cm dish at 500 cells or 2,000 cells per dish and cultured in normal medium or treated with 1 μg/mL of ADM for 1 hr. Colonies were counted after 2 weeks.
FACS analysis
The status of the cells in cell cycle was detected on a Guava system (Guava Technologies) using the Guava Cell Cycle Reagent.
ROS assay
ROS levels under normal or stress conditions were determined using the MitoSOXTM RED mitochondrial superoxide indicator live-cell imaging kit (Invitrogen) following the supplier’s protocol. Live cells were stained with 5 μM MitoSOXTM RED for 10 min at 37°C and observed with a Nikon Eclipse TE20000E fluorescence microscope. Alternatively, ROS was measured using a 2’,7’-dichlorofluorescein diacetate (DCFDA) - Cellular Reactive Oxygen Species Detection Assay Kit (abcam) following the protocol provided by the supplier. Briefly, cells were labeled with 20 μM DCFDA for 45 min and then treated with 1 μg/mL ADM or DMSO for 2 hr. Cells were then washed once with PBS, transferred to a microplate and read on a spectrophotometer at wavelength of 485 nm. Tert-butyl Hydrogen Peroxide (tbHP) that mimics ROS activity and oxidizes DCFDA to fluorescent DCF was used as a positive control.
Mitochondria Isolation
Cytosolic and mitochondrial fractions were separated using a Mitochondria Isolation Kit (Thermo Scientific). Briefly, 10 million PAGE4-expressing CWR22rv1 cells or control cells were plated in a 150 mm Petri dish and cultured in glucose-depleted medium or normal medium for 48 hr. The cells were then harvested on ice using rubber scrapers and mitochondria were isolated following the manufacture’s protocol. Cytosolic and mitochondrial protein fractions were used to detect PAGE4 expression by immunoblotting using the WER2 PAGE4 antibody.
Confocal microscopy
CWR22rv1 cells overexpressing PAGE4 were transfected with pDsRed2-Mito (Clontech) for 24 hr, and then re-plated on the glass bottom 6-well plates in the medium with or without glucose supplement for 48 hr. Co-localization of mitochondrial and cytoplasmic PAGE4 were observed under a Zeiss 710NLO Meta Confocal Microscope (Carl Zeiss).
Statistical analysis
Comparisons were made using the Student’s t-test, Mann-Whitney U test (for comparing mRNA levels in tissue samples), Wilcoxon signed-rank test (for comparing mRNAs levels in paired LCM samples), or χ2 test (for comparing PAGE4 staining in TMA). Two-sided P values of less than 0.05 were considered to be significant.
Results
PAGE4 expression in adult and fetal human tissues
The PAGE family of CT-X antigens consists of five members (PAGE1-5). However, to our knowledge, other than PAGE4, their expression in the normal or diseased human prostate has not been discerned. Thus, the mRNA expression of the genes encoding the PAGE family members in various normal adult human tissues as well as in some fetal tissues was determined. As expected, all members of this family of CT-X antigens were expressed in the testis albeit, at differing levels (Figure 1A). However, although collectively referred to as PAGE genes, only PAGE4 was expressed in the prostate (Figure 1A). Further, as shown in Figure 1A, only PAGE4 was dramatically up-regulated (10~1000 fold) in the fetal prostate and fetal testis when compared to the adult prostate or testis.
Figure 1.
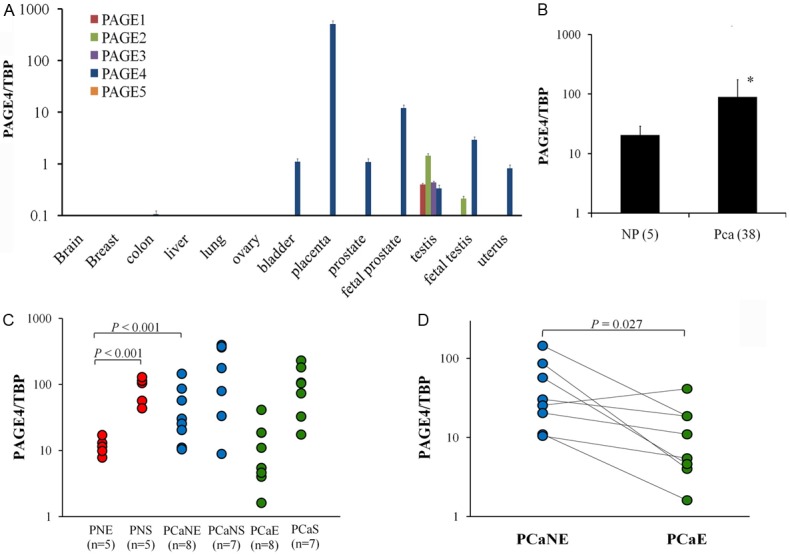
PAGE4 mRNA expression in different human normal tissues and prostate cancer samples. A: mRNA levels of PAGE1-5 in different normal human tissue samples obtained from healthy donors. B: PAGE4 mRNA expression in the whole tissue samples of normal prostate and primary prostate cancer (PCa). C: PAGE4 mRNA expression in isolated cells obtained by laser capture microdissection. PNE, epithelia in normal prostate; PNS, stroma in normal prostate; PCaE, cancer epithelia in cancer prostate; PCaS, stroma surrounding PCaE; PCaNE, normal epithelia adjacent to cancer lesions in cancer prostate; PCaNS, stroma surrounding PCaNE. D: PAGE4 expression pattern in matched PCaE and PCaNe samples. Solid line links the samples obtained from the same patient. Values are expressed relative to the expression of TATA box binding protein (TBP) mRNA. Data are represented as mean±SD. All experiments were repeated a minimum of three times.
PAGE4 expression is upregulated in the adjacent normal cells in PCa
To further demonstrate the differential expression of PAGE4 in normal prostate and PCa, qPCR was performed on PCa samples obtained surgically. As shown in Figure 1B, PAGE4 is significantly upregulated in prostate specimens from patients with primary PCa compared to the normal prostate. Since cancer cells were not separated from stromal cells or from adjacent non-cancer cells the increased expression of PAGE4 observed in these PCa samples, is likely to represent an average of PAGE4 expression in different cell types in the cancer microenvironment. To discern whether PAGE4 is expressed in prostate epithelial or stromal cells, we separated these cell types using LCM of paraffin sections. A qPCR analysis of the isolated cell types demonstrated that PAGE4 mRNA was expressed in both epithelial and stromal cells (Figure 1C). However, PAGE4 mRNA was upregulated in epithelial cells obtained from the adjacent apparently “normal” tissue when compared to either the normal prostate or PCa (Figure 1C). Further, in matched samples obtained from each patient, the levels of PAGE4 mRNA were typically higher in the adjacent normal epithelia than in the cancer epithelia (Figure 1D). Thus, the upregulation of PAGE4 in the PCa samples shown in Figure 1B is potentially due to the existence of adjacent non-cancer cells that overexpress PAGE4. Together, these results suggest that PAGE4 expression is up-regulated in the cancer-adjacent apparently “normal” epithelia more than in the cancer lesions themselves.
PAGE4 protein is upregulated in the PIA lesions in PCa
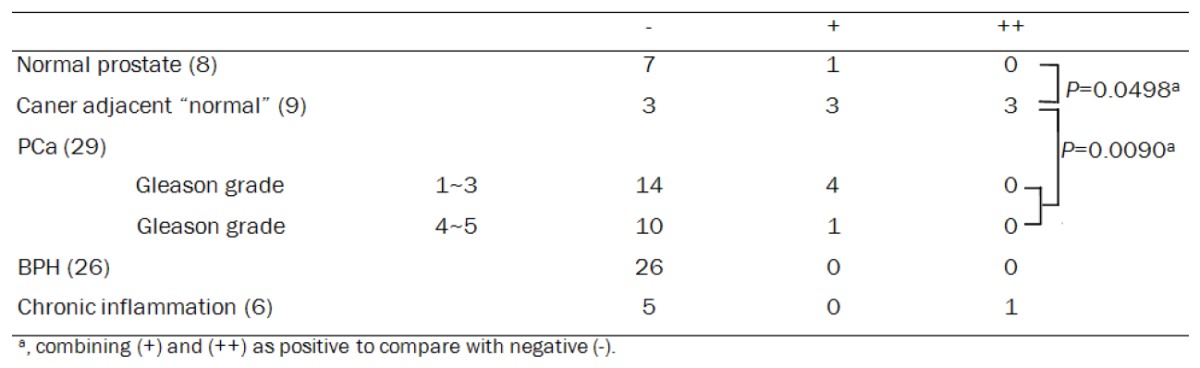
To examine the expression patterns of PAGE4 at the protein level immunohistochemical analysis (Supplemental Figure 1) was performed on TMAs constructed from a total of 29 primary cancer and 8 normal prostatic specimens, as well as tissues isolated from men with other prostatic diseases. As shown in Table 1 and Figure 2, PAGE4 protein was barely detectable in normal prostatic glands (1/8), but was exclusively expressed in the stroma in BPH (Figure 2B). However, the expression of PAGE4 in PCa appeared more complex. Consistent with the qPCR results, PAGE4 expression was detectable in some of localized PCa area (5/29) but not in metastatic PCa (0/2) (Figure 2C-F), with the exception of the cancer adjacent “normal” epithelia, where a positive staining (+~++) was more frequently observed (6/9, P=0.0498 as compared with epithelia in the normal prostate and P=0.0090 as compared with cancerous epithelia) (Table 1). Further investigation revealed that the staining of PAGE4 in these epithelial cells was predominantly associated with inflammation in atrophic glands with inflammatory cell infiltration (PIA lesions) (Figure 2G & 2H).
Table 1.
PAGE4 staining in the prostate diseases
|
Figure 2.
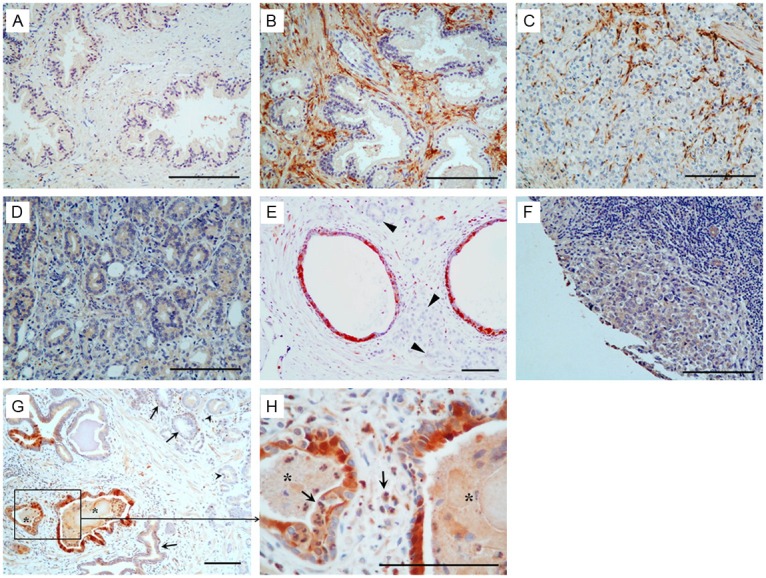
Immunohistochemistry analysis of PAGE4 in prostate cancer. (A) Negative staining (-) in the normal prostate. (B) Intense staining (++) shown in the stromal tissue in BPH. (C) Positive staining (++) in the stromal cells but negative in the cancer cells in some PCa specimens. (D) Moderate staining (+) in the cancer cells but negative in the stromal cells in some PCa specimens. (E) Positive staining (++) in the atrophic glands but negative in the cancer cells (arrowhead). (F) Negative staining in metastatic PCa. (G) Intense staining (++) shown in cancer adjacent “normal” glands (asterisk) associated with inflammation but only moderate staining in the cancer cells (arrowhead). (H) High power view of boxed area in (G). Asterisk, PIA lesions; arrows, inflammatory cells. Scale bars in all panels, 100 μm.
PAGE4 expression is increased in response to stress
Since PAGE4 expression in PCa was associated with inflammation-related lesions, we conjectured a correlation between PAGE4 overexpression and stress conditions that exist in the diseased prostate. To test this idea, we subjected normal prostate epithelial cells and PCa cell lines to various stress conditions including nutrient (glucose) deprivation, drug treatment (ADM), and treatment with the proinflammatory cytokine, TNF-α. As shown in Table 2, PAGE4 mRNA was upregulated by each of the stress factors in the cell lines employed albeit, to varying degrees. Unfortunately, given the extremely low levels of PAGE4 mRNA in these cell lines (Supplemental Figure 2) even under conditions of stress stimulation, we could not detect the endogenous PAGE4 protein by immunoblotting. However, when we subjected CWR22rv1 cells that stably overexpress the PAGE4 cDNA to the same stress conditions, ectopically expressed PAGE4 protein level was increased (Figure 3A). A similar result was also observed in the HEK293T cells after TNF-α treatment following transient transfection of pcDNA3.1-PAGE4-nV5 (Figure 3B). At the same time, mRNA expression of PAGE4 that is driven by the CMV promoter in these HEK293T cells was not significantly increased after TNF-α treatment (Figure 3C). To determine whether the increase of PAGE4 protein levels after stress stimulation is due to the decreased degradation that is commonly associated with protein ubiquitin and/or SUMOlation (small ubiquitin-related modifi er proteins) cells were treated with a proteasome inhibitor MG132, or Ubiquitin E1 Inhibitor PYR41, or a SUMO inhibitor Ginkgolic Acid. However, no significant changes in the PAGE4 protein level were observed following treatment with any of these inhibitors. On the other hand, the level of the p53 protein whose degradation is known to be regulated by the proteasome was altered in response to treatment with the inhibitors indicating that indeed, the inhibitors were effective. Therefore, these results suggest that, in addition to the mRNA level, the expression of PAGE4 might be regulated by stress stimulations at a post-transcriptional level other than proteasome-mediated protein degradation such as mRNA stability and or translational effciences. Given that PAGE4 is a highly intrinsically disordered protein, these results are not surprising; in fact, it is a paradox as to how IDPs that are tightly regulated in normal cells [31,32] evade the cellular degradation machinery and contribute to the toxic/pathologic effects upon overexpression [33].
Table 2.
PAGE4 mRNA is increased by varied stress stimulations in prostatic cell lines
| Glucose-deprivation | ADM | TNF-α | |
|---|---|---|---|
| LNCaP | 3.7±0.2 | 3.3±0.1 | 4.2±0.1 |
| CWR22rv1 | 113.1±48.9 | 3.1±0.1 | 3.5±0.1 |
| PrEC | 3.8±0.4 | 2.2±0.1 | 8.6±0.7 |
Values indicate fold change compared to untreated controls (P<0.05 in all the comparisons).
Figure 3.
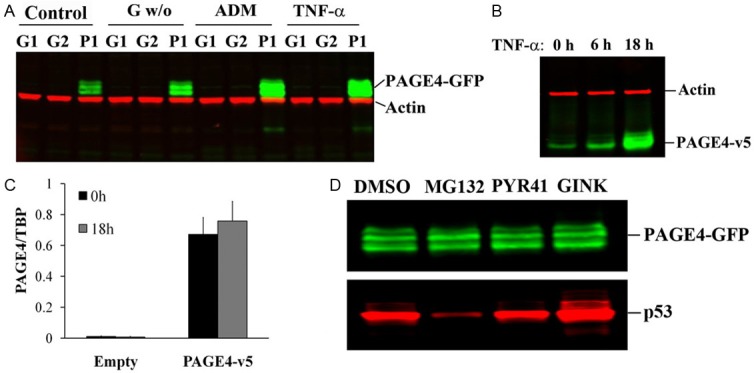
PAGE4 is a stress-response protein. (A) CWR22rv1 cells stably expressing PAGE4 (P1) or GFP (G1 and G2) were cultured in medium without glucose (G w/o) for 48 hr, or treated with Adriamycin (ADM) or TNF-α for 24 hr. PAGE4 expression was detected by Western blot. (B) HEK293T cells were transiently transfected with pcDNA3.1-PAGE4-v5 and treated with TNF-α for 0-18 hr. PAGE4 expression was detected by Western blot. (C) Same experiment with (B) but the mRNA levels of PAGE4 was detected by RT-PCR. Data are represented as mean±SD. (D) P1 cells were cultured with proteasome inhibitor MG132, Ubiquitin E1 Inhibitor PYR41, small ubiquitin-related modifi er proteins (SUMOs) inhibitor Ginkgolic Acid (GINK), or DMSO for 24 hr, the expressions of PAGE4 or p53 were detected by Western blot. All experiments were repeated a minimum of three times.
PAGE4 overexpression protects cells from stress-induced cell death
We next examined the effect of PAGE4 overexpression on stress-response in PCa cells. Since PAGE4 expression was found to be significantly increased in response to glucose deprivation in CWR22rv1 cells (Table 2), we cultured CWR22rv1 cells that stably expressed PAGE4 in medium with or without glucose supplement. As shown in Figure 4A, depleting glucose resulted in massive cell death in the empty vector transfected cells (5-10% survival) but in contrast, in those cells overexpressing PAGE4, >40% of the cells survived. Notably, in comparison to cells transfected with the empty vector, PAGE4 overexpressing cells were blocked before the G2/M-phase of the cell cycle under glucose-deprivation stress (Figure 4B). This suggests that PAGE4 appears to protect against stress-induced cell death by slowing cell cycle progression. A clonogenic assay further substantiated this conclusion in that, survival was significantly higher in PAGE4 overexpressing cells compared to control cells not expressing PAGE4 after treatment with ADM, a commonly used anti-cancer drug capable of inducing DNA damage (Figure 4C). This results is consistent with our previous finding using a transient PAGE4 overexpressing system and suggest an anti-apoptotic effect of PAGE4 overexpression [27].
Figure 4.
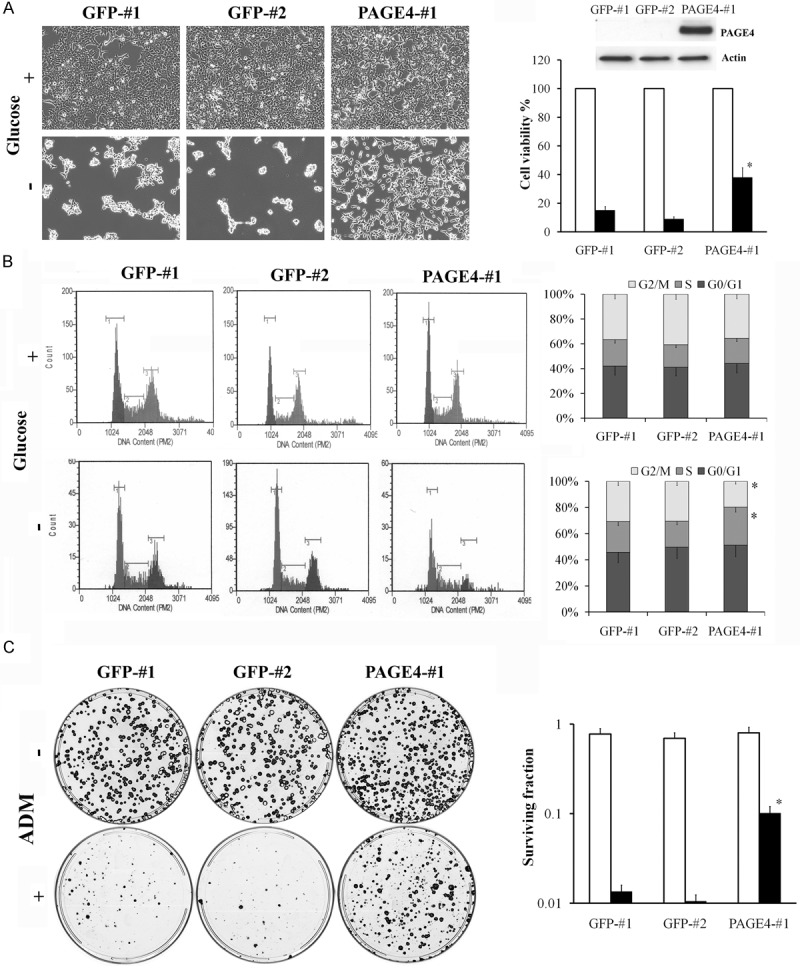
Overexpression of PAGE4 protects cells from stress-induced apoptosis. A: CWR22rv1 cells were transfected with pCMV6-PAGE4-GFP or pCMV6-GFP and the clones stably expressing PAGE4 were selected. PAGE4 expressing clone (PAGE4-#1), or GFP control clones (GFP-#1 and -#2) were cultured in normal medium with (□) or without (■) glucose for 72 h. Cells were observed under phase-contrast microscope and the representative images are shown. Right panel, cell viability was evaluated by WST-1 assay. B: PAGE4-#1, GFP-#1 and -#2 cells were stained with Propidium Iodide (PI) 48 hr after cultured in medium with or without glucose supplement and subjected to cell cycle analysis. Right panels, cell population in each phase of cell cycle was determined. C: PAGE4-#1, GFP-#1 and -#2 cells were plated in 10-cm dish at 500 cells (medium) or 2000 cells (ADM) per dish, and cultured in normal medium or treated with 1 μg/mL of ADM for 1 hr, respectively. Right panel, colonies were counted after 2-week growing. (□), medium; (■), ADM treatment. Data are represented as mean±SD. All experiments were repeated a minimum of three times. An asterisk indicates P<0.05 compared to control cells.
PAGE4 suppresses ROS production and protects DNA damage
Furthermore, as shown in Figure 5A, protein levels of the CDK inhibitor p21 that is a checkpoint protein involved in DNA damage response, were increased in PAGE4 overexpressing cells compared to control cells, especially when cells were subjected to glucose deprivation or ADM treatment, although this increase of p21 apparently was not dependent on p53 activation. At the same time, the DNA damage response marker γ-H2A.X was found to be less activated by the stress stimulants in those cells overexpressing PAGE4, underscoring the protective function of PAGE4. On the other hand, the activation of cell survival-related signaling molecule pAkt, was higher in the PAGE4 overexpressing clones than those not expressing PAGE4 (Figure 5B) suggesting that PAGE4 overexpression attenuates the stress-induced damage caused by glucose deprivation or drug treatment resulting in enhanced cell survival under these conditions. However, when these cells were treated with hydrogen peroxide (H2O2), which is a highly ROS, the observed protective effects of PAGE4 overexpression, namely the increase of p21, the decrease of γ-H2A.X and the activation of Akt, were missing, suggesting that PAGE4 overexpression is insufficient to inhibit ROS-induced cellular stress once ROS is generated. Since many stress factors including nutrition deprivation and chemical irritants induce DNA damage through generating ROS [5], we asked whether PAGE4 protects stress by suppressing ROS generation. Indeed, PAGE4-overexpressing cells showed a reverse correlation between the PAGE4 level and the ROS level when cells were cultured in medium without glucose supplement (Figure 5C and Supplemental Figure 4). In addition, treating cells with ADM readily induced ROS while this process was inhibited by PAGE4 overexpression (Figure 5D). Taken together, these results strongly suggest that one of the mechanisms by which PAGE4 protects stress-induced cellular damage is by inhibiting ROS generation.
Figure 5.
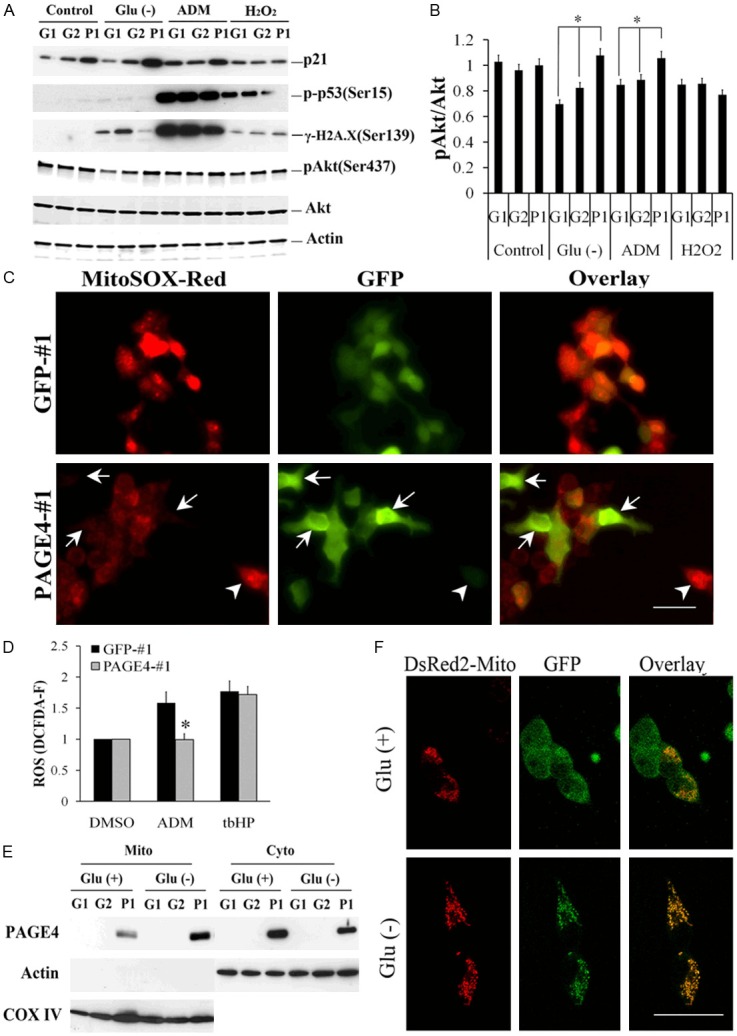
PAGE4 protects DNA damage and transfers to mitochondria upon glucose deprivation. A: CWR22rv1 cells overexpressing PAGE4 (P1) or GFP (G1 and G2) were cultured in medium with (+) or without (-) glucose (Glu) for 48 h, or treated with 1 μg/mL of ADM for 4 hr, or treated with 200 μM of H2O2 for 45 min followed by recovering in normal medium for 24 hr. Cells were then collected, and cell lysate were subjected to Western blot. B: Quantitative analysis of expression of p-AKT relative to actin. C: CWR22rv1 cells were transfected with either GFP (GFP-#1) or GFP-tagged PAGE4 (PAGE4-#1), and then cultured in the medium without glucose supplement for 48 hr. Cells were stained with MitoSOXTM Red and observed under fluorescence microscopy. Arrows indicate cells that highly express PAGE4 (green) but generate low ROS (red), while arrowheads indicate cells with high ROS level but low PAGE4 level. D: GFP-#1 and PAGE4- #1 cells were treated with 1 μg/mL of ADM or DMSO for 2 hr, and then ROS levels were measured. tbHP 50 μM was used as positive control. All the values are presented as relative to GFP-#1-DMSO. E: Cells were cultured with or without glucose supplement for 48 hr, and mitochondrial component were isolated. Cytoplasmic fractions (Cyto) and mitochondrial fractions (Mito) of cell lysate were subjected to Western blot. Actin level is used as protein loading control for Cyto component, while COX IV is used as a loading control for Mito component. F: PAGE4-#1 cells were transfected with pDsRed2-Mito and then cultured in the medium with or without glucose supplement for 48 hr. Localization of mitochondrial (red) and PAGE4 (green) were observed under confocal fluorescence microscopy. Data are represented as mean±SD. All experiments were repeated at a minimum of three times. An asterisk indicates P<0.05 compared to control cells. Scale bars in all panels, 10 μm.
PAGE4 translocates to mitochondria upon stress stimulation
PAGE4 has been shown to be a predominantly cytoplasmic protein in prostatic tissue samples (Figure 2) as well as in the cell lines that overexpress PAGE4 [23] (Supplemental Figure 3). In light of the importance of mitochondria in ROS production and the cellular response to stress stimulation, we asked if PAGE4 expression in the cytoplasm is related to mitochondrial function. Thus, mitochondrial fractions isolated from CWR22rv1 cells grown with or without glucose supplement were interrogated for the presence of PAGE4. As shown in Figure 5E, ectopically expressed PAGE4 was detected in the mitochondrial fraction although its expression level was still high in the cytosol fraction. But more importantly, when cells were cultured in the medium without glucose supplement, increased PAGE4 protein was detectable in the mitochondrial fraction with a concomitant decrease in the cytosol fraction. Consistent with subcellular fractionation results, we observed an accumulation of PAGE4 protein in the mitochondria by confocal microscopy when cells were subjected to glucose deprivation (Figure 5F). Taken together, these results indicate that PAGE4 translocates to the mitochondria in response to stress although, the mechanisms by which the translocation is achieved remain poorly understood.
Discussion
In the present study we have demonstrated that PAGE4 which is highly upregulated in the fetal prostate but barely detectable in the healthy adult prostate is upregulated in both BPH and PCa. However, in contrast to being exclusively expressed in the stroma in symptomatic BPH, in PCa, PAGE4 was detected more in the inflammation-related, non-cancerous epithelial cells particularly, those associated with PIA lesions, a potential precursor of PCa. Further, we demonstrated that PAGE4 is a stress-response gene as evidenced by its accumulation and translocation to the mitochondria in prostatic cell lines under various stress stimulations. In support of this point, PAGE4 expression has been previously shown to be stimulated in both prostatic stromal and epithelial cell lines by transforming growth factor (TGF)-β, which is a multi-functional cytokine that has been implicated to play roles during embryonic development and prostate diseases development [34]. Two other well-established stress-related proteins namely, cyclooxygenase-2 (COX-2) and glutathione S-transferase-π (GSTP1) are both overexpressed in PIA lesions but decreased in PIN and frank cancer epithelia [18,35]. Interestingly, while COX-2 converts arachidonic acid to various proinflammatory prostaglandins and therefore, enhances carcinogenesis related to chronic inflammation [36,37], GSTP1 has been proposed to be a caretaker gene, protecting cells against genome damage mediated by oxidants and electrophiles from inflammation or dietary exposures [15,38]. Similarly, PAGE4 overexpression, as shown in the present study protects cells from apoptosis induced by various stress factors, indicating a stress-protective role of PAGE4. These results corroborate well our previous finding that depleting PAGE4 expression in PCa cells resulted in the accumulation of DNA damage and greatly impedes cell survival under stress stimulations [39].
Although the mechanism underlying PAGE4 signaling remains unclear, we found that PAGE4 overexpression suppressed ROS production induced by cytotoxic agent. The increased translocation of PAGE4 from cytosol to mitochondria in response to stress also supports its regulation on ROS generation, because the mitochondrion is the most important organelle for ROS generation. ROS is a powerful promoter for oxidative DNA damage. Thus, by suppressing ROS production PAGE4 could contribute to attenuating DNA damage under stress stimulations as is shown in the present study. On the other hand, our data show that PAGE4 overexpression induced the expression of p21 that may attenuate cell cycle progression under stress stimulation. p21 is a critical checkpoint protein that induces cell cycle arrest during stress response reportedly following p53 activation, DNA damage, and ROS production [40,41]. However, we found that p21 elevation in PAGE4-overexpressing cells was not dependent on p53 activation, DNA damage or ROS production, because while p21 levels were increased, all these stress-responsive indicators were attenuated under conditions of stress. This suggests that PAGE4 may help cells respond to stress by increasing p21 and subsequently, cell cycle arrest prior to the resulting cellular damage. Thus, in this context, PAGE4 overexpression in the microenvironment of the prostate is a protective response to stress stimulation to reduce cellular damage potentially through inhibiting ROS production and increasing the levels of p21.
However, it still remains elusive how PAGE4 is involved in the development of prostate diseases, especially PCa. Nonetheless, the accumulation of PAGE4 in the microenvironment of PCa is associated with PIA lesions, which are widely recognized as the ‘hot spots’ for PCa. Interestingly, a recent report on PAGE4 suggests that overexpression of epithelial PAGE4 may attenuate androgen receptor (AR) signaling in PCa [42]. Given that AR signaling is essential to maintain the volume of luminal cells in the prostate gland, and the PIA lesions are atrophic glands with attenuated AR signaling [43], this may underscore the potential role of PAGE4 in the development of PIA lesions in a stress-enriched microenvironment of the prostate. In summary, given the remarkable tissue specificity in the adult male and the important role of PAGE4 in both the benign and malignant diseases of the prostate, it is tempting to conclude that PAGE4 may represent an ideal therapeutic target for these diseases that are among the most common ailments in the male population. Additional studies in the future should help elucidate the detailed mechanism underlying the function of PAGE4.
Acknowledgements
We thank Dr. William B. Isaacs for his interest in our work, Dr. Alan Meeker for his helpful suggestions regarding the IHC, and Mr. Don Vindivich for the technical assistance with this project. We also thank Dr. Takahiro Inoue for his helpful suggestions on the tissue mRNA expression analysis. This work is partly supported by the National Natural Science Foundation of China (Grant No. 81372766); Work in PK’s laboratory was supported by the Patrick C Walsh Prostate Cancer Research Fund.
Disclosure of conflict of interest
The authors declare no competing financial interests.
Abbreviations
- PAGE4
Prostate-associated gene 4
- CTA
Cancer/Testis Antigen
- PCa
Prostate cancer
- BPH
Benign prostatic hyperplasia
- ROS
Reactive oxygen species
- PIA
Proliferative Inflammatory Atrophy
- TMA
Tissue microarray
Supporting Information
References
- 1.Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, Ozgok Y, Dimovski A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–179. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Tam NN, Nyska A, Maronpot RR, Kissling G, Lomnitski L, Suttie A, Bakshi S, Bergman M, Grossman S, Ho SM. Differential attenuation of oxidative/nitrosative injuries in early prostatic neoplastic lesions in TRAMP mice by dietary antioxidants. Prostate. 2006;66:57–69. doi: 10.1002/pros.20313. [DOI] [PubMed] [Google Scholar]
- 3.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 4.Pace G, Di Massimo C, De Amicis D, Corbacelli C, Di Renzo L, Vicentini C, Miano L, Tozzi Ciancarelli MG. Oxidative Stress in Benign Prostatic Hyperplasia and Prostate Cancer. Urol Int. 2010;85:328–33. doi: 10.1159/000315064. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 6.Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 2005;37:1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Gosselin K, Martien S, Pourtier A, Vercamer C, Ostoich P, Morat L, Sabatier L, Duprez L, T’Kint de Roodenbeke C, Gilson E, Malaquin N, Wernert N, Slijepcevic P, Ashtari M, Chelli F, Deruy E, Vandenbunder B, De Launoit Y, Abbadie C. Senescence-associated oxidative DNA damage promotes the generation of neoplastic cells. Cancer Res. 2009;69:7917–7925. doi: 10.1158/0008-5472.CAN-08-2510. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 10.Maeda H, Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Mosc) 1998;63:854–865. [PubMed] [Google Scholar]
- 11.Espey MG, Miranda KM, Thomas DD, Xavier S, Citrin D, Vitek MP, Wink DA. A chemical perspective on the interplay between NO, reactive oxygen species, and reactive nitrogen oxide species. Ann N Y Acad Sci. 2002;962:195–206. doi: 10.1111/j.1749-6632.2002.tb04068.x. [DOI] [PubMed] [Google Scholar]
- 12.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner WA Jr, Bennett BD. The prostate--overview: recent insights and speculations. Monogr Pathol. 1992;34:129–148. [PubMed] [Google Scholar]
- 15.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 16.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons JK, Nelson CP, Gage WR, Nelson WG, Kensler TW, De Marzo AM. GSTA1 expression in normal, preneoplastic, and neoplastic human prostate tissue. Prostate. 2001;49:30–37. doi: 10.1002/pros.1115. [DOI] [PubMed] [Google Scholar]
- 18.Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, Faith DA, Nelson WG, De Marzo AM, Isaacs WB. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 19.Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, Lee C, Marberger M. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83:1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 20.Schenk JM, Kristal AR, Neuhouser ML, Tangen CM, White E, Lin DW, Kratz M, Thompson IM. Biomarkers of systemic inflammation and risk of incident, symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;171:571–582. doi: 10.1093/aje/kwp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 22.Brinkmann AO, Kuiper GG, Bolt-de Vries J, Mulder E. In situ photolabelling of the human androgen receptor. J Steroid Biochem. 1988;30:257–261. doi: 10.1016/0022-4731(88)90102-1. [DOI] [PubMed] [Google Scholar]
- 23.Iavarone C, Wolfgang C, Kumar V, Duray P, Willingham M, Pastan I, Bera TK. PAGE4 is a cytoplasmic protein that is expressed in normal prostate and in prostate cancers. Mol Cancer Ther. 2002;1:329–335. [PubMed] [Google Scholar]
- 24.Prakash K, Pirozzi G, Elashoff M, Munger W, Waga I, Dhir R, Kakehi Y, Getzenberg RH. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A. 2002;99:7598–7603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannon GW, Mullins C, Lucia MS, Hayward SW, Lin V, Liu BC, Slawin K, Rubin MA, Getzenberg RH. A preliminary study of JM-27: a serum marker that can specifically identify men with symptomatic benign prostatic hyperplasia. J Urol. 2007;177:610–614. doi: 10.1016/j.juro.2006.09.023. discussion 614. [DOI] [PubMed] [Google Scholar]
- 26.Shah US, Arlotti J, Dhir R, Lu S, Pirozzi G, Prakash K, Getzenberg RH. Androgen regulation of JM-27 is associated with the diseased prostate. J Androl. 2004;25:618–624. doi: 10.1002/j.1939-4640.2004.tb02832.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Y, He Y, Yang F, Mooney SM, Getzenberg RH, Orban J, Kulkarni P. The Cancer/Testis Antigen Prostate-associated Gene 4 (PAGE4) Is a Highly Intrinsically Disordered Protein. J Biol Chem. 2011;286:13985–13994. doi: 10.1074/jbc.M110.210765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Y, Kakehi Y, Nouh MA, Tsunemori H, Sugimoto M, Wu XX. Gene expression profiles of lysophosphatidic acid-related molecules in the prostate: relevance to prostate cancer and benign hyperplasia. Prostate. 2009;69:283–292. doi: 10.1002/pros.20879. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Y, Kulkarni P, Inoue T, Getzenberg RH. Down-regulating cold shock protein genes impairs cancer cell survival and enhances chemosensitivity. J Cell Biochem. 2009;107:179–188. doi: 10.1002/jcb.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gsponer J, Futschik ME, Teichmann SA, Babu MM. Tight regulation of unstructured proteins: from transcript synthesis to protein degradation. Science. 2008;322:1365–1368. doi: 10.1126/science.1163581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards YJ, Lobley AE, Pentony MM, Jones DT. Insights into the regulation of intrinsically disordered proteins in the human proteome by analyzing sequence and gene expression data. Genome Biol. 2009;10:R50. doi: 10.1186/gb-2009-10-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vavouri T, Semple JI, Garcia-Verdugo R, Lehner B. Intrinsic protein disorder and interaction promiscuity are widely associated with dosage sensitivity. Cell. 2009;138:198–208. doi: 10.1016/j.cell.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Sampson N, Untergasser G, Lilg C, Tadic L, Plas E, Berger P. GAGEC1, a cancer/testis associated antigen family member, is a target of TGF-beta1 in age-related prostatic disease. Mech Ageing Dev. 2007;128:64–66. doi: 10.1016/j.mad.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama M, Bennett CJ, Hicks JL, Epstein JI, Platz EA, Nelson WG, De Marzo AM. Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 37.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Friedman SL, Whalen R, Boyer TD. Cellular sources of glutathione S-transferase P in primary cultured rat hepatocytes: localization by in situ hybridization. Biochem J. 1994;299:79–83. doi: 10.1042/bj2990079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiraishi T, Terada N, Zeng Y, Suyama T, Luo J, Trock B, Kulkarni P, Getzenberg RH. Cancer/Testis Antigens as potential predictors of biochemical recurrence of prostate cancer following radical prostatectomy. J Transl Med. 2011;9:153. doi: 10.1186/1479-5876-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Zou P, Zou J, Wang J, Zhou D, Liu L. Autophagy regulates ROS-induced cellular senescence via p21 in a p38 MAPKalpha dependent manner. Exp Gerontol. 2011;46:860–867. doi: 10.1016/j.exger.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TB, von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sampson N, Ruiz C, Zenzmaier C, Bubendorf L, Berger P. PAGE4 Positivity Is Associated with Attenuated AR Signaling and Predicts Patient Survival in Hormone-Naive Prostate Cancer. Am J Pathol. 2012;181:1443–54. doi: 10.1016/j.ajpath.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 43.Penney KL, Sinnott JA, Fall K, Pawitan Y, Hoshida Y, Kraft P, Stark JR, Fiorentino M, Perner S, Finn S, Calza S, Flavin R, Freedman ML, Setlur S, Sesso HD, Andersson SO, Martin N, Kantoff PW, Johansson JE, Adami HO, Rubin MA, Loda M, Golub TR, Andren O, Stampfer MJ, Mucci LA. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J. Clin. Oncol. 2011;29:2391–2396. doi: 10.1200/JCO.2010.32.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


