Abstract
Androgen receptor (AR) plays an important role in the tumorigenesis and progression of prostate cancer (PCa), and is the primary therapeutic target for PCa treatment. AR activity can be regulated via phosphorylation at multiple phosphorylation sites within the protein. Modifications by phosphorylation alter AR function, including its cellular localization, stability and transcriptional activity, ultimately leading to changes in cancer cell biology and disease progression. Here we present a brief overview of AR phosphorylation sites in PCa, focusing on functional roles of phospho-AR (p-AR) species, relevance in PCa disease progression, and potential as biomarkers and/or therapeutic targets through the use of kinase inhibitors. Additionally, recent evidence has shown the important role of AR activity in the cancer associated stroma on PCa growth and progression. The phosphorylation status of epithelial and stromal AR may be distinct; however, the current data available on stromal AR phosphorylation is limited. Further research will determine global view on the synergistic effects of phosphorylation across multiple AR sites in both epithelial and stromal cells and validate whether together they can be used as prognostic markers and/or effective therapeutic targets for PCa.
Keywords: Androgen receptor, phosphorylation, prostate cancer
Androgen receptor (AR) has been known to play an important role in the tumorigenesis and progression of prostate cancer (PCa) for decades. Androgen signaling acts through the AR to regulate a number of cellular processes via genomic [1-3] and non-genomic [4,5] pathways. Although AR is robustly activated by ligand binding, a number of post-translational modifications (PTMs) that alter AR activity in the cell have been discovered. These AR modifications include phosphorylation, acetylation, SUMO-ylation, methylation, and ubiquitination [6]. Phosphorylation of AR has been the most extensively studied AR PTM, with the first phospho-AR (p-AR) species discovered almost 30 years ago [7]. To date over 15 distinct sites within AR have been found to be modified by phosphorylation, most of which reside in the N-terminal domain [8]. Even with the extensive studies of p-AR performed over the years there is still much not understood about the role of the various phosphorylated AR species. In particular, it would be of great value to determine which of these phosphorylated AR species could serve as potential PCa therapeutic targets with the use of specific kinase inhibitors or site specific phospho-antibodies as well as prognostic biomarkers. However, more research on p-AR functions and cell specific localizations is still needed. In this editorial, we aim to give a brief overview of AR phosphorylation in prostate cancer and how these studies could impact the direction of future research.
A number of sites throughout the AR protein are modified by phoshorylation as shown in Figure 1. Most of the identified phospho-sites in AR are serine residues, however there are also important threonine and tyrosine residues [8,9]. Phosphorylation of these sites has been implicated in a number of different cellular responses including AR transcriptional activity, regulation of AR expression, cell growth, and AR degradation. Serine-81 phosphorylation is increased in PCa and leads to increased AR expression in PCa cells [10,11]. Phosphorylation of Serine-81 also facilitates AR chromatin recruitment and transcriptional activity [12]. Functionally, phosphorylation at AR S81 promotes cell growth [13]. AR phosphorylation at S213 by Akt has been shown to either inhibit or promote cell growth in different cell contexts [14]. Phosphorylation of S213 by the PIM1S kinase isoform facilitates recruitment of E3 ubiquitin ligase Mdm2 and destabilizes AR protein [15]. Phosphorylation of S578 by PAK6 also leads to AR degradation [16].
Figure 1.
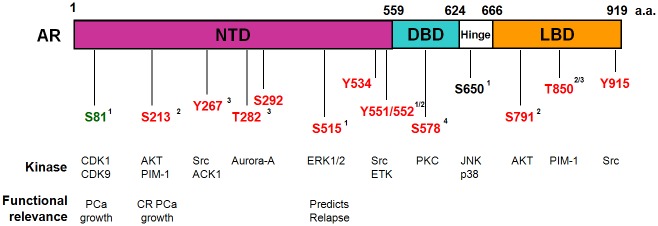
Androgen Receptor phosphorylation site and functional denotation. NTD: N-terminal Transactivation Domain; DBD: DNA Binding Domain; LBD: Ligand Binding Domain. Red: Growth factor induced phosphorylation; Green: Androgen induced phosphorylation; Black: Constitutive phosphorylation. AR functions: 1: nuclear/cytoplasmic shuttling; 2: stability; 3: transactivation; 4: DNA binding. PPase: S81 is regulated by PP2A and S650 is regulated by PP1.
Recurrent and in particular, castration resistant prostate cancer (CRPCa) is a clinically challenging condition that occurs when PCa becomes resistant to androgen ablation therapy. There is evidence that S515 phosphorylation may be a predictive marker for relapse in PCa [17]. Prognosis for patients with CRPCa is poor and multiple mechanisms have been proposed to explain the way PCa evades androgen ablation therapy. Even in the absence of androgens, AR is critical for the growth of CRPCa and still remains the primary therapeutic target [18-20]. Activation of AR by phosphorylation is one potential mechanism for the development of CRPCa. Even after androgen ablation therapy there is still evidence of low levels of androgens in the tumor microenvironment which aid in driving the formation of CRPCa [21]. Phosphorylation of AR at S81, S213 and S790 occurs in low androgen environments and may sensitize AR to low androgen conditions and be an indicator for CRPCa [11,22]. Further, phosphorylation of AR at T850 by the PIM1 isoform PIM1-L leads to recruitment of the E3 ligase RNF6 and promotes AR mediated transcription at low androgen levels [15]. Activation of AR by phosphorylation can also act as a means of cross-talk with other signaling pathways, particularly in a low androgen environment relevant to CRPCa. For example, EGF signaling results in phosphorylation at S515 to promote growth in androgen-free conditions [23]. A recent report shows that AR S213 phosphorylation likely identifies cells with catalytically active PIM1 and is correlated with CRPCa [24]. In addition, phosphorylation of AR at Y267 by Ack1 is correlated with PCa radiation treatment resistance in CRPCa patients [25]. In contrast to most p-AR associated with poor prognosis, a recent study showed phosphorylation at S308 and S791 can predict enhanced survival in CRPCa [26]. It is of great interest to determine whether any of these phosphorylation sites for AR are directly causal of CRPCa development. With increased understanding of the mechanisms and function of AR phosphoryation, there is greater potential for development of therapeutic targets or prognostic markers for PCa.
There is still much left to be understood about the role of AR phosphorylation in PCa. For example, splicing variants of AR have been discovered that are constitutively active in the absence of androgens [27]. The impact of AR phosphorylation on the expression and function of these recently identified truncated [28] and membrane [29] variants of AR is not known. Targeting kinases which phosphorylate sites in the truncated variants may be an effective means of treatment under conditions where androgen ablation therapy would have no effect.
AR expression and activity in cancer epithelial cells has been the primary focus of study in PCa research over the years. However, more recent studies implicated the increased importance of the stromal cells in regulating PCa growth. Multiple in vitro cell culture models are now available for the study of AR functions [30]. Conditioned media from stromal cells can lead to S81 phosphorylation of AR in PCa cells [31]. Additionally, AR signaling in the stromal cells is also important in the regulation of surrounding PCa epithelial cell proliferation. For example, stromal AR can inhibit growth of PCa cells in the presence of androgen in co-culture and co-xenograft studies [32]. Further, studies with P-S213 AR antibody showed increased detection of phosphorylation at S213 in epithelial cells but no expression in surrounding stromal cells in PCa [33]. There has been very limited research on the role of stromal AR in PCa, and particularly the phosphorylation status of stromal AR. Many potential therapies work very well in cell culture but fail in vivo and much of this could be due to activity in the stromal microenvironment. As continued studies show the importance of AR activity in PCa stromal cells, it will also be important to identify the role of phosphorylation in AR positive stromal cells on PCa progression. Increased understanding of the complex combination of phosphorylation signals in the AR in both cancer and cancer-associated stroma cells will undoubtedly lead to more successful treatments in PCa.
Of note, PCa has been described as a more aggressive cancer in African American (AA) compared to Caucasian (CA) patients [34-36]. Further, epithelial AR expression has been shown to be increased [37] and stromal AR decreased [38] more frequently in AA PCa in comparison to CA patients. It is of great interest to determine whether AA PCa possess changes in AR splice variants and if there are differences in phosphorylation status, both in the cancer epithelial and cancer associated stromal cells, that make these cancers more aggressive.
New research has shown unique roles of various kinases that regulate AR to ultimately modulate effects on cell growth and these kinases may be used as the primary targets for battling prostate cancer. As the roles of site-specific AR phosphorylation are elucidated, it is important to note that each site works in conjunction with others, as well as additional PTMs of AR. Continued research is necessary to evaluate the function of phosphorylation at each phospho-site but also learn how the various AR PTMs work together to modulate cell homeostasis in either genomic or non-genomic pathways. Identification of single p-AR modifications may not be predictive of cell behavior outside of the context of other PTMs of AR. Disparate activities at separate phospho-sites concurrently may explain some of the discrepancies found in the literature on p-AR functions. More detailed understanding of how the specific phosphorylation activities work together will ultimately lead to more effective combinatorial treatments.
Acknowledgements
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). This study is funded by NIH (1U01CA149556-01), DOD PCRP (PC080010 and PC11624) and VA Merit (1I01BX001505-01) grants to PL, NYU Molecular Oncology and Immunology Postdoctoral Training grant (T32 CA009161) to GD.
References
- 1.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 2.Green SM, Mostaghel EA, Nelson PS. Androgen action and metabolism in prostate cancer. Mol Cell Endocrinol. 2012;360:3–13. doi: 10.1016/j.mce.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu S, Tindall DJ. Androgen action in prostate cancer. Horm Cancer. 2010;1:223–228. doi: 10.1007/s12672-010-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 5.Bonaccorsi L, Nosi D, Quercioli F, Formigli L, Zecchi S, Maggi M, Forti G, Baldi E. Prostate cancer: a model of integration of genomic and non-genomic effects of the androgen receptor in cell lines model. Steroids. 2008;73:1030–7. doi: 10.1016/j.steroids.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. J Endocrinol. 2012;215:221–237. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 7.Goueli SA, Holtzman JL, Ahmed K. Phosphorylation of the androgen receptor by a nuclear cAMP-independent protein kinase. Biochem Biophys Res Commun. 1984;123:778–784. doi: 10.1016/0006-291x(84)90297-3. [DOI] [PubMed] [Google Scholar]
- 8.Gioeli D, Paschal BM. Post-translational modification of the androgen receptor. Mol Cell Endocrinol. 2012;352:70–78. doi: 10.1016/j.mce.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Ward RD, Weigel NL. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. Biofactors. 2009;35:528–536. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Szabolcs M, Terwilliger JD, Efstratiadis A. Prostatic intraepithelial neoplasia and adenocarcinoma in mice expressing a probasin-Neu oncogenic transgene. Carcinogenesis. 2006;27:1054–1067. doi: 10.1093/carcin/bgi324. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci U S A. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Gulla S, Cai C, Balk SP. Androgen receptor serine 81 phosphorylation mediates chromatin binding and transcriptional activation. J Biol Chem. 2012;287:8571–8583. doi: 10.1074/jbc.M111.325290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon V, Bhadel S, Wunderlich W, Zhang J, Ficarro SB, Mollah SA, Shabanowitz J, Hunt DF, Xenarios I, Hahn WC, Conaway M, Carey MF, Gioeli D. CDK9 regulates AR promoter selectivity and cell growth through serine 81 phosphorylation. Mol Endocrinol. 2010;24:2267–2280. doi: 10.1210/me.2010-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HK, Hu YC, Yang L, Altuwaijri S, Chen YT, Kang HY, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 15.Linn DE, Yang X, Xie Y, Alfano A, Deshmukh D, Wang X, Shimelis H, Chen H, Li W, Xu K, Chen M, Qiu Y. Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases. J Biol Chem. 2012;287:22959–22968. doi: 10.1074/jbc.M111.338350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Li Y, Gu H, Zhu G, Li J, Cao L, Li F. p21-Activated kinase 6 (PAK6) inhibits prostate cancer growth via phosphorylation of androgen receptor and tumorigenic E3 ligase murine double minute-2 (Mdm2) J Biol Chem. 2013;288:3359–3369. doi: 10.1074/jbc.M112.384289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willder JM, Heng SJ, McCall P, Adams CE, Tannahill C, Fyffe G, Seywright M, Horgan PG, Leung HY, Underwood MA, Edwards J. Androgen receptor phosphorylation at serine 515 by Cdk1 predicts biochemical relapse in prostate cancer patients. Br J Cancer. 2013;108:139–148. doi: 10.1038/bjc.2012.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J. Clin. Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 19.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–257. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saylor PJ. Prostate cancer: The androgen receptor remains front and centre. Nat Rev Clin Oncol. 2013;10:126–128. doi: 10.1038/nrclinonc.2013.14. [DOI] [PubMed] [Google Scholar]
- 21.Mitsiades N. A road map to comprehensive androgen receptor axis targeting for castration-resistant prostate cancer. Cancer Res. 2013;73:4599–4605. doi: 10.1158/0008-5472.CAN-12-4414. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 23.Ponguta LA, Gregory CW, French FS, Wilson EM. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J Biol Chem. 2008;283:20989–21001. doi: 10.1074/jbc.M802392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha S, Iqbal NJ, Mita P, Ruoff R, Gerald WL, Lepor H, Taneja SS, Lee P, Melamed J, Garabedian MJ, Logan SK. Phosphorylation of the androgen receptor by PIM1 in hormone refractory prostate cancer. Oncogene. 2013;32:3992–4000. doi: 10.1038/onc.2012.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahajan K, Coppola D, Rawal B, Chen YA, Lawrence HR, Engelman RW, Lawrence NJ, Mahajan NP. Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer. J Biol Chem. 2012;287:22112–22122. doi: 10.1074/jbc.M112.357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall P, Adams CE, Willder JM, Bennett L, Qayyum T, Orange C, Underwood MA, Edwards J. Androgen receptor phosphorylation at serine 308 and serine 791 predicts enhanced survival in castrate resistant prostate cancer patients. Int J Mol Sci. 2013;14:16656–16671. doi: 10.3390/ijms140816656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18:R183–196. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Guo Z, Sun F, Li W, Alfano A, Shimelis H, Chen M, Brodie AM, Chen H, Xiao Z, Veenstra TD, Qiu Y. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem. 2011;286:36152–36160. doi: 10.1074/jbc.M111.265124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson N, Neuwirt H, Puhr M, Klocker H, Eder IE. In vitro model systems to study androgen receptor signaling in prostate cancer. Endocr Relat Cancer. 2013;20:R49–64. doi: 10.1530/ERC-12-0401. [DOI] [PubMed] [Google Scholar]
- 31.Shigemura K, Isotani S, Wang R, Fujisawa M, Gotoh A, Marshall FF, Zhau HE, Chung LW. Soluble factors derived from stroma activated androgen receptor phosphorylation in human prostate LNCaP cells: roles of ERK/MAP kinase. Prostate. 2009;69:949–955. doi: 10.1002/pros.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Li CX, Ye H, Chen F, Melamed J, Peng Y, Liu J, Wang Z, Tsou HC, Wei J, Walden P, Garabedian MJ, Lee P. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12:2790–2798. doi: 10.1111/j.1582-4934.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280:40916–40924. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- 34.Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res. 2009;1:235–248. [PMC free article] [PubMed] [Google Scholar]
- 35.Mordukhovich I, Reiter PL, Backes DM, Family L, McCullough LE, O’Brien KM, Razzaghi H, Olshan AF. A review of African American-white differences in risk factors for cancer: prostate cancer. Cancer Causes Control. 2011;22:341–357. doi: 10.1007/s10552-010-9712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wachtel MS, Nelius T, Haynes AL, Dahlbeck S, de Riese W. PSA screening and deaths from prostate cancer after diagnosis--a population based analysis. Prostate. 2013;73:1365–1369. doi: 10.1002/pros.22680. [DOI] [PubMed] [Google Scholar]
- 37.Gaston KE, Kim D, Singh S, Ford OH 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol. 2003;170:990–993. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Zhang DY, Ren Q, Ye F, Zhao X, Daniels G, Wu X, Dynlacht B, Lee P. Regulation of a novel androgen receptor target gene, the cyclin B1 gene, through androgen-dependent E2F family member switching. Mol Cell Biol. 2012;32:2454–2466. doi: 10.1128/MCB.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


