Abstract
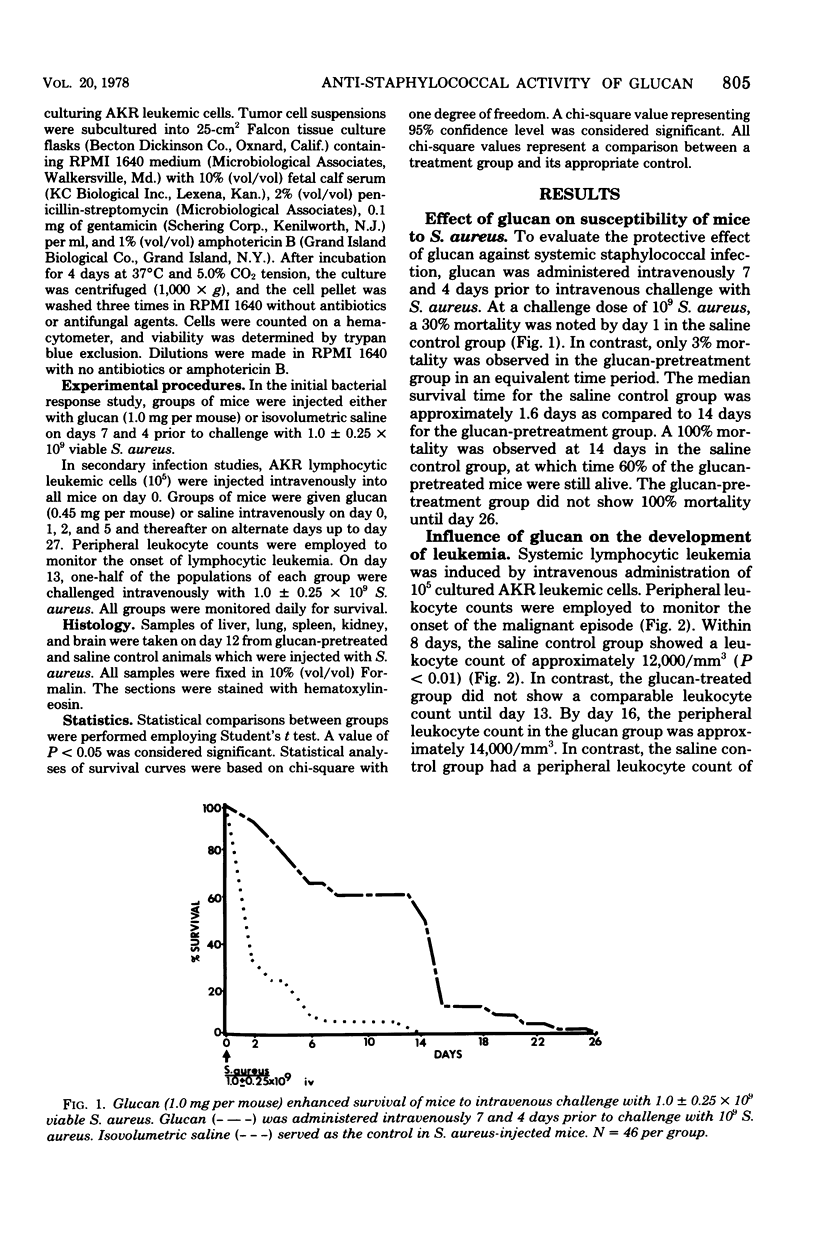
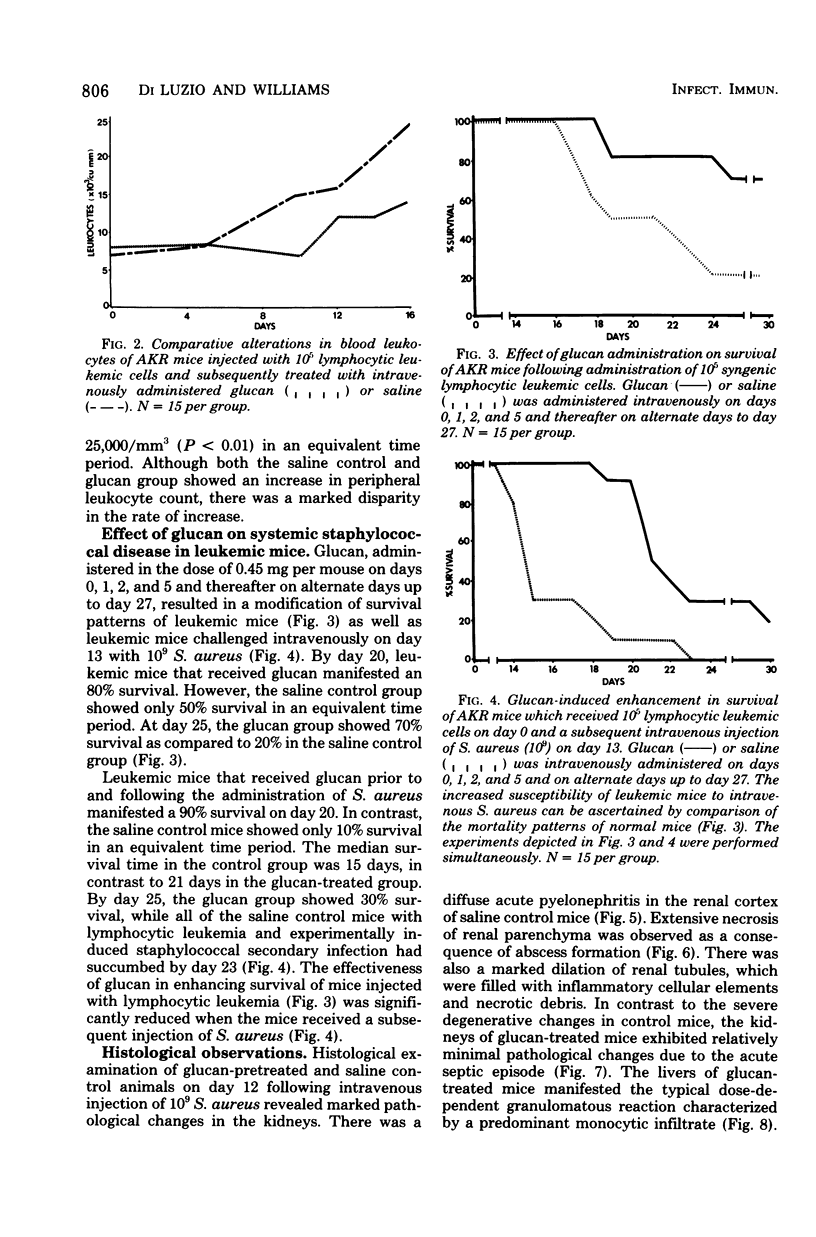
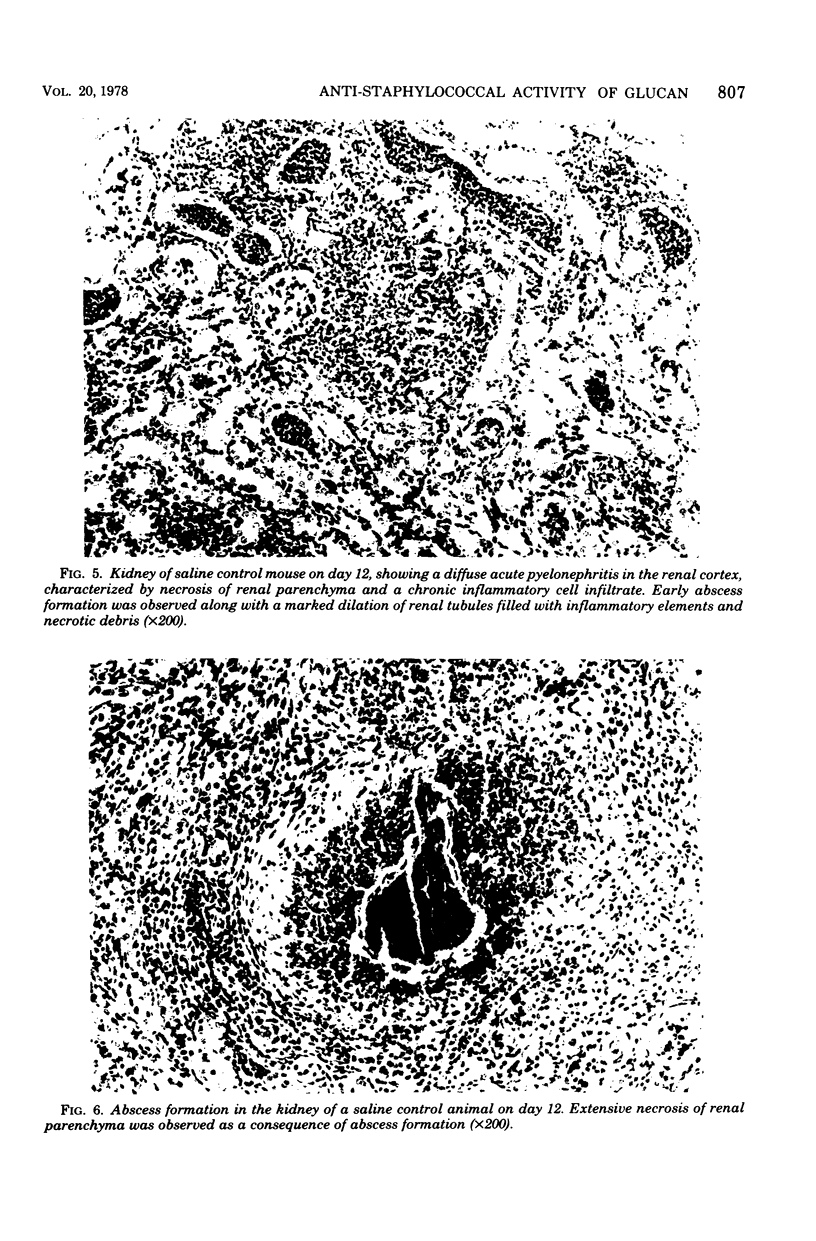
The reticuloendothelial stimulant glucan, a beta-1,3-polyglucose component of the cell wall of Saccharomyces cerevisiae, was evaluated for its ability to modify Staphylococcus aureus-induced lethality in normal and leukemic mice. In normal mice the intravenous injection of glucan (0.45 mg per mouse) 7 and 4 days prior to intravenous challenge with S. aureus (1.0 x 10(9)) resulted in a significantly increased survival. Histological examination of the kidneys revealed that glucan significantly inhibited renal necrosis associated with systemic staphylococcal diseases. Further studies indicated that glucan administration not only enhanced survival of leukemic mice, but also increased survival of leukemic mice with experimentally induced staphylococcal speticemia. These data denote that glucan enhances nonspecific resistance to S. aureus sepsis, promotes survival during leukemic episodes, and increases survival time of leukemic mice with experimentally induced staphylococcal infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Baughn R., Bonventre P. F. Phagocytosis and intracellular killing of Staphylococcus aureus by normal mouse peritoneal macrophages. Infect Immun. 1975 Aug;12(2):346–352. doi: 10.1128/iai.12.2.346-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar W. D., Buron S., Holmes B. Bactericidal mechanisms in rabbit alveolar macrophages: evidence against peroxidase and hydrogen peroxide bactericidal mechanisms. Infect Immun. 1976 Jul;14(1):6–10. doi: 10.1128/iai.14.1.6-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder W., Jones E., McNamee R., Di Luzio N. R. Inhibition of tumor growth by glucan, a nonspecific immunostimulant. Surg Forum. 1976;27(62):134–135. [PubMed] [Google Scholar]
- Burgaleta C., Golde D. W. Effect of glucan on granulopoiesis and macrophage genesis in mice. Cancer Res. 1977 Jun;37(6):1739–1742. [PubMed] [Google Scholar]
- Di Luzio N. R. Pharmacology of the reticuloendothelial system - accent on glucan. Adv Exp Med Biol. 1976;73(PT-A):412–421. [PubMed] [Google Scholar]
- GORRILL R. H. The establishment of staphylococcal abscesses in the mouse kidney. Br J Exp Pathol. 1958 Apr;39(2):203–212. [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T. Fatal infections in childhood leukemia. Am J Dis Child. 1971 Oct;122(4):283–287. doi: 10.1001/archpedi.1971.02110040067003. [DOI] [PubMed] [Google Scholar]
- Hughes W. T. Infections in the compromised host. Adv Intern Med. 1977;22:73–96. [PubMed] [Google Scholar]
- Lampert I. A., Jones P. D., Sadler T. E., Castro J. E. Intravascular coagulation resulting from intravenous injection of C. parvum in mice. Br J Cancer. 1977 Jul;36(1):15–22. doi: 10.1038/bjc.1977.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell P. W., DiLuzio N. R., McNamee R., Rowden G., Proctor J. W. Recognition factors and nonspecific macrophage activation in the treatment of neoplastic disease. Ann N Y Acad Sci. 1976;277(00):20–44. doi: 10.1111/j.1749-6632.1976.tb41689.x. [DOI] [PubMed] [Google Scholar]
- Mansell P. W., Ichinose H., Reed R. J., Krementz E. T., McNamee R., Di Luzio N. R. Macrophage-mediated destruction of human malignant cells in vivo. J Natl Cancer Inst. 1975 Mar;54(3):571–580. [PubMed] [Google Scholar]
- RIGGI S. J., DI LUZIO N. R. Identification of a reticuloendothelial stimulating agent in zymosan. Am J Physiol. 1961 Feb;200:297–300. doi: 10.1152/ajplegacy.1961.200.2.297. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Bernard S. Effects of BCG, Corynebacterium parvum, and methanol-extration residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect Immun. 1975 Dec;12(6):1325–1330. doi: 10.1128/iai.12.6.1325-1330.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLES W. R., DI LUZIO N. R. Influence of reticuloendothelial hyperfunction on bone marrow transplantation. Am J Physiol. 1962 Sep;203:404–408. doi: 10.1152/ajplegacy.1962.203.3.404. [DOI] [PubMed] [Google Scholar]
- WOOLES W. R., DILUZIO N. R. RETICULOENDOTHELIAL FUNCTION AND THE IMMUNE RESPONSE. Science. 1963 Nov 22;142(3595):1078–1080. doi: 10.1126/science.142.3595.1078. [DOI] [PubMed] [Google Scholar]






