Abstract
It is well documented that androgen receptor (AR), a steroid hormone receptor, is important for prostate cancer (PCa) growth. Conversely, however, there is increasing evidence that activation of AR by androgens can also lead to growth suppression in prostate cells. AR mediated transcription is regulated by a number of different transcriptional coactivators. Changes in expression level or cellular localization of specific coactivators may play a crucial role in this switch between proliferative and anti- proliferative processes regulated by AR target gene programs. In this review, we discuss the expression and function of several AR coactivators exhibiting growth suppressive function in PCa, including ARA70/ELE1/NCOA4, androgen receptor coactivator p44/MEP50/WDR77, TBLR1, and ART-27. In luciferase reporter assays, they all have been shown to activate AR mediated transcriptional activation. ARA70 exists in two forms, the full length nuclear ARA70α and internally spliced cytoplasmic ARA70β. For p44 and TBLR1, we identified nuclear and cytoplasmic forms with distinct expression and function. In comparison of their expression (ARA70α, p44, TBLR1 and ART-27) in prostate, these coactivators are expressed in the nucleus of benign prostate epithelial cells while they are more predominantly expressed in cytoplasmic form (ARA70β, cytoplasmic p44 and TBLR1) in PCa. Consistent with their nuclear expression in benign prostate, the nuclear form of these coactivators inhibit PCa growth targeting a subset of AR target genes. In contrast, the cytoplasmic versions of these proteins enhance PCa growth and invasion. Interestingly, first characterized as an AR coactivator in luciferase assays, ART-27 functions as corepressor for endogenous AR target genes. Importantly, the growth inhibitions by these nuclear proteins are androgen-dependent processes and the regulation of invasion is androgen-independent. Understanding the molecular switches involved in the transition from AR dependent growth promotion to growth suppression and dysregulation of these coactivator proteins promoting androgen-independent invasion may lead to identification of novel therapeutic targets for PCa.
Keywords: Androgen receptor, coactivator, growth inhibition, prostate cancer
Introduction
Androgen receptor (AR), a member of the nuclear hormone receptor family of transcription factors, plays a vital role in early and late stages of prostate cancer (PCa). The binding of steroidal androgens, testosterone and 5α dihydrotestosterone (DHT), causes receptor dimerization, recruitment to androgen response elements on DNA and recruitment of a series of cofactors to promote expression of the AR target genes. Cofactors of steroid receptors interact with androgen receptor to either enhance (coactivators) or reduce (corepressors) its trans-activation without any intrinsic DNA binding capacity or significant effect on the basal transcription rate. Hundreds of cofactors have been characterized across multiple pathways, including chromatin remodeling, histone modification, proteasomal degradation, DNA repair, signal transduction, and post translational modification [1,2]. Most of these studies have provided overwhelming evidence that AR coactivators primarily function in promoting PCa growth and progression. However, recent studies indicate that activation of the AR pathway also has a growth suppressive function.
AR inhibiting PCa
It is well known that androgen stimulation leads to increased proliferation in the prostate and withdrawal, through various methods, leads to a decrease in proliferation via decreased cell cycle activation and increased apoptosis. There are several examples suggesting that androgen activation of AR can also lead to inhibition of prostate cell growth in certain contexts [3-5]. For example, in the presence of androgen, the ectopic expression of AR cDNA can lead to slowed growth in PC3 prostate cancer cells [3,5,6].
Yuan et al. demonstrated that introduction of a full length human AR complementary DNA into AR negative PC3 cells resulted in decreased proliferation rate with a more differentiated phenotype in these cells [5]. The proliferation rate of these cells was reduced by 50% after incubation with exogenous androgen. Similar results were found in another study which demonstrated that the treatment of AR-expressing PC3 cells with physiological levels of dihydrotestosterone (DHT) for 3 days resulted in paradoxical inhibition of cell growth [3]. Whitacre et al. characterized the androgen growth response of an immortalized non-tumorigenic rat prostate cell line called CA25, which contains an AR cDNA expression cassette linked to a hygromycin resistance gene that had been stably transfected [4]. They found that the AR positive CA25 cells demonstrated slower growth in the presence of DHT, whereas AR negative CA25 cells were not affected by the hormone.
Hess-Wilson et al. showed that the response of PCa cells to taxane induced cell death is significantly enhanced by androgen stimulation in AR positive androgen-dependant PCa cells which was attributed to caspase dependent apoptosis [7]. Similar studies have shown that androgens are able to promote cell quiescence and cell arrest by inducing the expression of anti-proliferative genes in PCa cells [8,9]. Additionally, studies indicate activation of AR represses c-Met expression, an indicator of poor prognosis, in the AR positive prostate cancer cell line LNCaP [10]. Moreover, continuous androgen deprivation of LNCaP cells leads to outgrowth of a variant cell line which is independent of androgens for enhanced proliferation. Androgen replacement of this LNCaP variant results in decreased cell growth [11]. This suggests that AR functions remain intact, although with an altered transcriptional response.
Mechanisms underlying the growth inhibitory PCa growth suppressive effects are not well understood. Most of the experiments showing AR inhibits PCa growth are performed with PC3 cells. Although PC3 cells do not express endogenous AR, Litvinov et al. documented that PC3 cells retained the cofactors necessary for AR tumor suppressor activity in vivo which may halt PCa growth and justify PC3 as a model for AR activity in prostate cancer [12]. However, it cannot be ruled out that the effect of overexpression of AR in PC3 cells is due to other factors. In addition, the various levels of AR may influence the cell proliferation or growth inhibition in PCa [13]. Vander et al. identified AR as a replication licensing factor which must be degraded during mitosis in order to allow licensing in the subsequent cell cycle [14]. They suggested that an acute enhanced expression of AR in PCa cells results in its incomplete degradation in mitosis which subsequently inhibits cell proliferation. AR can also drive senescence in prostate cancer cells by induction of p21 and inhibition of Rb phosphorylation [15]. Additionally, AR has non-genomic functions that include promotion of apoptosis via downregulation of p21 [16].
AR coactivators inhibiting PCa growth
The exact mechanism responsible for the switch between cell proliferation and growth suppression is not fully understood, although transcriptional cofactors have been implicated to be important in the process. Changes in the levels of expression and localization of these cofactors can regulate distinct spectrum of AR target genes, either activated or repressed, and ultimately the fate of the cell. In this review, we will discuss AR coactivators that act as tumor suppressors to inhibit cell growth. The AR coactivators included in this review are ARA70/ELE1/NCOA4, AR coactivatorp44/MEP50/WD R77, TBLR1 and ART-27 (Figure 1). In luciferase assays, all of these proteins are characterized as AR coactivators as they increase AR mediated transcriptional activation in a dosage dependent manner, though degree of AR activation varies between them [17-21].
Figure 1.
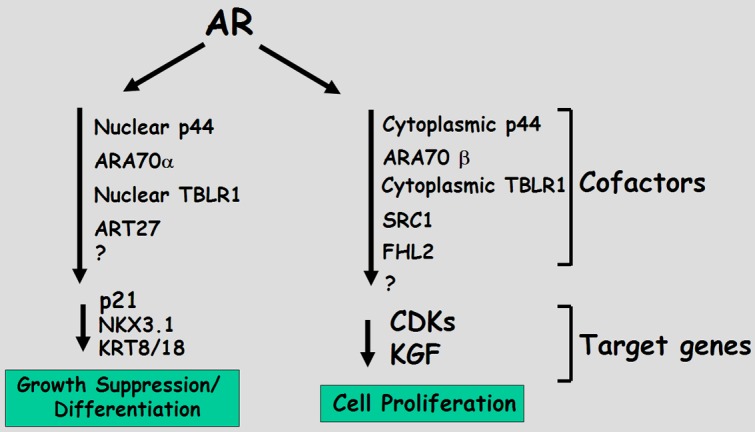
Representative model of transcriptional coactivators that can activate AR target genes either involved with cell proliferation (e.g. CDKs, KGF) or growth suppression/differentiation (e.g. p21, NKX3.1). Dysregulation of AR coactivators leads to PCa.
Expression and function of ARA70/ELE1/NCOA4
ARA70, first identified as a gene fused with the ret oncogene in thyroid carcinoma [22], was identified as an AR coactivator in the presence of ligand [17]. There are two major isoforms of ARA70, the full length 70 kDa ARA70α and internally spliced 35 kDa ARA70β. We and others have demonstrated that ARA70α is expressed as nuclear protein in benign prostate epithelial cells and there are reduction in ARA70α transcript and protein levels in PCa compared to benign prostate [23]. In contrast, the levels of ARA70β are increased in PCa [21,24].
ARA70α functions as an androgen-dependent tumor suppressor gene in PCa, both in vitro as well as in vivo studies [25]. Retroviral vector overexpressing full length ARA70α (pBabe-ARA70α) was constructed to establish clonal stable LNCaP and PC3 cell lines stably overexpressing ARA70α (LNCaP-ARA70α and PC3-ARA70α respectively). In the presence of the synthetic androgen, R1881, LNCaP-ARA70α cells grew significantly slower than control LNCaP-pBabe cells. In reciprocal, abolishing ARA70α expression in wild type LNCaP cells using ARA70α specific siRNA led to increased proliferation rate compared to control LNCaP cells. Modifications in ARA70α level had no effect on the growth of AR-negative PC3 cells or in LNCaP cells in the absence of androgen. Additionally, AR siRNA knockdown rescued the growth inhibitory effect of ARA70α on LNCaP cells further confirming AR dependence of ARA70α growth inhibition. Similar results were also obtained with anchorage independent growth assays. LNCaP-ARA70α cells formed fewer and smaller colonies than control LNCaP cells in the presence of R1881 but no difference was observed in the absence of androgen. In a nude mouse xenograft model, mice injected with LNCaP-ARA70α cells also showed significant reduction in tumor size compared to LNCaP-pBabe cells. These findings confirmed that AR coactivator ARA70α inhibits cell growth in PCa in an AR-dependent manner.
Growth inhibition by ARA70α is primarily due to increased apoptosis as opposed to decrease in cell cycle activation [25]. A sub-G1 population of cells was observed in flow cytometry indicating DNA fragmentation. Staining with cyanide dye DiOC2 (a dye which enters mitochondria and emits red and green signal on excitation due to dye stacking) followed by flow cytometric analysis showed LNCaP-ARA70α cells demonstrate reduced emission (a sign of a decreased mitochondrial membrane potential) indicating that certain cells in this population are subjected to mitochondrial damage. Western blot analysis confirmed apoptotic changes with increased levels of cleaved caspase 3 and Bax.
Expression and function of androgen receptor coactivatorp44/MEP50/WDR77 in PCa
p44 is an AR interacting protein that functions as an AR coactivator in the context of the protein arginine methyl transferase 5 (PRMT5) complex [19]. p44 acts as a coactivator of AR mediated transcription through a p44-AR-Smad1 complex [27]. Immunohistochemical studies showed expression of p44 is strong in the nuclei and low in the cytoplasm of benign epithelial cells. Conversely, in tumor cells, nuclear expression of p44 is significantly reduced and cytoplasmic levels are increased. Reduction of nuclear and increase in cytoplasmic p44 expression is observed as early as in high grade prostate epithelial neoplasia (HGPIN), a precursor of PCa, suggesting loss of nuclear p44 as an early event in tumorigenesis. The nuclear and cytoplasmic translocation of p44 from benign to cancerous prostate is mediated by two nuclear exporting and three nuclear localization signals and some of them are non-conventional [28]. Additionally, strong cytoplasmic p44 staining is associated with castration resistant PCa [29].
Our lab showed in both in vitro assays and in vivo tumor xenograft experiments that nuclear p44 inhibits PCa growth, whereas cytoplasmic p44 promotes PCa growth and that nuclear exclusion of p44 is associated with androgen-independent PCa growth. We dissected the functions of nuclear and cytoplasmic p44 by fusing either a nuclear import sequence (NLS, nuclear localization signal) or a nuclear export signal (NES) sequence to the N terminus of p44, resulting in NLSp44 or NESp44 respectively in LNCaP or PC3 cells. Proliferation of LNCaP-NLSp44 cells is significantly reduced compared to control cells in androgen media. Anchorage-independent assays also demonstrated a loss of transformation ability of LNCaP-NLSp44 cells. Nuclear p44 had no effect on proliferation or anchorage-independent growth in AR-negative PC3 cells. Further, AR knockdown by siRNA in LNCaP-NLSp44 cells rescued the inhibitory growth effect of nuclear p44 further confirming an AR-dependent mechanism. Nuclear p44 was also confirmed to have an inhibitory effect on in vivo growth by nude mouse xenograft model. Subcutaneous injection of LNCaP-NLSp44 showed significant reduction of size and tumor onset compared to control LNCaP cells. Consistent with its growth suppression function, transgenic mice, lacking one allele for p44 (p44+/−), exhibit excessive epithelial cell proliferation that results in HGPIN [30].
Immunohistochemistry showed decreased Ki67 expression but no significant difference between cleaved Caspase 3 in LNCaP-NLSp44 xenograft tumor cells relative to LNCaP-pBabe cells suggesting that the observed tumor growth is caused by reduced cell proliferation rather than increased apoptosis. Cell cycle analysis showed that nuclear p44 overexpression led to a significant reduction of S phase and increase in G0/G1 cells corresponding with growth inhibition by cell cycle arrest. Cells expressing NLSp44 demonstrated decreased levels of cyclin A and cyclin B with an increase in the levels of cyclin inhibitors p21 and p27. p21 knockdown by siRNA rescued LNCaP cells from the growth inhibitory effects of nuclear p44.
On the other hand, cytoplasmic p44 promotes growth in PCa cells. Cell proliferation assays performed on LNCaP-NESp44 cell lines showed both increased cell growth and anchorage-independent growth in soft agar [29]. Overexpression of cytoplasmic p44 in LNCaP cells led to increased S phase cells, slight decrease in expression of p21 and p27, increased expression of cyclin D2 and CDK6, although no difference in cyclin A or cyclin B in these cells. Knockdown of cyclin D2 or CDK6 led to reduced growth in LNCaP-NESp44 cells. These findings were consistent with the growth kinetics that cytoplasmic p44 increases cell proliferation. Surprisingly, there was no change in xenograft tumor growth with LNCaP-NESp44 cells compared with control LNCaP cells. However, cytoplasmic p44 does have a growth promotion role in LNCaP xenograft tumors. This is demonstrated by the growth inhibition with endogenous p44 in LNCaP cells knocked down by shRNAp44 as the p44 is expressed as cytoplasmic protein in LNCaP cells.
Expression and function of TBLR1 in PCa
Transducin beta like related protein-1 (TBLR1), a core component of the nuclear receptor corepressor (NCoR) complex, shows both corepressor and coactivator activities on different nuclear receptors in various cellular context [31-33]. Our data reveal TBLR1 is an AR coactivator in PCa cells by luciferase assays [18]. Coimmunoprecipitation studies also showed androgen-dependent physical interaction between TBLR1 and AR. TBLR1 coactivator activity of AR is 19S proteosome and PKCδ phosphorylation dependent. TBLR1 is primarily expressed in the nucleus of normal prostate cells and expression of nuclear TBLR1 is significantly reduced in human PCa compared to benign cells both at cell line and tissue levels. Similar to nuclear p44, there is also reduced nuclear TBLR1 in HGPIN compared to benign cells. Additionally, growth arrest induced by serum starvation leads to migration of TBLR1 from the cytoplasm to nucleus, consistent with nuclear TBLR1 as a growth arrest signal [18].
In order to study the functional effects of nuclear TBLR1 in prostate cells, our lab created the LNCaP-NLSTBLR1 cell line stably overexpressing nuclear TBLR1 in LNCaP cells. Nuclear TBLR1 dramatically reduced proliferation of LNCaP cells in the presence of androgen. Anchorage-independent assays in soft agar as a test for transformation ability also showed fewer and smaller colonies in LNCaP-NLSTBLR1 cells compared to control cells. LNCaP-NLSTBLR1 also showed significantly slower in vivo growth resulting in smaller tumors in nude mouse xenografts. Together, these data strongly support that TBLR1 functions as a tumor suppressor in PCa.
Cell cycle analysis by flow cytometry revealed that overexpression of nuclear TBLR1 in androgen containing media caused significant reduction of S phase cells, concurrent with a G0/G1 arrest mechanism as the cause of reduced proliferation. There was no evidence of increased apoptosis in these cells by caspase 3/7 activity assay.
Expression and function of ART-27 in PCa
Androgen receptor trapped clone-27 (ART-27) was first identified as an AR coactivator as it binds to the AR NH2 terminus in the NTD domain and enhances the transcriptional activity of AR in luciferase assays [20,34]. In the prostate, ART-27 expression is mainly restricted to the luminal epithelial cells [34]. Immunohistochemistry studies with human PCa specimens demonstrate reduced levels of ART-27 compared with nonmalignant tissue and expression is reduced significantly during PCa progression. Significantly, loss of ART-27 leads to bicalutamide resistance on AR activity and correlates with PCa recurrence.
ART-27 associates with a large, multiprotein complex whose constituents regulate transcription, genomic stability, apoptosis, and cell transformation [20,35]. ART-27 acts as a suppressor of cell transformation, and a nuclear factor-kB coregulator [36,37]. ART-27 has been shown to inhibit AR-dependent proliferation in LNCaP cells in response to androgen, suggesting that it has a tumor suppressive action on these cells [34]. Additionally, reduction of ART-27 protein levels enabled LNCaP cells treated with ART-27 siRNA, but not control siRNA, to grow in the presence of bicalutamide. These studies indicate that ART-27 functions as a tumor suppressor in PCa. While characterized as AR coactivator by luciferase assays, ART-27 functions as corepressor endogenously for AR target genes as discussed below.
Selective AR target genes affected by the growth inhibiting coactivators
To date, a large number of studies have been reported on the genome wide AR target gene signature in PCa [38-40], in particular using cell line models. These studies indicated a wide range of AR target gene profiles involved in many aspects of PCa biology including cell cycle activation, apoptosis, tumor suppression and cell differentiation. Thus, AR regulates a host of different target genes, including pro-proliferative but also differentiation and growth suppression genes. Androgen response elements (ARE) are not stringently conserved and new binding sequences are continually being characterized [41,42]. The coactivators are critical in dictating which AR target programs are activated upon androgen stimulation and ultimately the fate of the cell. The PCa inhibiting AR coactivators described in this review can selectively initiate the transcriptional activation of distinct AR target genes.
Overexpression of p44 leads to increased activation of AR transcription that leads to cell cycle arrest. Androgen regulated cell cycle regulators, p21 and p27, were both upregulated upon nuclear p44 overexpression in the presence of androgen [29]. Nuclear p44 also led to reduced expression of the pro-proliferative AR target gene CDK2 [30], signifying that not only can p44 act in selective activation of AR target genes for growth suppression but can have corepressor function for other target genes. Androgen signaling via AR also is important in regulation of genes involved in cellular differentiation, including KRT18, a differentiation marker present in luminal epithetial cells. As transgenic mice lacking one allele of p44 (p44 -/+) in mice form HGPIN in the prostate, examination of these HGPIN cells showed reduced KRT18 levels by immunohistochemistry [30].
AR target gene studies showed that overexpression of nuclear TBLR1 led to selective activation of AR target genes responsible for growth suppression and differentiation (ie. NKX3.1, KRT8) but had no effect on pro-proliferative AR target genes (ie. CDC6, cyclin A2) [18]. Chromatin immunoprecipitation studies showed direct interaction of TBLR1 with AR at growth suppressive AR target gene promoters in an androgen-dependent manner. Earlier, we examined the role of activated AR in growth suppression. This study showed that nuclear TBLR1 acts a PCa tumor suppressor and AR coactivator by selectively activating the growth suppressive AR regulated pathways.
Nwachukwu et al. examined the effects of ART-27 on genome wide transcription [44]. They found an enhancement of expression of a number of androgen regulated genes subsequent to the loss of ART-27, indicating a role of ART-27 in AR inhibiting target gene expression. Upregulated genes included regulators of DNA damage check point and cell cycle progression.
Androgen-dependent growth inhibition and androgen-independent invasion promotion
As discussed above, the coactivators reviewed here are all expressed as nuclear proteins in benign prostate and inhibit PCa growth. These coactivators interact with AR to regulate transcription and the growth suppressive processes facilitated are androgen-dependent. This was confirmed by experiments showing cofactor overexpression led to no significant proliferative effects upon growth in androgen free media, using LNCaP cells with AR knockdown by siRNA or PC3 cells with stable AR overexpression.
Interestingly, the regulation of invasion is an androgen-independent process as exemplified by ARA70. ARA70α inhibits and ARA70β promotes PCa invasion [21,25]. These processes observed are not regulated through AR and are confirmed androgen-independent by continuous ARA70α inhibition or ARA70β promotion of invasion after AR knockdown, removal of androgens, or AR expression in PC3 cells. These data suggest that in contrast to the androgen-dependent nature of growth inhibition, the ability of reduced invasion by ARA70α or enhanced invasion by ARA70β is an androgen-independent process [21,25]. Androgen-independent function of coactivators may represent one potential mechanism of continued activation of AR upon androgen ablation treatment and the development of CRPC. Therefore, androgen ablation therapy alone may have no effect on these cells ability to invade and metastasize.
Summary and future directions
The prevalent attitude in PCa treatment over the years has been that blocking androgen signaling will suppress PCa. Androgen signaling is complex. Androgens are predominately viewed as pro-proliferative signals in PCa, evident by regression of tumors on ablative treatment. However, they are also important in the activation of target genes responsible for differentiation and growth suppression. In this review, we discuss a subset of AR coactivators that do not drive proliferation, but inhibit growth by activating the non-proliferative AR target genes. Dysregulation of these coactivators lead to PCa growth and invasion (Figure 1). Though these cofactors all are defined as AR coactivators by luciferase assays, they may have either coactivator (ARA70, TBLR1) or corepressor (ART-27) functions on endogenous AR target genes or also play different roles on distinct target genes at the same time (p44/WDR77).
Additionally, the expression of these coactivators in benign and cancer epithelial cells are well studied, however, their expression and function in stromal cells remain to be characterized. In PCa, higher levels of AR expression in the stroma correlates with improved prognosis and stromal AR activity can inhibit growth of PCa epithelial cells [45]. It would be of great significance to evaluate the roles of coactivators on stromal AR activity and the effect on PCa growth and progression. Of note, TBLR1 is expressed in certain stromal cells and would be of interest to determine its function in these cells.
There is still much left to be understood in the complex regulation of AR mediated transcription and the role it plays in prostate cancer progression. We already know that merely blocking androgens or AR signaling alone is not adequate for treating prostate cancer. Androgen ablation may also abolish the AR activity necessary for growth suppression. Additionally, although it may initially reduce tumor burden it may not have any effect on invasion properties of residual PCa cells. Expression profiles of different cofactors will be important in improved targeting of specific AR pathways. Rather than turn off all AR signaling, selective activation or repression of specific AR target gene programs may be more desirable in developing better treatment strategies.
Acknowledgements
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). This study is funded by NIH 1U01CA149556-01A1, 3U01CA149556-01S1, DOD PCRP (PC080010 and PC111624) and VA Merit (1I01BX001505-01) grants to PL; and postdoctoral fellowships from NYU Molecular Oncology and Immunology Training grant (T32 CA009161) to GD.
References
- 1.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 2.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 3.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 4.Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]
- 5.Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- 6.Marcelli M, Haidacher SJ, Plymate SR, Birnbaum RS. Altered growth and insulin-like growth factor-binding protein-3 production in PC3 prostate carcinoma cells stably transfected with a constitutively active androgen receptor complementary deoxyribonucleic acid. Endocrinology. 1995;136:1040–1048. doi: 10.1210/endo.136.3.7532576. [DOI] [PubMed] [Google Scholar]
- 7.Hess-Wilson JK, Daly HK, Zagorski WA, Montville CP, Knudsen KE. Mitogenic action of the androgen receptor sensitizes prostate cancer cells to taxane-based cytotoxic insult. Cancer Res. 2006;66:11998–12008. doi: 10.1158/0008-5472.CAN-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnenschein C, Olea N, Pasanen ME, Soto AM. Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res. 1989;49:3474–3481. [PubMed] [Google Scholar]
- 9.Geck P, Szelei J, Jimenez J, Lin TM, Sonnenschein C, Soto AM. Expression of novel genes linked to the androgen-induced, proliferative shutoff in prostate cancer cells. J Steroid Biochem Mol Biol. 1997;63:211–218. doi: 10.1016/s0960-0760(97)00122-2. [DOI] [PubMed] [Google Scholar]
- 10.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–975. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 11.Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941–953. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 12.Litvinov IV, Antony L, Dalrymple SL, Becker R, Cheng L, Isaacs JT. PC3, but not DU145, human prostate cancer cells retain the coregulators required for tumor suppressor ability of androgen receptor. Prostate. 2006;66:1329–1338. doi: 10.1002/pros.20483. [DOI] [PubMed] [Google Scholar]
- 13.Yu SQ, Lai KP, Xia SJ, Chang HC, Chang C, Yeh S. The diverse and contrasting effects of using human prostate cancer cell lines to study androgen receptor roles in prostate cancer. Asian J Androl. 2009;11:39–48. doi: 10.1038/aja.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Griend DJ, Litvinov IV, Isaacs JT. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle. 2007;6:647–651. doi: 10.4161/cc.6.6.4028. [DOI] [PubMed] [Google Scholar]
- 15.Mirochnik Y, Veliceasa D, Williams L, Maxwell K, Yemelyanov A, Budunova I, Volpert OV. Androgen receptor drives cellular senescence. PLoS One. 2012;7:e31052. doi: 10.1371/journal.pone.0031052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Lu Z, Kokontis J, Xiang J. Androgen receptor primes prostate cancer cells to apoptosis through down-regulation of basal p21 expression. Biochem Biophys Res Commun. 2013;430:289–293. doi: 10.1016/j.bbrc.2012.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci U S A. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels G, Li Y, Gellert LL, Zhou A, Melamed J, Wu X, Zhang X, Zhang D, Meruelo D, Logan SK, Basch R, Lee P. TBLR1 as an androgen receptor (AR) coactivator selectively activates AR target genes to inhibit prostate cancer growth. Endocr Relat Cancer. 2014;21:127–142. doi: 10.1530/ERC-13-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosohata K, Li P, Hosohata Y, Qin J, Roeder RG, Wang Z. Purification and identification of a novel complex which is involved in androgen receptor-dependent transcription. Mol Cell Biol. 2003;23:7019–7029. doi: 10.1128/MCB.23.19.7019-7029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markus SM, Taneja SS, Logan SK, Li W, Ha S, Hittelman AB, Rogatsky I, Garabedian MJ. Identification and characterization of ART-27, a novel coactivator for the androgen receptor N terminus. Mol Biol Cell. 2002;13:670–682. doi: 10.1091/mbc.01-10-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Y, Li CX, Chen F, Wang Z, Ligr M, Melamed J, Wei J, Gerald W, Pagano M, Garabedian MJ, Lee P. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70. Am J Pathol. 2008;172:225–235. doi: 10.2353/ajpath.2008.070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alen P, Claessens F, Schoenmakers E, Swinnen JV, Verhoeven G, Rombauts W, Peeters B. Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1alpha with multiple steroid receptors and identification of an internally deleted ELE1beta isoform. Mol Endocrinol. 1999;13:117–128. doi: 10.1210/mend.13.1.0214. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y, Chiriboga L, Yee H, Pei Z, Wang Z, Lee P. Androgen receptor coactivator ARA70alpha and ARA70beta isoform-specific antibodies: new tools for studies of expression and immunohistochemical localization. Appl Immunohistochem Mol Morphol. 2008;16:7–12. doi: 10.1097/PAI.0b013e31802e91ea. [DOI] [PubMed] [Google Scholar]
- 24.Hu YC, Yeh S, Yeh SD, Sampson ER, Huang J, Li P, Hsu CL, Ting HJ, Lin HK, Wang L, Kim E, Ni J, Chang C. Functional domain and motif analyses of androgen receptor coregulator ARA70 and its differential expression in prostate cancer. J Biol Chem. 2004;279:33438–33446. doi: 10.1074/jbc.M401781200. [DOI] [PubMed] [Google Scholar]
- 25.Ligr M, Li Y, Zou X, Daniels G, Melamed J, Peng Y, Wang W, Wang J, Ostrer H, Pagano M, Wang Z, Garabedian MJ, Lee P. Tumor suppressor function of androgen receptor coactivator ARA70alpha in prostate cancer. Am J Pathol. 2010;176:1891–1900. doi: 10.2353/ajpath.2010.090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu Y, Yeh S, Miyamoto H, Li G, Altuwaijri S, Yuan J, Han R, Ma T, Kuo HC, Chang C. Tissue prostate-specific antigen facilitates refractory prostate tumor progression via enhancing ARA70-regulated androgen receptor transactivation. Cancer Res. 2008;68:7110–7119. doi: 10.1158/0008-5472.CAN-07-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Tian L, Ligr M, Daniels G, Peng Y, Wu X, Singh M, Wei J, Shao Y, Lepor H, Xu R, Chang Z, Wang Z, Lee P. Functional domains of androgen receptor coactivator p44/Mep50/WDR77and its interaction with Smad1. PLoS One. 2013;8:e64663. doi: 10.1371/journal.pone.0064663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu Z, Zhou L, Gao S, Wang Z. Nuclear transport signals control cellular localization and function of androgen receptor cofactor p44/WDR77. PLoS One. 2011;6:e22395. doi: 10.1371/journal.pone.0022395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y, Chen F, Melamed J, Chiriboga L, Wei J, Kong X, McLeod M, Li Y, Li CX, Feng A, Garabedian MJ, Wang Z, Roeder RG, Lee P. Distinct nuclear and cytoplasmic functions of androgen receptor cofactor p44 and association with androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2008;105:5236–5241. doi: 10.1073/pnas.0712262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Wu H, Lee P, Wang Z. Roles of the androgen receptor cofactor p44 in the growth of prostate epithelial cells. J Mol Endocrinol. 2006;37:283–300. doi: 10.1677/jme.1.02062. [DOI] [PubMed] [Google Scholar]
- 31.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 32.Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomita A, Buchholz DR, Shi YB. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol. 2004;24:3337–3346. doi: 10.1128/MCB.24.8.3337-3346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taneja SS, Ha S, Swenson NK, Torra IP, Rome S, Walden PD, Huang HY, Shapiro E, Garabedian MJ, Logan SK. ART-27, an androgen receptor coactivator regulated in prostate development and cancer. J Biol Chem. 2004;279:13944–13952. doi: 10.1074/jbc.M306576200. [DOI] [PubMed] [Google Scholar]
- 35.Delgermaa L, Hayashi N, Dorjsuren D, Nomura T, Thuy le TT, Murakami S. Subcellular localization of RPB5-mediating protein and its putative functional partner. Mol Cell Biol. 2004;24:8556–8566. doi: 10.1128/MCB.24.19.8556-8566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGilvray R, Walker M, Bartholomew C. UXT interacts with the transcriptional repressor protein EVI1 and suppresses cell transformation. FEBS J. 2007;274:3960–3971. doi: 10.1111/j.1742-4658.2007.05928.x. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, St Clair DK, Fang F, Warren GW, Rangnekar VM, Crooks PA, St Clair WH. The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-kappaB inhibition and enhanced by the presence of PTEN. Mol Cancer Ther. 2007;6:2477–2486. doi: 10.1158/1535-7163.MCT-07-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velasco AM, Gillis KA, Li Y, Brown EL, Sadler TM, Achilleos M, Greenberger LM, Frost P, Bai W, Zhang Y. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology. 2004;145:3913–3924. doi: 10.1210/en.2004-0311. [DOI] [PubMed] [Google Scholar]
- 39.Ngan S, Stronach EA, Photiou A, Waxman J, Ali S, Buluwela L. Microarray coupled to quantitative RT-PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene. 2009;28:2051–2063. doi: 10.1038/onc.2009.68. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mole Cell. 2007;27:380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia L, Berman BP, Jariwala U, Yan X, Cogan JP, Walters A, Chen T, Buchanan G, Frenkel B, Coetzee GA. Genomic androgen receptor-occupied regions with different functions, defined by histone acetylation, coregulators and transcriptional capacity. PLoS One. 2008;3:e3645. doi: 10.1371/journal.pone.0003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang Z, Niu Y, Cai Q, Chen J, Tian J, Yeh S, Lai KP, Chang C. Human kallikrein 2 (KLK2) promotes prostate cancer cell growth via function as a modulator to promote the ARA70-enhanced androgen receptor transactivation. Tumour Biol. 2013 doi: 10.1007/s13277-013-1253-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Nwachukwu JC, Mita P, Ruoff R, Ha S, Wang Q, Huang SJ, Taneja SS, Brown M, Gerald WL, Garabedian MJ, Logan SK. Genome-wide impact of androgen receptor trapped clone-27 loss on androgen-regulated transcription in prostate cancer cells. Cancer Res. 2009;69:3140–3147. doi: 10.1158/0008-5472.CAN-08-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Li CX, Ye H, Chen F, Melamed J, Peng Y, Liu J, Wang Z, Tsou HC, Wei J, Walden P, Garabedian MJ, Lee P. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12:2790–2798. doi: 10.1111/j.1582-4934.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


