Abstract
The microbiome is a new center of attention for studies on the pathogenesis of human disease by focusing on the alterations of all microorganisms living in a particular site or system of human body, referred as microbiota. Evidence suggests that microbiota could contribute to the pathogenesis of a number of chronic diseases, including cancers, both locally and remotely. Multiple mechanisms have been proposed and/or proven for the microbiota’s role in tumorigenesis, such as via induction of chronic inflammation, genotoxicity, bacterium-mediated cell proliferation, and activation of procarcinogens. Emerging data suggest that indigenous microbiota in the urinary tract may play an important role in the tumorigenesis of urothelial carcinoma, similar to other tumors. Future studies are needed to adequately define the microbiota composition and correlate its change with urothelial carcinoma.
Keywords: Microbiome, microbiota, bladder cancer, urothelial carcinoma
Introduction
The microbiome is currently one of the heated topics in medicine, particularly in cancer research, and has gained strong research support for its promising perspectives. As a result of technological advances in sequencing, our knowledge about the microbiome is ever expanding and new hypotheses are being formulated on idiopathic diseases, which could pave a new way for diagnosis, prevention and treatment of human diseases. The microbiome refers to the collection of genomes of all microorganisms in a particular site or system of our body, which are collectively named as microbiota [1]. Cultivation, coupled with use of selective media, has been the classical approach for isolation of bacterial pathogens. However, this approach is not adequate for characterization of a complex microbial community. Cultivation could miss bacteria which are in a viable but non-culturable (VBNC) state [2]. Further, cultivation favors bacteria that are capable of growing on artificial media over those whose nutritional needs are undetermined and therefore unsatisfied by in vitro methods. The limitation of cultivation had not been overcome until cultivation-independent technology became applied to the field of microbiology in the 1980s [3,4]. 16S rRNA genes, which are widely-used to estimate the evolutionary history and taxonomic assignment of individual organisms, formed the basis of the most popular cultivation-independent technique. All bacteria have one or more 16S ribosomal RNA genes. These genes are essential components for protein synthesis and are vital for life. Since all bacteria are descendants of a common ancestor, some of the regions of the 16S genes have remained conserved over billions of years of evolution. PCR based analyses using broad range primers to these regions can amplify 16S rRNA genes of nearly all bacterial species. The less conserved regions can serve as fingerprints for taxonomic classification. The combination of sequencing technology and comprehensive 16S rRNA gene databases has allowed 16S rRNA gene surveys to become the major format of cultivation-independent studies of simple and complex bacterial communities. With the recent advance of next generation sequencing technology, it is now feasible to characterize complex microbiomes with one shot of analysis of 16S rRNA genes in a sample.
Increasing data have shown that dysbiosis -- the imbalance or alteration of bacterial composition of microbiota in a disease condition compared with the healthy state, is implicated in the pathogenesis of local and even distant diseases [5]. The successful treatment of refractory Clostridium difficile infection with fecal microbiota transplantation provides a good example for the clinical application of microbiota in treatment [6]. Microbiota have been shown to be involved in the pathogenesis of cancers of many organs, including esophagus, stomach, colon, liver, gall bladder, pancreas, breast, and lymphoid tissue in animal and human studies [1]. With next generation sequencing technology, the “core” microorganisms of microbiota in different body sites will be defined and the role of microbiota in the pathogenesis of diseases, including cancer, in different body sites will be further elucidated. This new knowledge will help in the discovery of new strategies in cancer prevention, and perhaps also therapy.
The role of microbiota in the pathogenesis of urinary tract neoplasia is still under investigation. Here we will briefly discuss the mechanisms of microbiota in the pathogenesis of cancer, and then speculate on the possible role of microbiota in urothelial carcinoma.
Evolving microbiome in cancer research
The role of specific microorganisms in the pathogenesis of cancer has been extensively studied and well established [7]. Four major infectious agents, Helicobacter pylori, human papillomaviruses, and hepatitis B and hepatitis C viruses are responsible for 1.9 million of new cancer cases per year worldwide [8]. Studies of infectious cancers have largely focused on a single microorganism. The role of microbiota in carcinogenesis was initially observed in animal studies, which showed that microbiota of the gastrointestinal tract can activate or produce carcinogens, which act locally on the gastrointestinal tract or remotely on other organs through circulation or secretion in the urine [9]. Some mechanisms of microbiota in carcinogenesis have been suggested [1]: 1) Innate immunity: Microbiota may promote carcinogenesis by inducing chronic inflammation. An imbalance between microbiota and host immunity leads to quantitative and/or qualitative changes of microbiota, and therefore changes the composition of the essential bacterial molecules at the specific body site, which is called microorganism-associated molecular patterns (MAMPs). Changes in MAMPs activate pattern receptors of innate immunity, including Toll-like receptors (TLR) [10] and/or nucleotide-binding-oligomerization-domain (NOD)-like receptors [11]. Triggering the receptors activates the NF-kB pathway and induces expression of cytokines and interleukins. 2) Genotoxicity: Many bacteria produce genotoxins that may cause genomic damage. For example, cytolethal distending toxin produced by Gram-negative bacteria causes double stranded DNA damage and genomic instability [12,13]. Other genotoxins include colibactin [13,14], cytotoxic necrotizing factor 1, Bacteroides fragilis toxin, hydrogen sulphide and superoxide radicals [15,16]. 3) Bacterial virulence: Bacteria produce virulent factors that may have tumor promoting effects. Cytotoxin-associated gene A (CagA) and vacuolating toxin A (VacA) produced by Helicobacter pylori promote gastric cancer development [17]. Virulence factor Fad A of Fusobacterium nucleatum interacts with E-cadherin, activates β-catenin signaling, and promotes colorectal cancer development [18]. 4) Activation of procarcinogens: Bacteria can modulate cancer risk by metabolizing carcinogenic chemicals. Oral and intestinal microbiota promote carcinogenesis by regulation of obesity-induced inflammation, metabolic activation and generation of carcinogens (e.g. nitrosamine and acetaldehyde), activation of dietary phytochemicals, metabolism of hormones, xenobiotics, and modification of tumor promoting bile acids [19].
Microbiome in urothelial carcinoma
Urothelial carcinoma is the major type of cancer found in the urinary tract, with its most common site in the bladder. Urothelial carcinoma is also one of the most common cancers, with about 75,000 new cases and 16,000 cancer deaths each year in Unites States [20]. The most important risk factors known for urothelial carcinoma are cigarette smoking and various occupational exposures. Schistosoma haematobium infection is associated with the development of squamous cell carcinoma of bladder due to chronic inflammation. Clinically, the urine of healthy people is considered sterile, and the presence of bacteria with inflammatory cells is indicative of urinary tract infection (UTI). Asymptomatic bacteriuria is commonly observed clinically. However, the relationship of urinary tract infection with urothelial carcinoma has not been established.
Recent microbiome studies showed that microbiota may exist in the urinary tract of healthy individuals. A microbiome study with 8 culture-negative (<100,000 CFU/ml) healthy female urine specimens revealed a complex bacterial community with the predominant genera Lactobacillus, Prevotella and Gardnerella, and showed considerable variation between individuals [21]. However, a common microbial signature was not evident in this study, probably due to inadequate sequencing depth. Another microbiome study on the urinary microbiota of healthy individuals showed that samples from females were more diversified at the genus level than those of males [22]. This study suggests that a “core” urinary microbiome could potentially exist when samples are grouped by age with fluctuation in abundance between age groups, for instance, age-specific genera Jonquetella, Parvimonas, Proteiniphilum, and Saccharofermentans. An important feature of microbiota is dysbiosis in disease conditions, which has been observed in urinary tract pathology. A microbiome study of urine from women with interstitial cystitis, a chronic inflammatory condition of the bladder of unknown etiology, revealed differences in composition and distribution of bacteria compared with healthy individuals [23]. There was a reduced microbial richness and diversity in the urine samples from interstitial cystitis patients, with more than 90% sequences classified to Lactobacillus, which is much more abundant compared to 60% in healthy female urine. A cross-sectional study was performed to compare microbiome between healthy people and subjects at risk for asymptomatic bacteriuria due to spinal cord injury-related neuropathic bladder [24]. Urine microbiomes differ according to: whether there is normal bladder function vs. neuropathic bladder, gender, type of bladder catheter utilized for bladder drainage (voided normally, utilized intermittent catheterization, or utilized indwelling Foley urethral catheterization), and duration of neuropathic bladder. A variety of bacterial taxa changed in abundance between the conditions compared, of which the top ten most abundant bacterial taxa were Lactobacillales, Enterobacteriales, Actinomycetales, Bacillales, Clostridiales, Bacteroidales, Burkholderiales, Pseudomonadales, Bifidobacteriales and Coriobacteriales. This study indicated that healthy urine microbiota are characterized by a preponderance of Lactobacillales in women and Corynebacterium in men. It appears that urine microbiome is altered by neuropathic bladder and method of urinary catheterization. Of note are the controversial findings of the relationship between Lactobacillales/Lactobacillus and health status from the study of interstitial cystitis and the study of neuropathic bladder in female subjects [21,22]. Because Lactobacillales/Lactobacillus is the most abundant bacteria taxon in the vaginal microbiome [25,26], avoidance of contamination of urine samples by the vaginal microbiome represents a great challenge to studies of urine microbiome in females.
We performed a microbiome study on urine specimens from healthy individuals (n=6) and urothelial carcinoma patients (n=8) using 454 sequencing technology. Overall, 329 genera were observed, with an average of 42.6±19.4 genera per sample in normal subjects and 73.1±42.8 genera in cancer patients. Acinetobacter was the most abundant genus (0.22±0.21), while Streptococcus, Pseudomonas, Finegoldia, Gardnerella, Anaerococcus, Escherichia, or Enterococcus was occasionally found to be the most dominant genus in some samples. Streptococcus abundance was near zero (0-0.017) in most normal samples except one outlier (0.66) but significantly elevated in 5 of the 8 cancer samples (0.12-0.31) (Figure 1). Pseudomonas or Anaerococcus was the most abundant genus in 2 of the 3 cancer samples where Streptococcus abundance was low. The results suggest that urothelial carcinoma may be associated with altered microbiota of the urinary tract.
Figure 1.
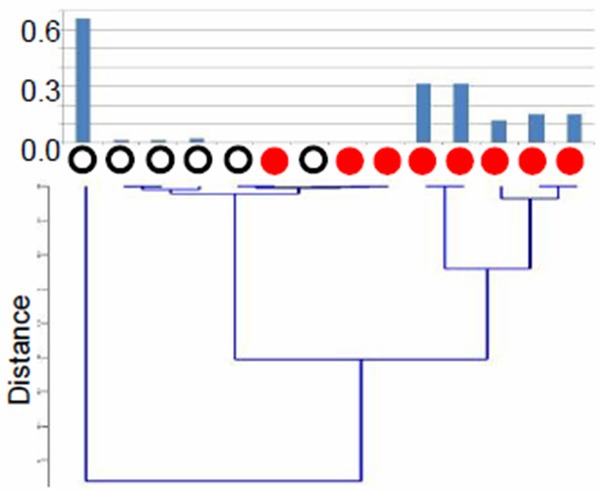
Difference in the relative abundance of Streptococcus in urine samples between healthy subjects (open black circles) and patients with urothelial carcinoma (solid red circles). Bacterial communities were analyzed from DNA extracted from mid-stream urine samples. Top panel shows the relative abundance of Streptococcus in each sample and the bottom panel is a dendrogram showing sample clusters based on the distances derived from the relative abundance of Streptococcus.
The major criticism against most studies of urine microbiome is the commonly used mid-stream urine collected by “clean catch” method. With this method, the urine is potentially contaminated with microorganisms in the terminal portion of urethra near or around the urethral orifice. Further studies are necessary to clarify this issue.
Summary and perspectives
Variations in microbiota may be a new risk factor for a variety of cancers. Studies indicate that the urinary tract is not sterile in healthy individuals. A complex microbiome exists in the urinary tract, varying with gender, age and disease conditions. A preliminary study with a small sample size showed an enrichment of Streptococcus in urines from patients with urothelial carcinoma. Urine microbiota may play an important role in the pathogenesis of urothelial carcinoma. Large scale studies are required to adequately characterize the urine microbiota and prospective studies are needed to resolve the temporal order between the change of the microbiome and the development of urothelial carcinoma, a key issue related to reverse causality. If microbiotas are demonstrated to contribute to the development of urothelial carcinoma, this new knowledge will help us to design novel measures for prevention as well as treatment of urothelial carcinoma, via normalization of the urine microbiome.
Acknowledgements
The authors would like to thank the support of the genitourinary fellowship program of NYUSOM. This work was supported in part by grants U01CA18237, UH3CA140233, and R01CA159036 from the National Cancer Institute and NIH Human Microbiome Project and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
References
- 1.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34:415–425. doi: 10.1111/j.1574-6976.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 3.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stackebrandt E, Goebel BM. A Place for DNA-DNA Reassociation and 16s Ribosomal-Rna Sequence-Analysis in the Present Species Definition in Bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 5.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman LA. Systematic review: faecal transplantation for the treatment of Clostridium difficile-associated disease. Aliment Pharmacol Ther. 2012;35:865–875. doi: 10.1111/j.1365-2036.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 7.Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, Roizman B. Infectious agents and cancer: criteria for a causal relation. Semin Cancer Biol. 2004;14:453–471. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 8.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 9.George SE, Chadwick RW, Kohan MJ, Allison JC, Williams RW, Chang J. Role of the intestinal microbiota in the activation of the promutagen 2,6-dinitrotoluene to mutagenic urine metabolites and comparison of GI enzyme activities in germ-free and conventionalized male Fischer 344 rats. Cancer Lett. 1994;79:181–187. doi: 10.1016/0304-3835(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 11.Werts C, Rubino S, Ling A, Girardin SE, Philpott DJ. Nod-like receptors in intestinal homeostasis, inflammation, and cancer. J Leukoc Biol. 2011;90:471–482. doi: 10.1189/jlb.0411183. [DOI] [PubMed] [Google Scholar]
- 12.Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004;429:429–433. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- 13.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrede JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood) 2004;229:586–597. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 17.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011;11:244. doi: 10.1186/1471-2180-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, Drake MJ. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. doi: 10.3389/fcimb.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui H, Lagesen K, Nederbragt AJ, Jeansson SL, Jakobsen KS. Alterations of microbiota in urine from women with interstitial cystitis. BMC Microbiol. 2012;12:205. doi: 10.1186/1471-2180-12-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, Huang ST, Ljungberg I, Sprague BM, Lucas SK, Torralba M, Nelson KE, Groah SL. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:174. doi: 10.1186/1479-5876-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fettweis JM, Serrano MG, Girerd PH, Jefferson KK, Buck GA. A new era of the vaginal microbiome: advances using next-generation sequencing. Chem Biodivers. 2012;9:965–976. doi: 10.1002/cbdv.201100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol. 2012;66:371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]


