Abstract
Intermittent androgen deprivation therapy (IADT) allows prostate cancer patients a break from the side-effects of continuous androgen deprivation therapy (ADT). Although clinical studies suggest that IADT can significantly improve patient quality of life over ADT, it has not been demonstrated to improve patient survival. Recently, increased survival has been demonstrated when 5α-reductase inhibitors have been used during the off-cycle of IADT in animal xenograft tumor models LNCaP and LuCaP35. In the current study, the sensitivity of LAPC4 xenograft tumor regrowth to the 5ARI dutasteride was determined. Tumor regrowth and gene expression changes in LAPC4 tumors were compared to the previously determined response of LNCaP and LuCaP35 xenograft tumors to 5ARI treatment during the off-cycle of IADT, LAPC4, LNCaP and LuCaP35 tumors were sensitive to androgen manipulation. However, in contrast to LNCaP and LuCaP35, dutasteride treatment during testosterone-stimulated prostate regrowth did not affect tumor regrowth or the expression of androgen responsive genes. Tumor response to dutasteride during the off-cycle of IADT is variable in xenograft prostate tumor models. Future studies will be required to elucidate the mechanisms contributing to the dutasteride resistance observed in the LAPC4 model during the off-cycle.
Keywords: Prostate cancer, androgen deprivation therapy, LAPC4, EAF2, 5α-reductase inhibitor
Introduction
Intermittent androgen deprivation therapy (IA-DT) has been proposed as an alternative treatment for prostate cancer in an effort to decrease the complications associated with continuous androgen deprivation therapy (ADT). ADT is associated with several side-effects including loss of libido, hot flashes, anemia, fatigue, loss of muscle and bone mass as well as endocrine and metabolic abnormalities (reviewed in [1]). IADT consists of multiple cycles of androgen suppression (on-cycle) and periods of testosterone recovery (off-cycle), where PSA and other clinical markers, are used to guide off-cycle duration. In several clinical trials, IADT has been shown to significantly reduce the side-effects associated with continuous ADT [2-7]. A majority of prostate cancer patients with sexual side effects reported an increased sexual activity and well-being during IADT [6,8]. Some preclinical studies have also suggested that IADT might delay prostate tumor progression to castration resistance. IADT was shown to prolong the time to androgen-independent PSA regulation in LNCaP xenografts [9]. In the Shinogi tumor model, IADT induced a 3-fold delay in progression to androgen independence [10]. However, the impact of IADT on prostate cancer progression and survival in clinical trials is less clear. Several randomized controlled trials failed to demonstrate a statistically significant difference in survival between IADT and ADT in patients [3,11-15]. Moreover, a recent large trial of 1535 men reported that IADT was associated with decreased survival compared to ADT in metastatic prostate cancer [16]. In a recent meta-analysis study comparing the efficacy of IADT versus ADT with respect to all-cause and disease-specific mortality, there was no difference in overall survival, but a small increased risk in disease-specific survival for men treated with IADT relative to ADT [17].
In an effort to maximize the potential benefits of IADT, the addition of 5α-reductase inhibitors (5ARI) has also been proposed. The regressed prostate responds differently to 5ARI treatment, which blocks the conversion of testosterone to DHT, than the intact prostate. 5ARI treatment in testes-intact rats reduced the expression of androgen-responsive genes [18], whereas addition of 5ARI during testosterone-stimulated prostate regrowth after castration increased androgen-responsive gene expression [19]. Addition of 5ARI to the off-cycle increased survival over IADT alone in LNCaP xenografts when the off-cycle interval was fixed [20]. However, when the off-cycle was terminated based on a pre-determined tumor volume, 5ARI treatment doubled the off-cycle interval but had no effect on survival [21]. We recently demonstrated that 5ARI treatment in LuCaP35 and LNCaP xenograft tumors suppressed initial reg-rowth of regressed prostate tumors, suggesting that a short off-cycle plus 5ARI treatment could maximize prostate tumor growth suppression and potentially prolong survival [22].
Prostate tumors are highly heterogeneous and variability among patients is great, which adds to the complexity of treating prostate cancer. Androgen-sensitive prostate cancer cell line models also have distinct differences and experimental results across various cell lines can vary widely. The androgen receptor in the most frequently used androgen-sensitive prostate cancer cell line, LNCaP, contains a single point mutation which has been shown to affect both binding specificity and the induction of gene expression [23]. LuCaP35 is an androgen-sensitive, PSA producing xenograft that was derived from a prostate cancer lymph node metastasis and expresses the wild-type androgen receptor [24]. In the current study, the effects of dutasteride on gene expression during testosterone stimulated prostate tumor regrowth were examined in the androgen-sensitive LAPC4 xenograft tumor model. LAPC4 is a more recently developed prostate cancer cell line derived from a lymph node metastasis that expresses wild-type AR and secretes PSA [25]. Understanding the variation in tumor response to IADT and ADT across multiple prostate cancer xenograft models will provide critical insight for maximizing the benefits of IADT across various prostate cancer patients in the clinical setting.
Materials and methods
Animals
BALB/c strain of athymic SCID and Hsd: Athymic Nude-Foxn1nu strain of Nude male mice were obtained from Charles River Laboratory, Wilmington, MA and Harlan labs, Indianapolis, IN respectively and were kept in accordance with the National Institute of Health guidelines under standard animal housing conditions for the Care and Use of Experimental Animals. Animal experiments were approved by the Institutional Animal Care Use Committee (IACUC) at the University of Pittsburgh.
Xenograft tumor implantation
The LAPC4 cell line, which produces PSA, has wild-type androgen receptor, and shows features of hormone-dependent growth and metastasis [25], was a gift from Charles Sawyers, Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA and Robert Reiter, Department of Urology, Jonsson Comprehensive Cancer Center, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA. LAPC4 were maintained in IMDM, supplemented with 10% fetal bovine serum (FBS), glutamine, penicillin and streptomycin. LAPC4 cells underwent 4-8 passages in culture prior to mouse inoculation. Approximately 106 LAPC4 cells suspended in 250 μL media were mixed 1:1 with Matrigel (Becton Dickinson Labware, Bedford, MA) and then inoculated subcutaneously in the flank region of 6~8 week old male athymic SCID/Nude mice using a 25-gauge needle as previously described for LNCaP and LuCaP35 tumors [21,22].
Construction of testosterone and dutasteride pellets
Pellets were prepared as previously described [21,22]. Approximately 7.5 mg of testosterone (Sigma Chemical, St. Louis, MO) was tightly packed into a silastic tube with an inner and outer diameter of 1.58 mm and 3.18 mm, respectively (Helix Medical, Carpenteria, CA). Dutasteride (gift from GlaxoSmithKline) and pellets were made similarly; ~8 mg of dutasteride was packed into silicone tubing with an inner and outer diameter of 1.47 mm and 1.96 mm, respectively. Pellet ends were plugged with wooden sticks and sealed with a silicone adhesive (Dow Corning, Midland, MI). Following overnight air-drying, pellets were sterilized with 70% ethanol for 10 min and stored in a light-free environment.
Treatment protocol and measurement of tumor growth and gene expression
The experimental design is outlined in Figure 1. Tumors were measured weekly and volume was calculated by the modified ellipsoid formula: length x width2 x 0.52 [26]. For the castration group, trans-scrotal castration was performed under isoflurane anesthesia with proper aseptic and antiseptic technique as previously [21]. Mice were initially randomized into two groups, testes-intact (I) and castration (C), when xenograft tumors reached a volume of 200 mm3. Mice were treated using 2 different protocols as either castrated (C) or testes-intact (I) (Figure 1A). Castrated mice were randomized 14 days post-castration into 4 groups: 1) castration only (C), 2) castration + testosterone replacement (C+T), 3) castration + testosterone replacement + dutasteride (C+T+D), and 4) castration + dutasteride (C+D). Testes-intact mice were randomized on the same time schedule as castrated mice into 2 groups: intact (I), and intact + dutasteride (I+D). After randomization, all groups were followed for either 18 or 28 days (Figure 1B). Animals in the castration (C) and intact (I) groups were followed without any further intervention. Testosterone and/or dutasteride pellets were surgically implanted subcutaneously 14 days post-castration in the groups receiving additional treatment (Figure 1B). Tumor volume was measured every 2 days. Tumors were collected at day 18 or day 28, either 4 days or 14 days after pellet implant. Each experimental group consisted of 5-6 animals and tumors were collected at sacrifice and flash-frozen in liquid nitrogen for further analyses.
Figure 1.
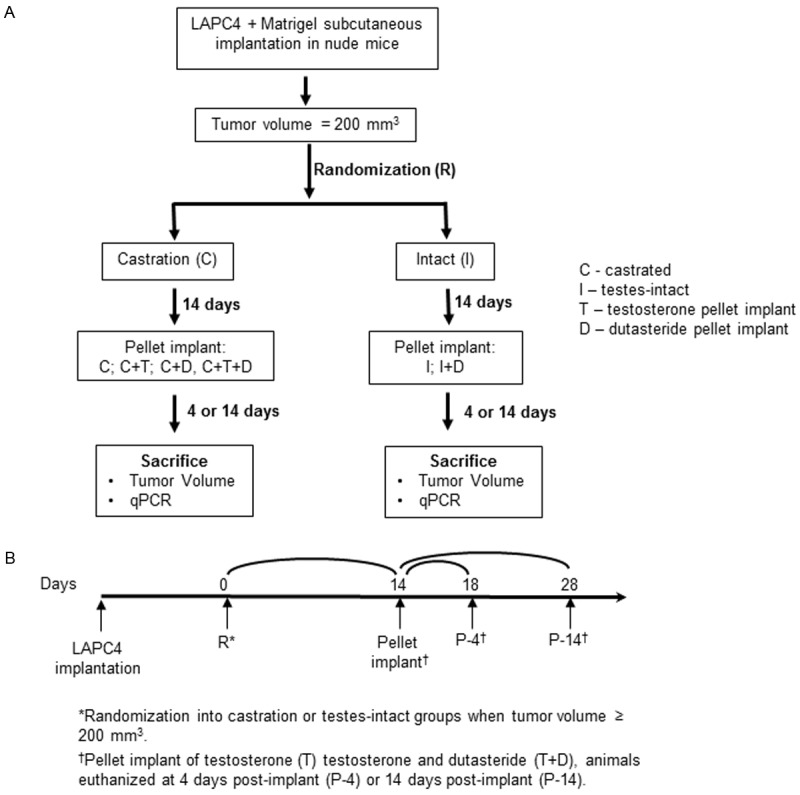
Experimental protocols. Tumor bearing nude mice were castrated and subjected to 2 different protocols, castrated (C) and testes-intact (I). Castrated animals were further randomized into 4 subgroups: implantation of testosterone (C+T), dutasteride (C+D), testosterone + dutasteride (C+T+D), and no intervention (C). Testes-intact animals were randomized into 2 subgroups: testes-intact (I) and testes-intact + dutasteride (I+D). Testosterone and/or dutasteride pellets were implanted on day 14 post-castration. Animals were euthanized and tumors were collected at day 18 and day 28 post-castration.
Androgen sensitivity was assessed as a statistically significant decrease in tumor volume in castrated compared to testes-intact animals within the first 14 days post-castration. Tumors that continued to grow within the 14 day period after castration were considered castration resistant and were excluded, as this study was designed to assess the ability of IADT coupled with 5ARI to inhibit tumor growth in LAPC4 xenograft tumor model.
Gene expression analysis
Quantitative real time reverse-transcriptase polymerase chain reaction (qPCR) was used to determine gene expression. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Approximately 2 μg of RNA was reverse transcribed with random primers using the high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Exon-exon junction spanning primers and Taqman probes were designed using Primer 3 software (Totowa, NJ) and synthesized by Integrated DNA Technologies (Coralville, IA). Primers used were: calreticulin For: 5’-GGATCGAATCCAAACACAAGTC-3’, Rev: 5’-TGGCTTGTCTGCAAACCTTTAT-3’; EAF2/U19 For: 5’-CCAGGACTCCCAATCTTGTAAA-3’, Rev: 5’-TAGCTTCTGCCTTCAGTTCTCTT-3’; ELL2 For: 5’-TGACTGCATCCAGCAAACAT-3’, Rev: 5’-TCGTTTGTTGCACACACTGTAA-3’; PSA For: 5’- GTCCCGGTTGTCTTCCTCA-3’, Rev: 5’-CACAATCCGAGACAGGATGAG-3’. Ex Taq™ 2X premix (Takara Bio Inc.) was used for real time PCR reactions with 0.25 μM of forward and reverse primers each. Reactions were run in triplicate on a Bio-Rad IQ5 (Bio-Rad Laboratories, Hercules, CA), and repeated on an ABI Step-One Plus (Applied Biosystems, Foster City, CA). ROX was used as passive reference dye. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as endogenous control, primer GAPDH: 5’-CATGTTCGTCATGGGTGTGA-3’, Rev: 5’-GGTGCTAAGCAGTTGGTGGT-3’. The specificity of the primer-probe combinations for their cDNA targets was confirmed by lack of amplification of human genomic DNA, mouse genomic DNA or mouse cDNA. qPCR data were exported into MS Excel and the expression of transcripts relative to GAPDH calculated by the ΔCP method: Relative Expression = 2-ΔCP, where ΔCP is the difference between the crossing point thresholds of target gene versus GAPDH, as described previously [27].
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc) and MS Excel 2003 (Microsoft) were used for statistical analysis and graphical composition. Data were expressed as the mean ± SEM, and statistical significance was determined by one-way ANOVA or Student’s t-test as appropriate. A P value of < 0.05 was considered statistically significant. The results were depicted on scatter plots to convey the expression patterns.
Results
LAPC4 xenograft tumors were sensitive to androgen manipulation
LAPC4 xenograft tumor volume in castrated animals (C) was significantly decreased compared to intact control animals (I) by day 14 post-castration, verifying the androgen sensitivity of LAPC4 xenograft tumors (Figure 2A). Intact animals displayed a steady growth increase to 140% of the original volume over 14 days, whereas tumor volume in castrated animals was reduced significantly by 20% by day 14 (**P < 0.01) (Figure 2A).
Figure 2.
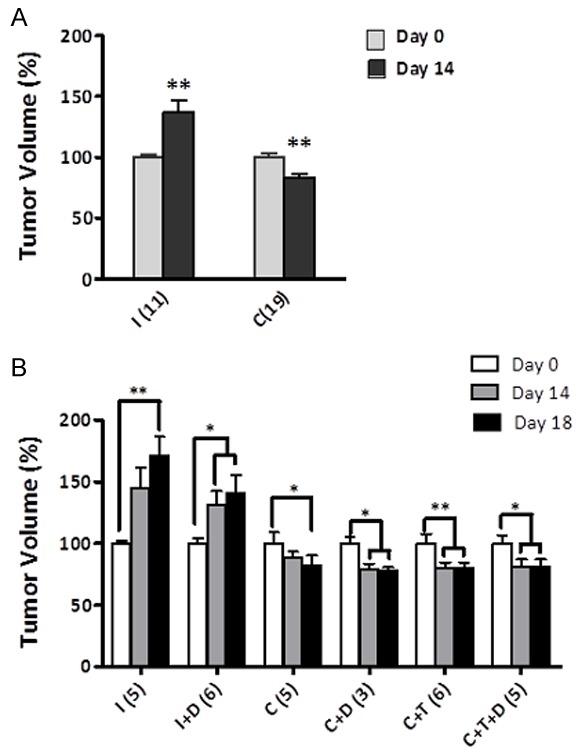
Response of LAPC4 xenograft tumors to androgen manipulation. A. Tumor volume in testes-intact (I) and castrated (C) at day 0 and day 14 post-castration. Values expressed as percentage of original volume (200 mm3). B. Response of LAPC4 tumors to 4 days of treatment. Tumor volume at day 0, day 14 and day 18 post-castration (4 days after pellet implant). Testes-intact (I), testes-intact plus dutasteride (I+D), castrated (C), castrated with testosterone replacement (C+T), castrated + dutasteride (C+D), castrated + testosterone replacement + dutasteride (C+T+D). Number of animals in each group is listed in parentheses. Values are expressed as mean ± SEM. (*, P < 0.05; **, P < 0.01).
In the LuCaP35 and LNCaP xenograft models, tumors in castrated animals treated with testosterone rapidly increased in volume within the first 4 days of testosterone replacement [22]. LuCaP35 and LNCaP xenograft tumor growth in castrated animals treated with testosterone plus dutasteride or finasteride was significantly inhibited during the first 4 days of treatment [22]. In contrast, LAPC4 xenografts in castrated animals treated with testosterone did not increase in volume during the first 4 days of treatment (Figure 2B). Furthermore, LAPC4 tumor growth was not significantly inhibited during the first 4 days of treatment with testosterone plus dutasteride or testosterone alone (p > 0.05). These findings suggested that although LAPC4 tumor growth is sensitive to castration, testosterone-stimulated response of regressed tumors differs from that of LNCaP or LuCaP35.
Dutasteride treatment had no effect on PSA, EAF2, CALR or ELL2 expression in LAPC4 xenograft after 4 days of testosterone replacement
Previous studies have demonstrated that dutasteride treatment during testosterone stimulated regrowth enhanced the expression of several androgen response genes, including EAF2 in the LNCaP [18] and LuCaP35 [22] xenograft models. Androgen responsive gene up-regulation was accompanied by a repression in growth of LNCaP and LuCaP35 xenograft tumors during the off-cycle when dutasteride was added. These studies suggested that short off-cycles coupled with 5α-reductase inhibition could suppress prostate tumor growth, potentially contributing to increased survival. In the current LAPC4 xenograft model, although PSA transcript level decreased significantly following castration and increased in response to testosterone replacement, dutasteride treatment alone had no significant effect (Figure 3A). Castrated animals treated with testosterone had a statistically significant increased expression of PSA compared to castrated controls. Castrated animals treated with testosterone replacement and dutasteride had a statistically significant increased expression in PSA over dutasteride treatment alone (C+D). However, in contrast to the previous findings in the LNCaP and LuCaP35 models [18,22], in castrated animals treated with testosterone plus dutasteride for 4 days, PSA expression was similar to testosterone replacement alone. Although not statistically significant, EAF2 expression was also increased in response to testosterone replacement in castrated animals (p=0.15), as well as in castrated animals treated with testosterone plus dutasteride compared to dutasteride alone (p=0.11) and not increased in C+T+D compared to C+T (Figure 3B). Androgen-response genes CALR and ELL2 were unaffected by castration in the LAPC4 xenograft model (Figure 3C, 3D). Dutasteride alone exerted no effect on the expression of this subset of androgen-responsive genes assayed in LAPC4 xenograft tumors of testes-intact (I+D), castrated mice (C+D), or castrated mice treated with testosterone and dutasteride (C+T+D). Furthermore, in contrast to the LNCaP [18] and LuCaP35 [22] models, CALR and ELL2 gene expression were not up-regulated by testosterone and dutasteride treatment. These results suggest that regressed LAPC4 xenograft tumors respond to dutasteride treatment differently than LNCaP and LuCaP35 xenografts with respect to induction of androgen-response genes during testosterone stimulated prostate re-growth.
Figure 3.
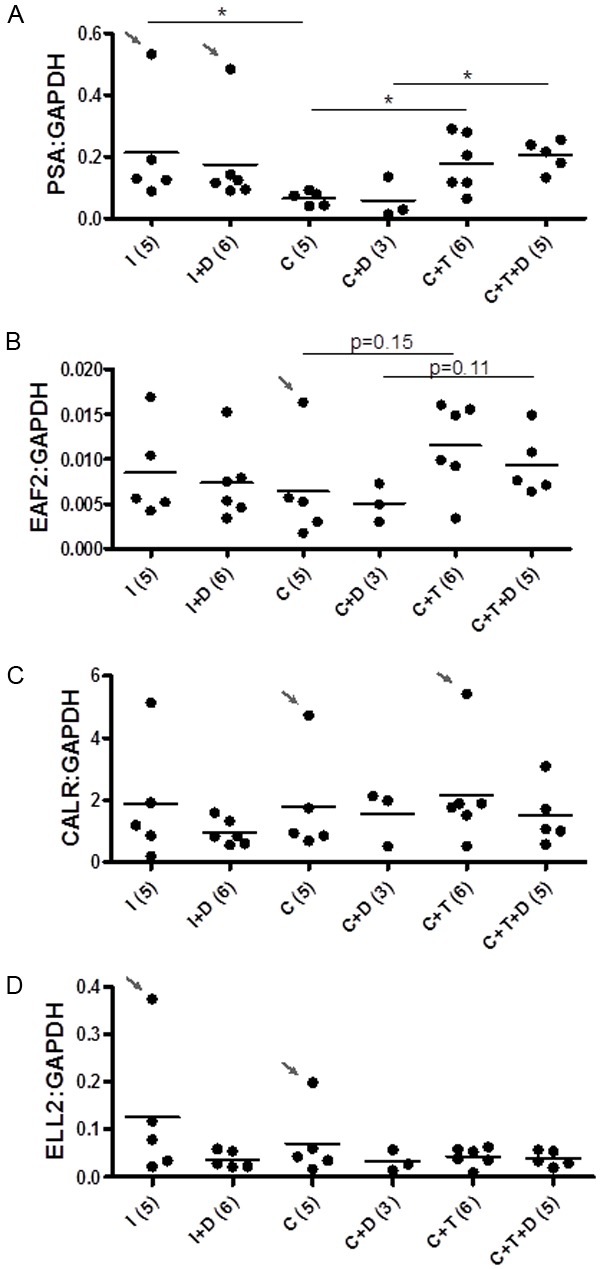
Effect of dutasteride on the expression of androgen-responsive genes in LAPC4 tumors 4 days after pellet implantation (day 18). QPCR analyses of A. PSA, B. EAF2, C. calreticulin (CALR) and D. ELL2, expression in LAPC4 xenograft tumors from the following groups: testes-intact (I), testes-intact plus dutasteride (I+D), castrated (C), castrated with testosterone replacement (C+T), castrated + dutasteride (C+D), castrated + testosterone replacement + dutasteride (C+T+D). Expression was normalized to GAPDH. Number of animals in each group is represented in parentheses.Each dot represents a single sample; line depicts the mean. (*, P < 0.05).
Dutasteride treatment did not inhibit LAPC4 xenografts tumor growth
LAPC4 tumor growth rate in both testes-intact animals as well as castrated animals with testosterone replacement was not altered by dutasteride treatment (Figures 2B and 4A). Although androgen-responsive gene expression was up-regulated during the initial phase of regrowth in regressed LAPC4 xenograft tumors, tumor growth was not affected by 5ARI during the off-cycle in this model. The overall change in tumor volume after 14 days of treatment was not significantly different between castrated animals treated with dutasteride during testosterone replacement (C+T+D) and testosterone alone (C+T). In comparison, previous findings demonstrated an inhibition of testosterone-induced regrwoth of LuCaP35 and LNCaP xenografts by dutasteride (Figure 4B, 4C).
Figure 4.
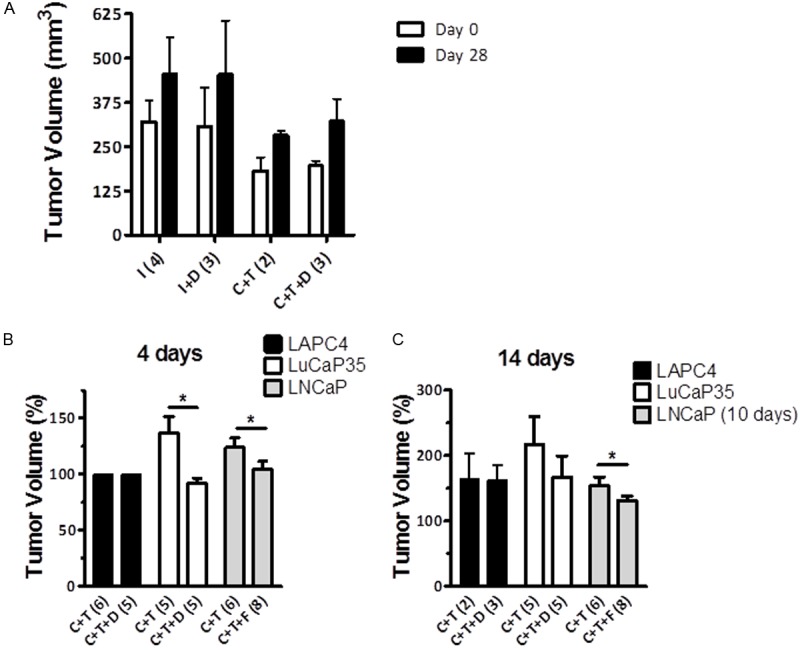
Effect of dutasteride on LAPC4 tumor regrowth 14 days after pellet implantation (day 28). A. Effect of dutasteride on LAPC4 tumor volume. Tumor volume in intact (I), intact + dutasteride (I+D), castrated with testosterone replacement (C+T), castrated + testosterone replacement + dutasteride (C+T+D) was determined at day 28 post-castration. Values are presented as mean ± SEM. Number of animals in each group is represented in parentheses. B. Response of androgen-sensitive xenograft tumors LAPC4, LuCaP35 and LNCaP to 5α-reductase inhibition during testosterone stimulated regrowth after 4 days of treatment (day 18). Tumor volume in castrated animals treated with testosterone (C+T) and testosterone plus dutasteride (C+T+D) for 4 days or finasteride (C+T+F) for 4 days. C. Response of androgen-sensitive xenograft tumors LAPC4, LuCaP35 and LNCaP to 5α-reductase inhibition during testosterone stimulated regrowth after 14 days of treatment (day 28). Tumor volume in castrated animals treated with testosterone (C+T) and testosterone plus dutasteride (C+T+D) for 14 days or finasteride (C+T+F) for 10 days. Data from LuCaP35 and LNCaP derived from previously published data [18,22]. Number of animals in each group is represented in parentheses. (*, P < 0.05).
Discussion
Prostate tumors are very heterogeneous in terms of grade, genetics, ploidy, and oncogene/tumor suppressor gene mutation/expression; which translates to tumor heterogeneity in biological, hormonal, and molecular characteristics [28]. Patients whose tumors are not suitable for surgical intervention or radiotherapy are frequently treated by hormonal intervention, either continuous or intermittent androandrogen deprivation, to suppress prostate cancer cell growth [29-31]. Androgen deprivation initially leads to tumor regression, but invariably, prostate cancer recurs and becomes castration resistant [32,33]. This is usually accompanied by alterations in the AR in the form of mutations [34,35], amplification [36], or hypersensitization [37]. Furthermore, recent studies have reported 5α-reductase isozyme differential expression in prostate cancer [38-40]. In one study, decreased expression of 5α-reductase 2 and increased expression of 5α-reductase 1 were observed in prostatic intraepithelial neoplasia (PIN) and prostate cancer [38]. Both 5α-reductase 1 and 2 were increased in high-grade compared to low-grade prostate cancer [41].
In previous studies, we demonstrated that 5α-reductase inhibition through finasteride or dutasteride treatment could prolong the survival of animals bearing LNCaP xenografts tumors on IADT when the off-cycle interval was fixed but not when off-cycle was prolonged. Short interval of off-cycles in the presence of finasteride or dutasteride stimulated prostatic differentiation, but not proliferation. In contrast, prolongation of the off-cycle in the presence of finasteride or dutasteride stimulated both prostatic differentiation and proliferation, with proliferation induction occurring subsequent to differentiation [22]. The inhibition of testosterone-stimulated regrowth by finasteride or dutasteride is associated with enhanced expression of tumor suppressive androgen-response genes such as EAF2, which only occurs during the initial phase but not prolonged regrowth during the off-cycle.
In the current study, LAPC4 xenograft growth was not affected by dutasteride treatment during testosterone replacement. LAPC4 tumors regressed in response to castration while tumors in intact animals continued to grow (Figure 2A). Although tumor growth did not differ between castrated controls and animals treated with dutasteride during testosterone-stimulated regrowth, LAPC4 xenografts in castrated animals had an increased expression of androgen-responsive gene PSA and EAF2 when treated with testosterone replacement (Figure 3). The induction of androgen-response genes during the initial regrowth of regressed tumors suggests that although tumor volume was not reduced, LAPC4 tumors were sensitive to testosterone during the first 4 days of treatment. However, LAPC4 tumor volume did not respond to dutasteride at 4 days of treatment, whereas LuCaP35 and LNCaP tumors had a statistically significant inhibition in tumor growth by dutasteride or finasteride (Figure 4B). Furthermore, at 14 days of treatment, there was no difference between the LAPC4 tumor xenografts treated with dutasteride plus testosterone and testosterone replacement only (Figure 4C). Both LuCaP35 and LNCaP tumors grew more slowly when treated with 5α-reductase inhibitor during testosterone replacement. These results suggest that tumor response to 5α-reductase inhibition during the off-cycle of IADT is variable in different androgen-sensitive tumors.
In summary, this study showed LAPC4 as a model for prostate cancer xenograft tumor insensitive to 5α-reductase inhibition. One potential mechanism is the inability of dutasteride to enhance the expression of androgen-regulated tumor suppressive genes such as EAF2. Recently, cultured LAPC4 cells were shown to have decreased sensitivity to dutasteride inhibition of AR signaling compared to LNCaP [42]. The ratio of DHT to testosterone varies among prostate cancer cell lines and is much higher in LAPC4 than in LNCaP cells [43], suggesting that the decreased sensitivity of LAPC4 to dutasteride might be due in part to a greater concentration of intracellular DHT.5α-reductase expression was increased in testosterone stimulated LNCaP cells but not in LAPC4 cells [43]. Regressed LAPC4 xenografts were also less sensitive to initial testosterone-stimulated regrowth compared to LuCaP35 and LNCaP tumors (see Figure 4B). Further studies will be required to fully elucidate the mechanisms involved in dutasteride resistance, particularly in testosterone-stimulated regrowth during the off-cycle of IADT. Understanding the mechanisms by which prostate cancer cells respond to or resist dutasteride inhibition of testosterone-induced regrowth may lead to the identification of molecular markers to identify patients who would be benefited from dutasteride administration in IADT.
Acknowledgements
We thank Charles Sawyers and Robert Reiter for LAPC4 cell line and GlaxoSmithKline (GSK) for providing dutasteride and members of Wang lab for critical reading. This study was supported by grants from the National Institute of Health, Prostate Cancer Specialized Program of Research Excellence (SPORE), CA90386 and R37 DK51193. This research was also supported by UPCI Animal Facility funded through NCI CCSG P30CA047904. KZM was recipient of a Mellam Fellowship and LEP is a Tippens Fellow.
Disclosure of conflict of interest
Authors have nothing to disclose.
References
- 1.Rashid MH, Chaudhary UB. Intermittent androgen deprivation therapy for prostate cancer. Oncologist. 2004;9:295–301. doi: 10.1634/theoncologist.9-3-295. [DOI] [PubMed] [Google Scholar]
- 2.Bruchovsky N, Klotz LH, Sadar M, Crook JM, Hoffart D, Godwin L, Warkentin M, Gleave ME, Goldenberg SL. Intermittent androgen suppression for prostate cancer: Canadian Prospective Trial and related observations. Mol Urol. 2000;4:191–199. discussion 201. [PubMed] [Google Scholar]
- 3.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, Frymire E, Malone S, Chin J, Nabid A, Warde P, Corbett T, Angyalfi S, Goldenberg SL, Gospodarowicz MK, Saad F, Logue JP, Hall E, Schellhammer PF, Ding K, Klotz L. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook JM, Szumacher E, Malone S, Huan S, Segal R. Intermittent androgen suppression in the management of prostate cancer. Urology. 1999;53:530–534. doi: 10.1016/s0090-4295(98)00547-0. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg SL, Gleave ME, Taylor D, Bruchovsky N. Clinical Experience with Intermittent Androgen Suppression in Prostate Cancer: Minimum of 3 Years’ Follow-Up. Mol Urol. 1999;3:287–292. [PubMed] [Google Scholar]
- 6.Klotz LH, Herr HW, Morse MJ, Whitmore WF Jr. Intermittent endocrine therapy for advanced prostate cancer. Cancer. 1986;58:2546–2550. doi: 10.1002/1097-0142(19861201)58:11<2546::aid-cncr2820581131>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Scholz MC, Jennrich RI, Strum SB, Johnson HJ, Guess BW, Lam RY. Intermittent use of testosterone inactivating pharmaceuticals using finasteride prolongs the time off period. J Urol. 2006;175:1673–1678. doi: 10.1016/S0022-5347(05)00975-4. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg SL, Bruchovsky N, Gleave ME, Sullivan LD, Akakura K. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology. 1995;45:839–844. doi: 10.1016/s0090-4295(99)80092-2. discussion 844-835. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Gleave ME, Bruchovsky N, Rennie PS, Goldenberg SL, Lange PH, Sullivan LD. Intermittent androgen suppression delays progression to androgen-independent regulation of prostate-specific antigen gene in the LNCaP prostate tumour model. J Steroid Biochem Mol Biol. 1996 May;58:139–46. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 10.Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782–2790. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Calais Da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA, Kirkali Z, Calais Da Silva FM, Robertson C. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 12.De Leval J, Boca P, Yousef E, Nicolas H, Jeukenne M, Seidel L, Bouffioux C, Coppens L, Bonnet P, Andrianne R, Wlatregny D. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163–171. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 13.Langenhuijsen JF, Badhauser D, Schaaf B, Kiemeney LA, Witjes JA, Mulders PF. Continuous vs. intermittent androgen deprivation therapy for metastatic prostate cancer. Urol Oncol. 2013;31:549–556. doi: 10.1016/j.urolonc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Mottet N, Van Damme J, Loulidi S, Russel C, Leitenberger A, Wolff JM. Intermittent hormonal therapy in the treatment of metastatic prostate cancer: a randomized trial. BJU Int. 2012;110:1262–1269. doi: 10.1111/j.1464-410X.2012.11120.x. [DOI] [PubMed] [Google Scholar]
- 15.Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TL. The FinnProstate Study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol. 2012;187:2074–2081. doi: 10.1016/j.juro.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 16.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Kanaga Sundaram S, Small EJ, Dawson NA, Donnelly BJ, Venner PM, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Ely B, Moinpour CM, Vogelzang NJ, Thompson IM Jr. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai HT, Penson DF, Makambi KH, Lynch JH, Van Den Eeden SK, Potosky AL. Efficacy of intermittent androgen deprivation therapy vs conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82:327–333. doi: 10.1016/j.urology.2013.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Wang Y, Ramos-Garcia R, Shevrin D, Nelson JB, Wang Z. Inhibition of 5alpha-reductase enhances testosterone-induced expression of U19/Eaf2 tumor suppressor during the regrowth of LNCaP xenograft tumor in nude mice. Prostate. 2010;70:1575–1585. doi: 10.1002/pros.21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dadras SS, Cai X, Abasolo I, Wang Z. Inhibition of 5alpha-reductase in rat prostate reveals differential regulation of androgen-response gene expression by testosterone and dihydrotestosterone. Gene Expr. 2001;9:183–194. doi: 10.3727/000000001783992551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggener SE, Stern JA, Jain PM, Oram S, Ai J, Cai X, Roehl KA, Wang Z. Enhancement of intermittent androgen ablation by “off-cycle” maintenance with finasteride in LNCaP prostate cancer xenograft model. Prostate. 2006;66:495–502. doi: 10.1002/pros.20297. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Gupta S, Hua V, Ramos-Garcia R, Shevrin D, Jovanovic BD, Nelson JB, Wang Z. Prolongation of off-cycle interval by finasteride is not associated with survival improvement in intermittent androgen deprivation therapy in LNCaP tumor model. Prostate. 2010;70:147–154. doi: 10.1002/pros.21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masoodi KZ, Ramos Garcia R, Pascal LE, Wang Y, Ma HM, O’Malley K, Eisermann K, Shevrin DH, Nguyen HM, Vessella RL, Nelson JB, Parikh RA, Wang Z. 5alpha-reductase inhibition suppresses testosterone-induced initial regrowth of regressed xenograft prostate tumors in animal models. Endocrinology. 2013;154:2296–2307. doi: 10.1210/en.2012-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–669. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 24.Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 2003;55:239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 25.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 26.Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229–234. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 27.O’Malley KJ, Langmann G, Ai J, Ramos-Garcia R, Vessella RL, Wang Z. Hsp90 inhibitor 17-AAG inhibits progression of LuCaP35 xenograft prostate tumors to castration resistance. Prostate. 2012;72:1117–23. doi: 10.1002/pros.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell P, Kingsley E. Human Prostate Cancer Cell Lines. In: Russell P, Jackson P, Kingsley E, editors. Prostate Cancer Methods and Protocols. New York: Springer; 2003. pp. 21–39. [Google Scholar]
- 29.Paul R, Breul J. Antiandrogen withdrawal syndrome associated with prostate cancer therapies: incidence and clinical significance. Drug Saf. 2000;23:381–390. doi: 10.2165/00002018-200023050-00003. [DOI] [PubMed] [Google Scholar]
- 30.Rambeaud JJ. Intermittent complete androgen blockade in metastatic prostate cancer. Eur Urol. 1999;35(Suppl 1):32–36. [PubMed] [Google Scholar]
- 31.Sciarra A, Casale P, Colella D, Di Chiro C, Di Silverio F. Hormone-refractory prostate cancer? Anti-androgen withdrawal and intermittent hormone therapy. Scand J Urol Nephrol. 1999;33:211–216. doi: 10.1080/003655999750015790. [DOI] [PubMed] [Google Scholar]
- 32.Laufer M, Denmeade SR, Sinibaldi VJ, Carducci MA, Eisenberger MA. Complete androgen blockade for prostate cancer: what went wrong? J Urol. 2000;164:3–9. [PubMed] [Google Scholar]
- 33.Lara PN Jr, Meyers FJ. Treatment options in androgen-independent prostate cancer. Cancer Invest. 1999;17:137–144. [PubMed] [Google Scholar]
- 34.Culig Z, Hobisch A, Hittmair A, Peterziel H, Cato AC, Bartsch G, Klocker H. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate. 1998;35:63–70. doi: 10.1002/(sici)1097-0045(19980401)35:1<63::aid-pros9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 35.Marcelli M, Ittmann M, Mariani S, Sutherland R, Nigam R, Murthy L, Zhao Y, DiConcini D, Puxeddu E, Esen A, Eastham J, Weigel NL, Lamb DJ. Androgen receptor mutations in prostate cancer. Cancer Res. 2000;60:944–949. [PubMed] [Google Scholar]
- 36.Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, Delprado W, Stricker PD, Grygiel JJ, Sutherland RL. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61:423–427. [PubMed] [Google Scholar]
- 37.Gregory CW, Johnson RT Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- 38.Thomas LN, Lazier CB, Gupta R, Norman RW, Troyer DA, O’Brien SP, Rittmaster RS. Differential alterations in 5alpha-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate. 2005;63:231–239. doi: 10.1002/pros.20188. [DOI] [PubMed] [Google Scholar]
- 39.Titus MA, Gregory CW, Ford OH 3rd, Schell MJ Maygarden SJ, Mohler JL. Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365–4371. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 40.Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, Titus MA, Mohler JL. 5alpha-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate. 2011;71:1033–1046. doi: 10.1002/pros.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas LN, Douglas RC, Lazier CB, Too CK, Rittmaster RS, Tindall DJ. Type 1 and type 2 5alpha-reductase expression in the development and progression of prostate cancer. Eur Urol. 2008;53:244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 42.Chhipa RR, Halim D, Cheng J, Zhang HY, Mohler JL, Ip C, Wu Y. The direct inhibitory effect of dutasteride or finasteride on androgen receptor activity is cell line specific. Prostate. 2013;73:1483–1494. doi: 10.1002/pros.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Godoy A, Azzouni F, Wilton JH, Ip C, Mohler JL. Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5alpha-reductase inhibitors. Prostate. 2013;73:1470–1482. doi: 10.1002/pros.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]


