Abstract
Recent clinical studies have raised the clinically important question of the relationship between dihydrotestosterone (DHT) and prostate cancer (PCa) progression. The significance of DHT or 5α-reductase inhibitors (5ARI) in PCa development and progression has not yet been fully characterized. The aim of this study was to determine whether the initiation of DNA replication was influenced by DHT in PCa. Three cell lines were used. LNCaP: a human PCa cell line that exhibits androgen-dependent proliferation, C4-2: a human PCa cell line that exhibits androgen-independent proliferation, and C4-2AT6: a castration resistant prostate cancer cell line. Two 5ARIs, finasteride and dutasteride, were used. We examined the mRNA expression of the components of pre-replication complex (Pre-RC), CDC6, CDT1, and MCM2-7. DHT induced cell proliferation of LNCaP accompanied by significantly increased CDC6, CDT1, and MCM2-7 expression. In contrast to LNCaP, DHT inhibited cell proliferation in C4-2AT6 cells accompanied by decreased expression of CDC6, CDT1, and MCM2-7. These reverse effects resemble the effects of 5ARIs in Pre-RC. Treatment with finasteride or dutasteride inhibited CDC6 expression in LNCaP, but both 5ARIs induced CDC6 expression in C4-2 and C4-2AT6 cells.These results indicate that DHT showed reversal effects on PCa cell proliferation among prostate cancer cells based on androgen-dependence, accompanied by regulation of the initiation of DNA replication. 5ARIs may modulate the DNA replication system in someaggressive PCa through up-regulation of CDC6 expression.
Keywords: Castration resistant prostate cancer, DNA replication, pre-replication complex, CDC6, 5α-reductase inhibitors, C4-2AT6
Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignant tumor in men living in the United States and Europe [1]. Androgen receptor (AR) activity is essential for prostate cancer development, growth, and progression [2]. The dependence of PCa on AR signaling at all stages of the disease has therefore been exploited in the treatment of advanced tumors, for which ablation of AR function by androgen deprivation therapy (ADT) is the goal of first-line therapy [3]. However a significant subset of these patients will develop evidence of biochemical relapse, leading to lethal metastatic castration resistant prostate cancer (CRPC). CRPC is an advanced form of the disease characterized by disease progression following surgical or medical castration [3]. The key molecular events associated with PCa progression remain to be elucidated.
Clinical evidence accumulated thus far indicates CRPC remains dependent on the expression and transcriptional activity of very low levels of androgens, suggesting a potential therapeutic target in CRPC [4-6]. Recent advances have raised the clinically important question of the relationship between dihydrotestosterone (DHT) and PCa [7]. The PCPT trial [8] and REDUCE trial [9], which investigated the role of 5α-reductase inhibitors (5ARIs) in the context of prostate cancer prevention have shed light on the relationship between DHT and PCa progression. Tumors found in patients treated with a 5ARI were of a higher grade than the tumors in those administered a placebo, although the significant reduction of low grade PCa were identified [10]. Therefore, the use of 5α-reductase inhibitors (5ARIs) to prevent the development of PCa continues to be widely discussed [11,12]. Despite this clinical importance, only a few studies have reported the significance of DHT in human CRPC.
Recently we have reported that human CRPC cells exhibited significantly reduced cell viability when treated with DHT, which wa accompanied by reduced 5α-reductase activities [13]. Our results showed that CRPC could be treated with androgens due to the inhibitory action of excess androgens. However, the associations of DHT and cancer cell proliferation in CRPC progression are poorly understood. CRPC cells may have an unknown regulation system to protect themselves from the androgenic suppressive effect mediated by unknown mechanisms.
In this study, we investigated the reversal effects of DHT on the induction of androgen-dependent proliferation of the prostate cancer cell line: LNCaP and the suppression of androgen-dependent proliferation of the CRPC cells: C4-2AT6. We especially focused on the components of the initiation of DNA replication, which is a well coordinated process that ensures duplication of the genome only once per cell division cycle in mammal cells [14,15]. Their activities are strictly regulated by pre-replicationcomplex (Pre-RC) and origin recognition complex (ORC) [16,17]. Therefore, we sought to determine whether the initiation of DNA replication may be influenced by DHT in PCa.
Materials & methods
Cell lines and culture
LNCaP cells were obtained from ATCC. C4-2 cells were obtained from UroCor (Oklahoma City, OK). LNCaP and C4-2 cells were routinely maintained in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, at 37°C in a humidified atmosphere with 5% CO2. C4-2AT6 cells were established from C4-2 as previously reported [18]. Briefly, C4-2 cells were grown in RPMI-1640 containing 10% charcoal stripped fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere. Cells were passaged upon attaining confluence during a 6 month period. We named this cell line C4-2AT6; that is, C4-2 cells subjected to androgen ablated treatment for 6 months.
Chemicals
DHT was purchased from Sigma (Tokyo, Japan). Finasteride and dutasteride were obtained from SantaCruz Biotechnology (Santa Cruz, CA, USA).
WST cell viability assay
LNCaP cells were plated in 96-well plates and then allowed to attach for 24 h. After overnight serum starvation, the cells were treated with different concentrations of DHT for 96 h. At the end of the incubation period, water soluble tetrazolium (WST) reagents were added to each well followed by incubation for 1 hr. Cell viability was estimated by colorimetry, using a plate reader at 570 nm.
Real-time quantitative PCR
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Hilden, Germany), and the quantity and quality were evaluated by spectrophotometry. Reverse transcription of RNA to cDNA was conducted using a High Capacity cDNA Archive Kit (Applied Biosystems). The reaction mixture (1 μL) was then used as a template in a TaqMan Fast real-time quantitative PCR assay using Taqman Universal PCR Master Mix and the 7500 Fast Real-time PCR system (Applied Biosystems). The primers and TaqMan probe sets (TaqMan Gene Expression Assays) for PSA (Hs02576345_m1), Nkx3.1 (Hs00171834_m1), TMPRSS2 (Hs01120965_m1), CDC6 (Hs00154374_m1), CDT1 (Hs00368864_m1) and human GAPDH endogenous control (Hs99999903_m1) were purchased from Applied Biosystems (sequences not disclosed).The cycling conditions were 50°C for 10 minutes, 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and at 60°C for 1 minute.
Statistics
Experiments were performed with 3 or more replicates. Statistical analysis was performed using the Mann-Whitney U test and Dunnett’s test for multiple comparison with p<0.05 considered significant. These analyses were performed with the SPSS Version 21.0 statistical software package (SPSS Corporation, Chicago, IL, USA).
Results
Effects of DHT on prostate cancer cell proliferation
We investigated and compared the viability of prostate cancer cells treated with DHT at various concentrations for 96 h (Figure 1A). Increasing the concentration of DHT on LNCaP cells had a positive effect on cell proliferation in a dose-dependent manner, as previously shown [13]. When treated with 0.1 nM DHT, LNCaP cells demonstrated significant maximal increased cell viability compared with that at lower DHT concentration. On the other hand, C4-2AT6 cells showed significantly lower cell viability at the same concentration of DHT compared to LNCaP cells. When treated with 0.1 nM DHT, C4-2AT6 cells showed significantly decreased cell viability compared with that at lower DHT concentrations. Increasing concentrations of DHT did not have a positive effect on C4-2AT6 cell proliferation. As shown in Figure 1A, the inhibitory effects on C4-2AT6 cells were more marked than those on LNCaP cells treated with 0.1 nM DHT.
Figure 1.
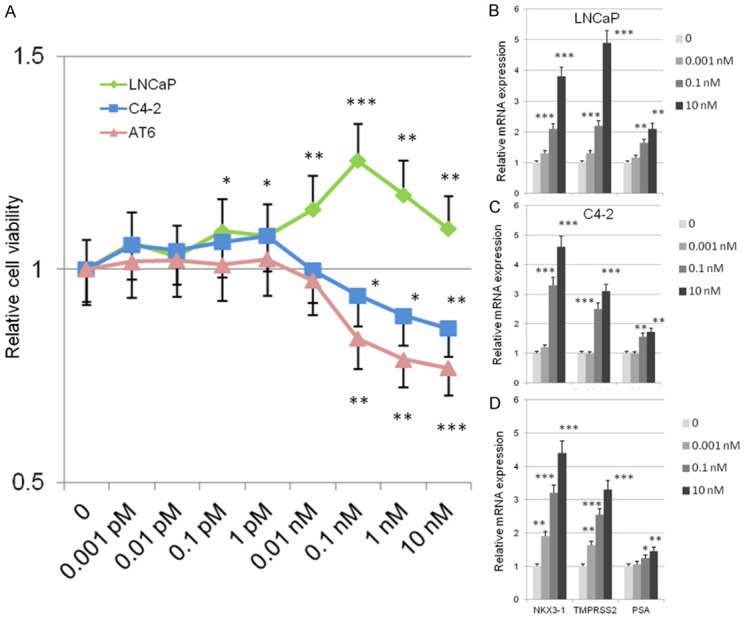
Effects of DHT on prostate cancer cell proliferation. A: WST cell viability assay exhibited a significant proliferative response to DHT at 0.1 nM DHT in LNCaP cells. When treated with 10-10 M to 10-8 M DHT, C4-2AT6 cells exhibited significantly decreased cell viability compared with that at lower DHT concentrations. B-D: mRNA expression of PSA, Nkx3.1, and TMPRSS2 in LNCaP, C4-2 and C4-2AT6 cells. The expression of each probe was normalized to 0 h to yield fold changes. *p<0.05, **p<0.01, ***p<0.001.
Next, we investigated the expression of AR target genes: PSA, Nkx3.1, and TMPRSS2. The mRNA expression was determined by qPCR at different concentrations of DHT at 24 h (Figure 1B-D). All of these AR target genes were significantly increased in a dose-dependent manner in all the cell lines. These results suggest the existence of an inverse effect of DHT on cancer cell proliferation which was not paralleled by the induction of AT target genes.
Reverse effects of DHT on DNA replication system among prostate cancer cells
To investigate the mechanism of the inverse effect of DHT on cancer cell proliferation, we searched for an androgen-induced gene in relation to the initiation of DNA replication related factors. The DNA replication system is strictly regulated by a pre-replication complex (Pre-RC) and an origin recognition complex (ORC) [16,17]. In eukaryotic cells, the replicative helicase, MCM (2-7), is loaded as a double hexamer on double-stranded DNA at replication origins [14,15]. MCM loading is dependent on ORC that interacts with CDC6 to mediate recruitment of MCM (2-7) and CDT1 [16,17]. These components are critical to DNA replication in cancer cells. We performed quantitative real-time PCR (qPCR) for CDC6, CDT1 and MCM2, MCM3, MCM4, MCM5, MCM6, and MCM7.
qPCR analysis demonstrated that CDC6 mRNA expression in LNCaP cells was significantly induced in a DHT dose-dependent manner, which was paralleled by the increase of LNCaP cell proliferation (Figure 2A). There was no significant difference in CDC6 mRNA expression among C4-2 cells treated by various concentrations of DHT (Figure 2A). On the other hand, CDC6 mRNA expression was significantly inhibited in C4-2AT6 cells in a DHT dose-dependent manner to the same extent as the decrease of C4-2AT6 cell proliferation by DHT (Figure 2A). These findings suggest DHT has an inverse effect on the initiation of DNA replication in LNCaP and C4-2AT6 cells. With respect to CDT1 expression, qPCR analysis revealed CDT1 mRNA in LNCaP cells was significantly induced ina DHT dose dependent manner to the same extent as the increase of LNCaP cell proliferation by DHT (Figure 2B). There was no significant difference in CDT1 mRNA expression in C4-2 cells treated by DHT, a result which is the same as that observed in the mRNA expression of CDC6. On the other hand, CDT1 mRNA expression was significantly inhibited in C4-2AT6 cells in a DHT dose-dependent manner (Figure 2B), and this was paralleled by the decrease of C4-2AT6 cell proliferation, suggesting DHT has an inverse effect on the initiation of DNA replication between LNCaP and C4-2AT6 cells.
Figure 2.
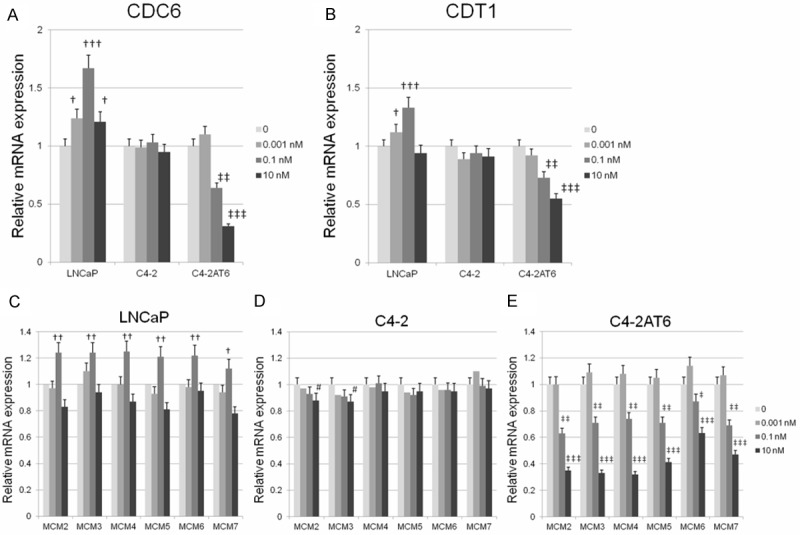
Effects of DHT on DNA replication system in LNCaP, C4-2, and C4-2AT6 cells. (A, B) qPCR analysis of CDC6 and CDT1 mRNA expression in LNCaP, C4-2, and C4-2AT6 cells after treating the cell with 0.001-10 nM DHT. (C-E) mRNA expression of MCM2-7 in LNCaP (C), C4-2 (D) and C4-2AT6 (E) cells. The expression of each probe was normalized to 0 h to yield fold changes. †,‡p<0.05; ††,‡‡p<0.01; †††,‡‡‡p<0.001.
Next, we investigated the mRNA expression of the replicative helicase, MCM (2-7). qPCR analysis revealed mRNA expression of MCM2, MCM3, MCM4, MCM5, MCM6, and MCM7 in LNCaP cells was significantly induced by DHT at a dose of 0.1 nM. At 10 nM DHT showed significant reduction of MCM2, MCM5, and MCM7 which was paralleled by a decrease in LNCaP cell proliferation (Figure 2C). In C4-2 cells treated with various concentrations of DHT, there was no significant difference in MCM4, MCM5, MCM6, or MCM7 mRNA expression treated by various concentrations of DHT. The mRNA expression of MCM2 and MCM3 was significantly but slightly inhibited in a DHT dose-dependent manner (Figure 2D). On the other hand, the mRNA expression of MCM2, MCM3, MCM4, MCM5, MCM6, and MCM7 was significantly inhibited in C4-2AT6 cells in a DHT dose-dependent manner, which was paralleled by a decrease in C4-2AT6 cell proliferation (Figure 2E).
These results indicate DHT has an inverse effect on the initiation of DNA replication among PCa cell lines through its regulation of the expression of the components of the pre-replication complex (Pre-RC) and origin recognition complex (ORC).
Different effects of DHT on DNA replication system among prostate cancer cells
A recent study shed light on the coordination of DHT and cell proliferation in human PCa and the use of 5ARIs for PCa prevention [7,8,13]. The use of 5α-reductase inhibitors (5ARI) may have a negative effect on preventing PCa development, otherwise 5ARIs may induce higher grade tumors with aggressiveness. Our previous reports showed that neither dutasteride nor finasteride had an inhibitory effect on C4-2 and C4-2AT6 cells at clinically achievable 5ARI concentrations, although these two 5ARIs exhibited significant inhibitory actions in LNCaP cells within the clinically achievable 5ARI concentration. As shown in Figure 3A, which summarizes the change in mRNA expression of Pre-RC at the dose of 0.1 nM DHT relative to each control, DHT induced the expression of components of Pre-RC in androgen-dependent cells, but not in castration-resistant cancer cells. We attempted to ascertain whether treatment with a 5ARI affects the expression of components of Pre-RC within the clinically achievable 5ARI concentration range of 1-100 nM. qPCR analysis demonstrated that CDC6 mRNA expression in LNCaP cells was slightly but significantly inhibited at the dose of 100 nM dutasteride and 100 nM finasteride (Figure 3B). On the other hand, slightly increased CDC6 mRNA expression was observed at concentrations of 10-100 nM dutasteride and 100 nM finasteride in C4-2 cells (Figure 3B). Moreover, in C4-2AT6 cells, CDC6 expression was significantly induced by 10-100 nM dutasteride and 10-100 nM finasteride (Figure 3B). qPCR analysis demonstrated that CDT1 mRNA expression in LNCaP cells was slightly but significantly inhibited at 100 nM dutasteride and 100 nM finasteride (Figure 3C). On the other hand, there was no significant change in CDT1 mRNA expression in C4-2 and C4-2AT6 cells (Figure 3B). No significant change in expression of MCMs was observed in LNCaP, C4-2, or C4-2AT6 cells (data not shown).
Figure 3.
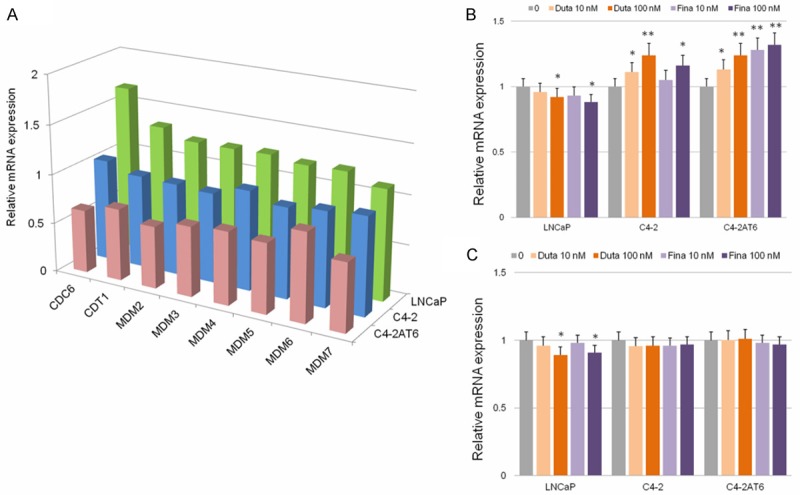
Effects of dutasteride and finasteride on DNA replication system in LNCaP, C4-2, and C4-2AT6 cells. A: Summarized mRNA expression change of pre-RC components treated with DHT at 0.1 nM. The expression was normalized to 0 h to yield fold changes. B: qPCR analysis demonstrated that CDC6 mRNA expression in LNCaP cells was inhibited by dutasteride and finasteride. In contrast to LNCaP cells, dutasteride and finasteride did induce CDC6 mRNA expression in C4-2 and C4-2AT6 cells. C: qPCR analysisdemonstrated that CDT1 mRNA expression in LNCaP cells was inhibited by dutasteride and finasteride. There was no significant change in CDT1 mRNA expression in C4-2 cells and C4-2AT6 cells. *p<0.05, **p<0.01, ***p<0.001.
Discussion
The initiation of DNA replication in mammal cells is a well coordinated process that ensures duplication of the genome only once per cell division cycle. Furthermore. it is strictly regulated by Pre-RC and ORC [14-17]. The present results clearly show that DHT has an inverse effect on the initiation of DNA replication in PCa, accompanied by elevated mRNA expression of almost all the components involved in the initiation of DNA replication, which are critical to DNA replication in cancer cells. Moreover, we clearly showed that treatment with 5ARI affects CDC6 mRNA expression within the clinically achievable concentrations in PCa cells. A simplified schema of the relationship among DHT, 5ARI, and DNA replication in PCa is presentedin Figure 4.
Figure 4.
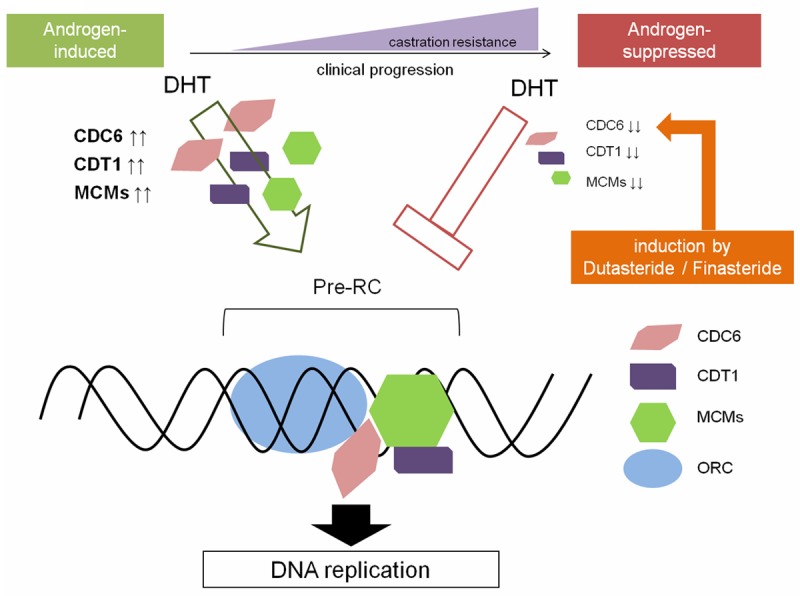
Simplified schema of the relationship between DHT and DNA replication in PCa, with a particular focus on castration resistance of prostate cancer.
AR axis plays an important role in the development and progression of prostate cancer by promoting prostate cancer cell proliferation. AR acts as one of the regulators of G1-S phase progression in androgen-dependent prostate cancer cells, suggesting AR can act as an initiator for DNA replication in androgen-sensitive prostate cancer cells [19,20]. Androgen-ablation therapy for prostate cancer initially triggers cell-cycle arrest of prostate cancer cells. However, nearly all invasive or metastatic prostatecancers eventually progress into a fatal androgen-independent and castration-resistant disease, yet most of these cancers paradoxically continue to express AR and remain dependent on AR for growth and survival [3]. Actually, a recent clinical study of human CRPC with new agents targeting AR axis found that AR axis is essential for CRPC progression [21,22]. Therefore, identifying the specific genes regulatied by AR axis is critical for understanding the mechanisms of androgen-dependent and -independent prostate cancer cell growth and proliferation. In order to investigate the mechanism of the inverse effect of AR axis and DHT on prostate cancer cell proliferation, we attempted to identify the DHT-induced or repressed genes involved in the initiation of DNA replication related factors in this study. We used three cell lines; LNCaP, which are an androgen-dependent, C4-2 which are androgen-independent and C4-2AT6 cells as a CRPC cell line [13,18,23-25].
The initiation of DNA replication in mammal cells is a well coordinated process that ensures duplication of the genome only once per cell division cycle [14,15,17]. Their activity is strictly regulated by Pre-RC and ORC. CDC6 and CDT1 mRNA expression in LNCaP cells was significantly induced by DHT in a dose-dependent manner. On the other hand, CDC6 and CDT1 mRNA expression in C4-2AT6 cells was significantly inhibited by DHT in a dose dependent manner. The inverse effect of DHT on CDC6 and CDT1 expression parallels the change in cancer cell proliferation, suggesting DHT has an inverse effect on the initiation of DNA replication among cancer cells through its modification of the components of Pre-RC and ORC, which are critical to DNA replication in cancer cells (Figures 3A, 4).
The use of 5ARIs to prevent progression of PCa continues to be widely discussed because the potential risk of more aggressive tumors outweighs their potential for chemoprevention [7,11,12]. Two large randomized, placebo-controlled trials, the ProstateCancer Prevention Trial (PCPT) with finasteride and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial have been conducted [8,9]. The PCPT trial was the first large-scale study to investigate the role of finasteride in prostate cancer development. Tumors detected in patients treated with finasteride were of a higher grade than the tumors in those administered a placebo. The REDUCE trial showed an overall reduction in the number of tumors with a low Gleason score in patients receiving dutasteride, however, tumors with a high Gleason score of 8-10 were more frequent in the dutasteride-treated group than in the placebo group. The FDA reanalyzed these two major trials and stated that the absolute incidence of tumors with Gleason scores between 8 and 10 was increased by 0.7% with finasteride and by 0.5% with dutasteride, resulting in it recommending against an indication for 5ARI to reduce PCa risk [7]. These observations still cannot be fully explained and whether or not 5ARIs increase the rates of high-grade disease remains a matter of debate. The decision by FDA not to approve the use of 5ARIs to prevent prostate cancer indicates that further basic and clinical investigations exploring the role of 5-ARIs in the development and progression of PCa are warranted. In this study, we clearly showed that 5ARIs within clinically achievable concentrations have an effect on CDC6 expression. In androgen-dependent LNCaP cells, CDC6 mRNA expression was slightly but significantly inhibited,however, in C4-2 and C4-2AT6 cells, 5ARIs induced significant CDC6 mRNA expression. These results indicate that 5ARIs may contribute to acceleration of adaptation to aggressive phenotypes in some PCa populations (Figure 4).
The present results clearly show DHT has an inverse effect on the initiation of DNA replication in PCa, accompanied by elevated mRNA expression of the components of initiation of DNA replication. These findings may represent a new potential mechanism for the development of aggressive PCa by 5ARIs.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
Disclosure of conflict of interest
The authors declare no conflict of interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 2009;10:981–991. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 6.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 7.Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365:97–99. doi: 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA Jr. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 9.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 10.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120:1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 11.Walsh PC. Chemoprevention of prostate cancer. N Engl J Med. 2010;362:1237–1238. doi: 10.1056/NEJMe1001045. [DOI] [PubMed] [Google Scholar]
- 12.Parker C. What (if anything) to do about low-risk prostate cancer. Lancet. 2012;379:1078–1080. doi: 10.1016/S0140-6736(12)60066-X. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka T, Miyajima A, Nagata H, Maeda T, Kikuchi E, Oya M. Human castration resistant prostate cancer rather prefer to decreased 5alpha-reductase activity. Sci Rep. 2013;3:1268. doi: 10.1038/srep01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blow JJ, Gillespie PJ. Replication licensing and cancer--a fatal entanglement? Nat Rev Cancer. 2008;8:799–806. doi: 10.1038/nrc2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlado LR, Mendez J. CDC6: from DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis. 2008;29:237–243. doi: 10.1093/carcin/bgm268. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Cid A, Riera A, Tognetti S, Herrera MC, Samel S, Evrin C, Winkler C, Gardenal E, Uhle S, Speck C. An ORC/Cdc6/MCM2-7 complex is formed in a multistep reaction to serve as a platform for MCM double-hexamer assembly. Mol Cell. 2013;50:577–588. doi: 10.1016/j.molcel.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Frigola J, Remus D, Mehanna A, Diffley JF. ATPase-dependent quality control of DNA replication origin licensing. Nature. 2013;495:339–343. doi: 10.1038/nature11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosaka T, Miyajima A, Shirotake S, Kikuchi E, Hasegawa M, Mikami S, Oya M. Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate. 2010;70:162–169. doi: 10.1002/pros.21049. [DOI] [PubMed] [Google Scholar]
- 19.Litvinov IV, Vander Griend DJ, Antony L, Dalrymple S, De Marzo AM, Drake CG, Isaacs JT. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci U S A. 2006;103:15085–15090. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin F, Fondell JD. A novel androgen receptor-binding element modulates Cdc6 transcription in prostate cancer cells during cell-cycle progression. Nucleic Acids Res. 2009;37:4826–4838. doi: 10.1093/nar/gkp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 23.Shirotake S, Miyajima A, Kosaka T, Tanaka N, Kikuchi E, Mikami S, Okada Y, Oya M. Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am J Pathol. 2012;180:1008–1016. doi: 10.1016/j.ajpath.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Yasumizu Y, Miyajima A, Kosaka T, Miyazaki Y, Kikuchi E, Oya M. Dual PI3K/mTOR Inhibitor NVP-BEZ235 Sensitizes Docetaxel in Castration Resistant Prostate Cancer. J Urol. 2014;191:227–34. doi: 10.1016/j.juro.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka T, Miyajima A, Shirotake S, Suzuki E, Kikuchi E, Oya M. Long-Term Androgen Ablation and Docetaxel Up-Regulate Phosphorylated Akt in Castration Resistant Prostate Cancer. J Urol. 2011;185:2376–81. doi: 10.1016/j.juro.2011.02.016. [DOI] [PubMed] [Google Scholar]


